Abstract
Objective
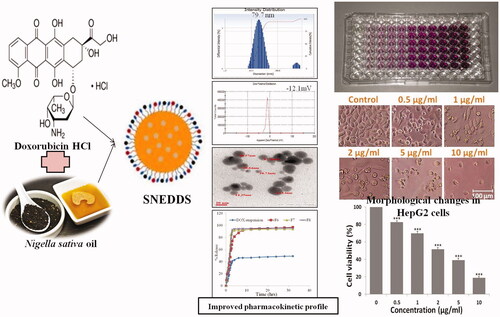
The development of self nano emulsifying co-delivery system of doxorubicin and Nigella sativa oil for potentiating the anticancer effects against HepG2 cell lines.
Materials and methods
SNEDDS were formulated by using Labrafil and N. sativa oil (3:2% w/w), Kolliphor RH40 (15% w/w), glycerol (5% w/w) as oil phase, surfactant and co-surfactant while deionized water (75% v/v) used as an aqueous phase. Optimized SNEDDS was evaluated for drug release and in vitro anticancer efficacy in liver cancer (HepG2) cell line.
Results and discussion
The selected formulation (F6) has a mean particle size of 79.7 nm with PDI 0.098 and the minimum viscosity of 16.42 cps with % transmittance of 1.332 with maximum drug release of 96.968% in 32 h as compared to DOX alone. Stability data showed stable emulsion in both 250C and -40C. F6 showed improved efficacy in HepG2 cells by cytotoxicity, showed significant results p<.05 with 2.5 μg/ml of (inhibitory concentration) IC50.
Conclusion
The overall study displayed that co-delivery of DOX and Nigella sativa oil in the form of SNEDDS may be an efficient carrier for further in vivo studies using oral delivery in human hepatocellular carcinoma in mammals.
Graphical Abstract
Introduction
Hepatocellular carcinoma (HCC) has a rising incidence as the sixth most frequent type of primary cancer of the liver in the world; however, the third common cause of cancer-mediated death is because of the high prevalence of alcoholism, hepatitis, aflatoxin and its complications [Citation1]. HCC may affect more than 100 out of 100,000 people who live in some parts of Asia and Africa. In Europe and the United States, secondary liver cancers are more frequent than primary liver cancer, but in Asia and Africa, primary liver cancer is more common [Citation2]. Several risk factors, such as alcoholism, viral hepatitis (hepatitis B infection is more common in Asia and hepatitis C is more common in the United States), aflatoxin in stapled foods, diethylnitrosamine, nonalcoholic steatohepatitis, diabetes and obesity, hemochromatosis, alpha-1-antitrypsin deficiency and minor risk factors such as anabolic steroids, vinyl chloride, arsenic, parasitic infection, smoking and dietary sugar. HCC develops in the functional cells of the liver called the hepatocytes and accounts for 82% of all liver cancers diagnosed [Citation3].
Four decades ago, doxorubicin renowned as early anthracyclines was isolated from Streptomyces peucetius and it is one of the effective drugs for cancer chemotherapy. According to reports, doxorubicin follows seven different mechanisms of producing cellular dysfunction or death [Citation4]. It is commonly believed that the anticancer activities of doxorubicin on cancer cells are associated with nucleotide base intercalation. The DOX molecule stabilizes the topoisomerase II enzyme and breaks the DNA chain for copying, stopping the DNA double helix from resealing and, therefore, prevents the progression of replication [Citation5]. Crystallographic information recommends that the planar tetracyclic fraction of the molecule intercalates among two base pairs of the DNA, whereas the amino sugar sites within the minor groove and interacts with flanking base pairs instantly adjacent to the intercalation site. Furthermore, the tumor-suppressor protein p53 is responsible for many forms of genotoxic agent-induced apoptosis [Citation6]. Doxorubicin-induced programmed cell death could lead to therapeutic effects and toxicities [Citation4]. In spite of the good efficacy of doxorubicin, cardiotoxicity is the serious side effect following treatment. In addition, anthracyclines are likely to cause alopecia and myelosuppression and oral ulcerations [Citation7].
It has been confirmed that DOX induces programmed cell death in normal cells and tumor cells through various mechanisms. Various possibilities have been investigated to reduce the toxicity, modify drug distribution, and enhance the antitumor effect of DOX. Different types of nanoparticulate delivery approaches of DOX have been developed to reduce the exposure of drug to the normal cells [Citation8]. Some combination regimens were recommended with DOX therapy to deal with its side effects [Citation9], as well as therapeutic efficacy of doxorubicin. So, liver cancer therapy could be improved by using nanoparticles as drug carriers, such as doxorubicin-intercalated nano-hydroxy apatite drug-delivery system, hematoporphyrin-modified, doxorubicin-loaded nanoparticles, injectable pH sensitive DOX-chitin-poly (l-lactic acid) composite biodegradable nanogels (PLA-CNGs), DOX-folic acid-chitosan (CHI) modified single-walled carbon nanotubes, proteins like transferrin and bovine serum albumin [Citation10,Citation11]. A further promising approach to increase the efficacy and lower the side effects of antineoplastic drugs like DOX is delivering them in nanocarriers such as emulsification systems [Citation12–14]. Anticancer drugs loaded in nanoemulsion systems have greater activity against cancer cells in comparison to other emulsification systems. Thus, via nano delivery system, the particle size can be reduced, which produced stable water dispersion, with reduced polydispersity index and greater stability of the drug in NE [Citation15].
Nigella sativa is an important herb/drug in the Unani and Ayurveda, Chinese and Arabic system of medicine [Citation16]. Nigella sativa seeds mainly contain 30–35% fixed oil, alkaloid, saponin and proteins and 0.5–2.5% of essential oil. The main phytochemicals of black seeds are thymoquinone, thymol, cymene, carvacrol, g-tocopherol, t-anethole, 4-terpineol, longifoline and all-trans retinol [Citation17]. NS oil was found to induce oxidative stress in a dose-dependent manner, which was specified by induction of ROS (reactive oxygen species) and LPO (lipid peroxidation) along with decline in reduction of glutathione (GSH) and metalloproteinase (MMP) Quantitative real-time polymerase chain reaction (PCR) data confirmed that following the exposure of Hep G2 cells to Nigella oil, the level of mRNA expression of apoptotic marker genes (p53, bax, caspase-3, and caspase-9) was up-regulated, while anti-apoptotic gene bcl-2 was down-regulated [Citation18]. The Nigella sativa seed lipid extract inhibited skin carcinogenesis in mice as topical application while it delayed the onset of papilloma formation when given as an intraperitoneal injection at a dose of 100 mg/kg, body weight. N. sativa alcoholic extract was also found to cure oral cancers [Citation19], inhibit endothelial cells progression so inhibit cancer cells growth [Citation20] and efficiently influence the survival rate of MCF-7 cells against H2O2 induced oxidative stress with 50% LC50 (lethal concentration) of 796.7 µg/ml [Citation21]. In addition, lipid extract of N. sativa has the cytotoxic effect to MCF-7 cells with LC50 (lethal concentration) at 2.72 ± 0.232 mg/ml, whereas aqueous extract produced cytotoxicity at a concentration of about -50 mg/ml. The Nigella sativa oil is a promising antitumor drug and its cytotoxicity action was enhanced in nanoemulsion formulation. Mahmoud et al. revealed that the antitumor activity of doxorubicin was enhanced when given with N. sativa extracts as adjunct therapeutic agents. The addition of DOX to the lipid nanoemulsion has a valuable impact on their therapeutic effects [Citation22]. Many researchers have confirmed that Nigella sativa has shown protective effects on doxorubicin-induced toxicity in h9c2 (cardiac myoblast) cells and isoproterenol-induced myocardial infarction by alleviating oxidative stress, biochemical alterations and histological damage [Citation23]. N. sativa oil also produced a cardio-protective effect against lead-induced cardiotoxicity by reducing the pro-inflammatory cytokines; in oxidative stress and cardiac tissue damage and shield the action of antioxidant enzymes [Citation24]. Ebru et al. confirmed that pretreatment with N. sativa oil diminished the cyclosporine-A induced cardiotoxicity [Citation25]. Moreover, recent studies of thymoquinone against doxorubicin-induced cardiotoxicity also revealed a protective effect of thymoquinone [Citation26].
Self-nano emulsifying drug delivery systems (SNEDDS) have a particular attention to various research groups, due to their ability to increase the oral bioavailability of the drug. Such delivery system can enhance the bioavailability of the drug by avoiding the first pass effect, protect drug in GI environment, reduces gastric irritation and facilitates intestinal lymphatic transport of drugs, controlled and sustained drug delivery, preventing pre-absorptive metabolism by gut membrane-bound cytochrome enzymes and hindered P-gp mediated drug efflux [Citation27]. Some reports suggest the bioavailability of some poorly water-soluble drugs that will be improved while formulating their SNEDDS such as docetaxol, cefpodoxime proxetil, atorvastatin and rosuvastatin calcium, candesartan cilexetil, Sunitinib, Rivaroxaban, glyburide, darunavir [Citation28,Citation29]. In comparison to the mortality rate, the available chemotherapeutic options are limited for HCC. Chemotherapy using doxorubicin appears to be one of the non-curative therapeutic approaches in liver cancer patients. DOX systemic therapy has been evaluated for many years but the response rate is below 30% and the survival benefit is also minimal. The uses of adjuvant or neoadjuvant systemic chemotherapy for liver resection may show the clinical benefits [Citation4]. However, based on our knowledge, enhancement in the doxorubicin bioavailability and reducing side effects mainly cardiotoxicity using Nigella sativa oil through SNEDDS formulation has yet not been approached. In the present study, SNEDDS comprised of Labrafil, Kolliphor RH 40, and glycerol for co-delivery of doxorubicin and Nigella sativa oil was designed, characterized and evaluated in HepG2 cells. It would be particularly valuable to explore the best use of DOX in combination with NS oil as SNEDDS for therapy of HCC.
Materials and methods
Chemicals and reagents
Labrafil® M1944 CS (oleoyl polyoxyl-6 glycerides) and Capryol 90 (propylene glycol monocaprylate) were gift samples from Gattefosse India Pvt. Ltd., while Kolliphor RH-40 and Sefsol 218 (propylene glycol monocaprylic ester) were gifted sample from Nikko Chemicals, Tokyo, Japan, respectively. Polyethylene glycol (PEG-200, 400, 600), distilled, deionized water and methanol (HPLC-Grade) were purchased from E-Merck, Mumbai, India. Ethanol, isopropyl alcohol, Tween 20 (Polyoxyethylene sorbitan monolaurate), Tween 80 (polyoxyethylene sorbitan monooleate), span 80 (Sorbitan monooleate) and glycerol were purchased from SD Fine Chemicals (Mumbai, India). The Nigella sativa seeds and brand of edible oil used for solubility study were based on the highest availability in the market. Doxorubicin hydrochloride, fluorescent dye DCFH-DA (2,7-dichlorodihydrofluorescein diacetate) and DAPI (4',6'-diamidino-2 phenylindole) were procured from Sigma Aldrich, (St. Louis, Missouri). Fetal bovine serum (FBS), Eagle’s minimal essential medium (MEM), MTT (3-[4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] dye and antibiotic solutions were obtained from Himedia, India. HepG2 cells and Chang liver cells were obtained from the National Cell Repository NCCS, Pune, India. All the other chemicals were of analytical grade.
Fixed oil extraction
Nigella sativa seeds were obtained from the local supermarket and authenticated by Department of Pharmacognosy and Phytochemistry, Faculty of Pharmacy, Integral University, Lucknow, U.P. (The accession no. IU/PHAR/HRB/17/02). About 50 g of Nigella seeds were taken, slightly crushed and added to the soxhlet condenser. In 500 ml capacity round bottom flask, 250 ml hexane was taken and the temperature was maintained at 70 °C. The extraction process was allowed to run for 48 h, then the viscous liquid was collected in a round bottom flask. These were taken out and evaporated at 65 °C on a water bath until evaporation of hexane.
Physicochemical analysis of Nigella sativa oil
The different physicochemical properties [i.e. color, odor, taste, % oil yield, pH, boiling point, refractive index, specific gravity, iodine number, saponification value (mg KOH/g) and total acid number (mg KOH/g)] of extracted Nigella sativa oil were investigated according to AOAC [Citation30].
GC-MS analysis
The GC-MS analysis of extracted Nigella sativa oil was performed on a GC-MS instrument GCMS-QP2010 Ultra. TR 5-MS capillary standard non-polar column, dimension: 30 m × 0.25 mm ID × 0.25 µm film thickness was used and flow rate of mobile phase carrier gas (Helium) was set at 0.5 ml/min. The temperature of the GC oven was raised from 100 °C–260 °C at 10 °C min−1 and 5 μl sample was injected. Sample dissolved in n-hexane was run fully at a range of 10–850 m/z and the results were interpreted by Wiley spectral library search program [Citation31]. The name, molecular formula, molecular weight, and structure of the component of the sample were determined while the percentage amount of each component was intended by evaluating its average peak area to the total areas.
Screening of SNEDDS formulation components
Solubility studies
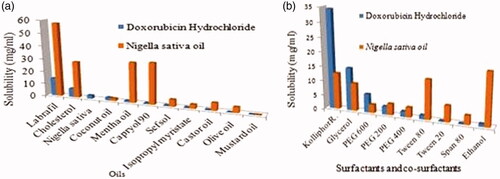
The solubility of DOX was measured by adding an excess amount of drug into 1 ml of the vehicle followed by vortex mixing for 5 min (vortex mixer Remi cyclo, CM-101plus, India). One millilitre of different oils and a mixture of oils (Capryol 90, Labrafil M 1944 CS, cholesterol, isopropyl myristate, Nigella oil, coconut oil, mentha oil, castor oil, mustard oil and olive oil) was taken in small vials separately and an excess amount of the drug was added to each vial. The vials were tightly stoppered and then these were kept at 25 ± 1.0 °C in an isothermal shaker for 72 h to reach equilibrium. After the equilibrium samples were removed from the shaker and centrifuged at 3000 rpm for 15 min. The supernatant was filtered through 0.45 µm Millipore membrane filter paper to remove the remaining insoluble drugs. Subsequently, supernatants were appropriately diluted with mobile phase and drug concentrations were measured at 494 nm by HPLC. Each experiment was repeated in triplicate. All the excipients selected for SNEDDS formulation were generally regarded as a safe category (GRAS) and finally, excipients were selected on the basis of the highest solubility and miscibility of drug and oil in different excipients [Citation32,Citation33].
Screening of surfactants for emulsifying ability
Different surfactants were screened for emulsification ability. The oil was mixed with each surfactant (Labrasol, Kolliphor RH 40, PEG 600, PEG 400, PEG 200 Tween 80, Tween 20, Span 80, and ethanol) at different ratio and vortexed for 5 min to homogenize the components. Then, 0.5 ml of each mixture was diluted with 50 ml double deionized water to obtain SNEDDS. After standing for 2 h, these SNEDDS were visually observed for physical appearance, phase separation, and clarity and their transmittance were evaluated at 494 nm on a UV-vis spectrophotometer (Schimadzu) using double distilled water as a blank. These measurements were performed in triplicate.
Screening of co-surfactants
The selected oil phase and surfactant were used to screen co surfactants based on emulsification capability. Particularly, 200 mg of surfactant, 100 mg of co-surfactant (ethanol, glycerol, isopropyl alcohol, n-butanol, carbitol and propylene glycol) and 300 mg of oil were thoroughly mixed by stirring at 37°C to obtain SNEDDS [Citation34,Citation35]. All evaluations were performed in triplicate ().
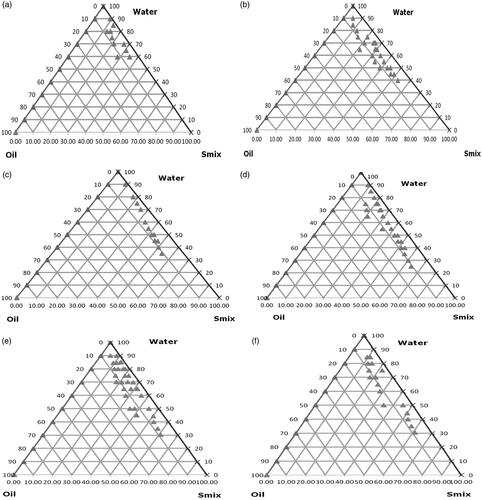
Construction of pseudo-ternary phase diagrams
Construction of pseudo-ternary phase diagrams were based on the solubility studies of DOX HCl in several components, Labrafil and Nigella sativa oil (3:2) as oil phase and Kolliphor RH 40 and Glycerol were selected as surfactant and co-surfactant for the development of SNEDDS of DOX HCl whereas deionized water was selected as the aqueous phase. These Smix ratios were chosen to reflect increasing concentrations of co-surfactant with respect to surfactant and increasing concentrations of surfactant with respect to co-surfactant for detailed study of the phase diagrams in the SNEDDS formation. Kolliphor RH 40 and glycerol (Smix) were mixed in ratios of 1:0, 1:1, 1:2, 2:1, 3:1 and 4:1. For each phase diagram, oil and definite Smix ratio were mixed well with volume ratios ranging from 1:9 to 9:1 and titrated with deionized water for constructing self-nano emulsifying zone. The concentrations of all excipients were used within acceptable limits which are recommended for oral usage [Citation36].
Development of SNEDDS
SNEDDS were developed using phase titration method keeping the water as dispersion media. SNEDDS formulations were selected for final studies, according to ternary phase diagram, based on the region that the concentration of oil phase should be such that it is able to dissolve 2 mg of the DOX easily. From the developed pseudo-ternary phase diagrams, various amount of Labrafil that solubilized 2 mg of DOX was chosen to develop different SNEDDS of DOX. Furthermore, the formulations were selected from the ternary phase diagrams by giving an eminence that has a minimum concentration of surfactants. Suitable nanoemulsions of DOX and Nigella were developed by phase titration method. While the ratio of Smix, oil and aqueous medium of SNEDDS was selected on the basis of the emulsifying regions obtained from the pseudo-ternary phase diagrams [Citation32]. Finally, Labrafil, Kolliphor RH 40 and glycerol was selected as oil, surfactant and co-surfactant, respectively and were added into the oil phase in the chosen concentration, further deionized water was added drop by drop till the sample become clear and transparent liquid with continuous vortexing. The detailed ratio of prepared nano emulsifying drug delivery system of DOX is mentioned in .
Table 1. Composition of SNEDDS formulation using labrafil: Nigella sativa oil (3:2), kolliphor RH 40, glycerol and deionized water.
Physicochemical characterization of SNEDDS
Stability studies of selected SNEDDS via thermodynamic methods
Developed SNEDDS of DOX HCl were subjected to different stability testings in order to discard unstable SNEDDS for further studies. These tests were carried out through the following methods like heating and cooling, centrifugation and freeze-thaw cycles by following the method reported in the literature [Citation32].
Heating-cooling cycle
Heating-cooling cycles involved six cycles of storage at 40 °C and 40 °C for 48 h. The formulations were evaluated either for any drug precipitation or phase separation. Thereafter, the formulations were diluted with distilled water up to 1:25 and the resulting SNEDDS were evaluated for any instability issues [Citation32]. Passed formulations were selected for further stability studies like centrifugation.
Centrifugation study
All formulations were diluted with distilled water up to 1:25 and centrifuged at 5000 rpm for 30 min. Then, instability problems of SNEDDS were observed for whether there was any phase separation, cracking or creaming occurred or not. If formulations showed any type of instability that were rejected and those passed the test were selected for further studies [Citation35].
Freeze-thaw cycle/accelerated aging
This stability study includes storage of formulation at temperatures -21 °C and -25 °C for 48 h, thereafter, the SNEDDs were centrifuged for 5 min at 3000 rpm to evaluate for any phase separation and drug precipitation. Further, all formulations were diluted up to 1:25 with distilled water and then the diluted SNEDDS were evaluated for any type of instability problems [Citation29]. Suitable stable SNEDDS were selected for characterization and further studies.
Assessment of robustness to dilution
This study comprised of dilution of SNEDD formulations up to 50-, 100- and 1000-fold with various solvents like distilled water, phosphate buffer (pH 6.8) and with 0.1 N HCl. Then, SNEDDS were stored for 24 h and observed for any alterations like precipitation, cloudiness or if there was any phase separation. Formulations which appear clear or slightly bluish tint at different dilution folds in dissimilar media were considered for further evaluation [Citation29].
Evaluation of self-nanoemulsification propensity
Self-nanoemulsification propensity was performed in order to assess the rate of efficiency at which self-emulsification was possible when 1 ml of formulation (F1–F8) diluted, when added dropwise to 250 ml of distilled water, 0.1 N HCl and phosphate buffer (pH 6.8) in a USP 24 paddle-type dissolution apparatus II with 50 rpm, while the temperature remained at 37 ± 0.5 °C. Self-emulsification time was confirmed when the appearance changed from a homogenous dispersion to the disappearance of SNEDDS was observed. Self-emulsification efficiency of various formulations was given in grades according to a grading system () [Citation37]. Formulations of those that passed the dispersibility test in all vehicles showed grade A, which were selected for further studies. Based on the results of stability studies and self-emulsification potency of formulations, six formulations (F2, F3 and F5–F8) were selected for further characterization.
Table 2. Observations for dispersability testing in SNEDDS.
SNEDDS characterization
Droplet size and zeta potential analysis
All physicochemical characterization of the sample was performed in triplicates manner. The mean droplet size and polydispersity index of selected (F2, F3, F5–F8) formulations were determined by dynamic light scattering (DLS) method using a “Nano ZS, Malvern Zetasizer” (Malvern Instruments, Malvern, UK). DLS analyzed fluctuations in the intensity of light scattering due to the Brownian movement of the particles [Citation29]. The scattering angle for measurement was set at 90°. Zeta potential was also measured by photon correlation spectroscopy. The prepared SNEDSS were diluted by dispersing (0.1 ml of formulation in 10 ml (100 dilutions) of Milli-Q® water in a volumetric flask and gently mixed by inverting the flask and measurement done at 90° angle.
Viscosity
The viscosity of the preferred formulations was measured at 25 ± 0.5 °C by Brookfield Viscometer DV III ultra V6.0 RV cone and plate rheometer (Brookfield Engineering Laboratories, Middleboro, MA) [Citation36].
Percentage transmittance
Percentage transmittances of the selected SNEDDS were measured after dilution and keeping distilled water as a blank by spectrophotometrically using Shimadzu UV-visible spectrophotometer [Citation37].
Measurement of drug contents
The selected formulations were analyzed for the drug contents from SNEDDS formulations that were extracted in ethanol. The solutions were filtered, using Whatman filter paper of 0.45 μm pore size and analyzed for the drug contents through HPLC [Citation36].
Transmission electron microscopy (TEM)
Details on the morphology and other structural features of the SNEDDS formulations were viewed using Transmission Electron Microscope (TEM), Model EM-410m LS facility available at CSIR-Indian Institute of Toxicology Research, Lucknow (India). Prior to the analysis, the samples (F6) were diluted at 10–100 times with water and applied on the grids which were stained with 2% (w/v) phosphotungstic acid for 30 s and then grids were observed after drying, using a combination of bright field imaging at increasing magnification to reveal the form and size of SNEDDS droplets [Citation29,Citation36].
In vitro drug release study
Comparative in vitro release profile of the selected DOX SNEDDS (F6) and the pure DOX in phosphate buffer (pH 6.8), were studied using dialysis bag techniques at 50 rpm rotational speed. The temperature was maintained at 37 ± 0.5 °C. Drug release was carried out by placing 1 ml of DOX SNEDDS in a treated dialysis bag (MWCO 14,000 g/mole, Sigma, USA). 1 ml aliquots were withdrawn at predetermined time intervals (0, 0.25, 0.5, 0.75, 1, 1.25, 1.5, 1.75, 2, 2.25, 2.5, 2.75, 3, 4, 6, 8, 12, 16, 20, and 24 h) and same volume was replaced with fresh dissolution media to maintain the sink condition. The samples withdrawn were filtered using 0.45 μm filter paper and the drug was analyzed by reported HPLC method [Citation36].
In vitro cytotoxicity effect of DOX SNEDDS on HepG2 cell
The methodologies used for in vitro testing are followed as done by Di et al. and confirmed that Diallyl disulfide enhances carbon ion beams-induced apoptotic cell death in cervical cancer cells through regulating Tap73/ΔNp73 [Citation38].
Cell viability assay
To detect cell viability of DOX SNEED approximately 10,000 cells/well of Chang liver cells as control and HepG2 were seeded in 100 μl culture medium in 96-well culture plate and incubated overnight in humidified air. The stock solution was prepared in phosphate buffer saline (PBS) and diluted into culture medium to the required concentrations of 0.5, 1, 2, 5 and 10 μg/ml, then added to the wells. After 24 h of incubation, 10 μl of MTT (5 mg/ml in PBS) reagent was added and re-incubated at 37 °C until purple formazan crystals developed. Formazan blue crystals were dissolved in 100 μl of DMSO and read at 540 nm using microplate ELISA reader (BIORAD 680 , USA). The plot of percent cell viability versus SNEDDS concentrations was used to calculate the concentration lethal to 50% of the cells (IC50). The cellular morphological changes were observed under inverted phase contrast microscopy (Nikon ECLIPSE Ti-S , Japan) [Citation39,Citation40].
Cell morphology study
Alterations in HepG2 and Chang liver cells morphology were observed after treatment with DOX SNEDDS at 0.5, 1, 2, 5 and 10 μg/ml concentrations. Concisely, 10,000 HepG2 and Chang liver cells per well were seeded in 96 wells culture plate and treated with DOX SNEDDS at 0.5, 1, 2, 5 and 10 μg/ml concentrations for 24 h, then cellular morphology of HepG2 cells was observed with the help of Nikon inverted phase-contrast microscope [Citation39].
Nuclear fragmentation assays
The nuclear fragmentation or nuclear cell death in HepG2 cells was observed with the help of fluorescent nuclear dye stain DAPI as per previous protocol. Apoptotic effect of DOX SNEED was added at a selected concentration (1, 2 and 5 μg/ml), following the incubation period, cells were washed and fixed in 4% paraformaldehyde for 15 min followed by permeabilization with permeabilizing buffer (3% paraformaldehyde and 0.5% Triton X-100) for 10 min. After staining with DAPI dye (50 μg/ml), images of condensed nuclei undergoing apoptosis were captured with an inverted fluorescent microscope (Nikon ECLIPSE Ti-S, Japan). Apoptosis was quantified by morphological changes of nuclei with approximately 500 cells/well representing one sample [Citation41].
Intracellular reactive oxygen species (ROS) production analysis
The intracellular ROS production was analyzed by using fluorescence microscopic imaging technique in HepG2 cells. Cells (10,000 per well) were exposed at three effective concentrations i.e. 1, 2 and 5 μg/ml of DOX SNEDDS for 12 h. Subsequently, cells were incubated with DCFH-DA (10 mM) at 37 °C for 15 min and washed with PBS. Intracellular fluorescence intensity of cells was visualized by an inverted fluorescent microscope (Nikon ECLIPSE Ti-S, Japan). For quantitative fluorometric analysis, cells (1 × 104 per well) were seeded and treated with SNEDDS in 96-well black bottom culture plate. Fluorescence intensity was measured with a multiwell microplate reader (Synergy H1 Hybrid Multi-Mode Microplate Reader, BioTek , USA) at an excitation wavelength of 485 nm and at an emission wavelength of 528 nm. Data were expressed as the percentage of fluorescence intensity relative to the control wells [Citation42].
Statistical analysis
The results were expressed as mean ± SD and were analyzed statistically (graph pad prism for Windows, version 5) using one-way analysis of variance (ANOVA) followed by Turkey’s test and considered statistically significant when p<.05.
Results and discussion
Compatibility studies
Differential Scanning Calorimetry (DSC) appeared as two endothermic peaks of pure DOX at 196.6 and 252 °C. The peak at 196.6 °C corresponds to the melting endothermic of DOX. The sharp endothermic peak at 252 °C is most likely due to slight moisture in DOX [Citation43]. The compatibility study between the DOX, Nigella oil and selected excipients was evaluated using FTIR. There was no significant change in FTIR spectrums indicating no interference between drug and excipients.
Solubility studies
The most important criteria to select several components were based on the solubility of DOX in different components. Solubility studies were designed for selecting a suitable oily phase for the development of DOX SNEDDS. Identifying the appropriate oil having a maximum solubilizing capability for the drug is very important to achieve optimum drug loading [Citation44]. All the examined surfactants in this study were non-ionic hydrophilic. The non-ionic surfactants are considered as biocompatible and safe and also with HLB values >10 are good in developing fine, uniform droplets which can rapidly clear from the stomach and give a large surface area that helps in rapid drug release and absorption [Citation45]. The solubility studies of Nigella sativa oil and DOX HCl in different components are outlined in . On the basis of solubility study, Labrafil was selected as oil phase as it showed the highest solubility for N. sativa and DOX HCl, i.e. 57.743 ± 1.098 and 14.0987 ± 2.703 mg/ml, Kolliphor-RH40 (12.547 ± 1.657 and 34.32 ± 2.512 mg/ml) was selected as a surfactant whereas glycerol (9.5801 ± 1.542 and 14.758 ± 2.758 mg/ml) as co-surfactant respectively and both also exhibited good solubility of N. sativa oil and DOX HCl that was sufficient to develop transparent and stable SNEDDS of DOX HCl.
Construction of pseudo-ternary phase diagrams
The pseudo-ternary phase diagrams were drawn to determine the Smix ratio and its ratio with oil phase, which provides the region for the development of suitable SNEDDS of DOX HCl [Citation35] and results are mentioned clearly in , on the basis of which it can be illustrated that at ratio 1:0 of Smix, nanoemulsions zones were moderately low (). Though, in case of Smix ratio of 1:1 when glycerol (co-surfactant) was used with Kolliphor RH 40 (surfactant) (), further indicates Smix ratio of 1:2 where the emulsion zones were further increased as that of ratio 1:1 of Smix. In case of S zones were seen to be improved as compared to 1:0 but lesser than 1:1 ratio. showed further enhanced SNEDDS zones as compared to 1:0, 1:1, and 1:2 ratios. While showed zones of Smix of 3:1, which showed further increase, rather in this ratio, the zones of emulsion were found to be maximum as compared to all Smix ratios (1:0, 1:1, 1:2 and 2:1). However, showed highest Smix of 4:1, but the zones seen were more than from Smix 1:0 and 1:2 but lesser than zones produced in Smix 1:1, 2:1 and 3:1 ratio, respectively.
Stability studies of DOX SNEDDS
Emulsification efficacy
The emulsification times and appearances after dilution with an aqueous phase are listed in . The blank SNEDDS (F2, F3, F5–F8) showed transparent appearances after dilution and found that emulsification times less than 180 s, which indicated they could disperse quickly and completely when subjected to aqueous dilution under mild agitation [Citation32]. After the drug loading, the emulsification time of F1, F4 increased from 110 s to 178 s, representing that the drug had a strong influence on the flow ability of the interfacial film [Citation45]. However, the emulsification times of F5 and F6 slightly increased, showing the fine drug loading capacity of these two SNEDDS formulations. Moreover, F2 and F3 took longer emulsification time than F5, F6 which was possibly caused by the low amounts of co-surfactants in this formulation, and the longer emulsification time may have a moderate ability to delay drug absorption [Citation46]. Thus, most of the DOX SNEDDS (F2, F3, and F5–F8) passed this test with grade A in the presence of water, 0.1 N HCl and PBS (6.8). Grade A is regarded as the best grade for this evaluation, however, F1 and F4 passed this evaluation with grade B.
Thermodynamic stability
The results of thermodynamic stability studies were evaluated and reported in . The thermodynamic studies were done to investigate the dispersion efficiency and stability of SNEDDS in gastrointestinal fluids [Citation47]. It was found that formulations (F1–F3 and F5–F8) of DOX SNEDDS were thermodynamically stable at centrifugation, while F4 failed this test, whereas F2, F3 and F5–F8 were found to be thermodynamically stable at freeze-thaw cycles, while formulations F1 and F4 failed in the freeze-thaw test. So, finally, F2, F3, F5, F6, F7 and F8 passed all the tests and selected for further studies.
Table 3. Thermodynamic stability tests of prepared nanoformulation.
Drug content
Drug content of the selected DOX SNEDDS (F6) formulation was found to be 97.66 ± 0.64%.
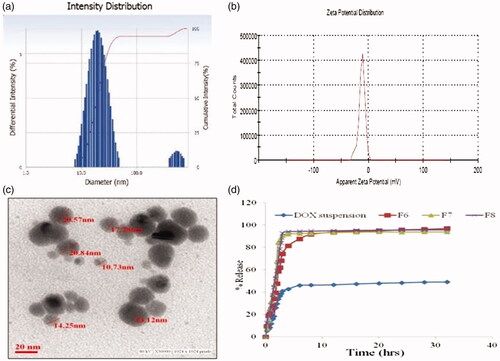
Droplet size analysis and zeta potential
The mean droplet size of diluted blank SNEDDS was <200 nm with a narrow distribution (PDI <0.3). All formulations exhibited zeta potentials around -15 mV, which indicated the stability of SNEDDS. After DOX was incorporated, the droplet sizes of the six formulations (F2, F3 and F5–F8) increased from 153 nm, 148 nm, 121 nm, 76 nm, 69 nm and 87 nm to 189 nm, 167 nm, 142 nm, 79.7 nm, 82.5 nm and 98.2 nm, respectively, represented in . The droplet size of F6 was comparatively stable after drug loading. The zeta potential values of DOX SNEDDS were found as -6.54 to -12.25 mV. The highest zeta potential was found in F6 as -12.1 ± 0.674. All selected formulations exhibited zeta potentials in the range, which signify the stability of SNEDDS [Citation32]. The viscosity of DOX SNEDDS F2, F3 and F5–F8 were found as 16.42–26.84 cps. The refractive index of DOX SNEDDS F2, F3 and F5–F8 were observed as 1.332–1.347 and percentage transmittance of F2, F3 and F5F8 were found to be 94.12–98.24%, respectively.
Table 4. Droplet size, polydispersity index, zeta potential, viscosity, refractive index, conductivity, and percent transmittance of selected DOX SNEDDS formulations.
Surface morphology
TEM images of formulation F6 () clearly show the spherical nature of SNEDDS with particle sizes of less than 50 nm observed in the image, which was slightly smaller than the DLS measurements shown in . This reduced particle size was possibly due to treating procedures of the different sample, i.e. the drying process used in preparing the TEM samples may have decreased the volatile composition of the SNEDDS, whereas the DLS sample preparation was maintained in aqueous conditions [Citation48].
In vitro release studies of optimized DOX SNEDDS
The in vitro drug release of DOX from SNEDDS was found to be significantly prolonged drug release for 32 h as compared to the pure DOX HCl as shown in . The formulation coded as F6 showed 96.9682% drug release while pure DOX showed 54.9584% drug release, respectively, within 32 h.
In vitro cell viability and alteration in cells morphology
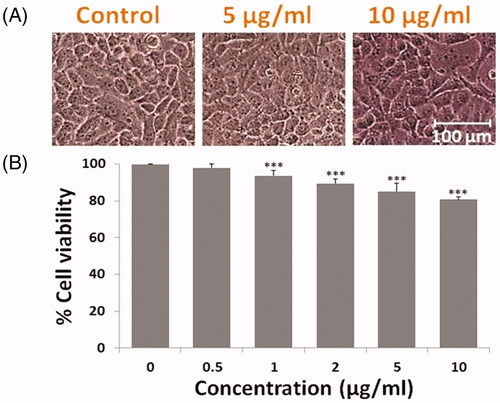
The cell viability assay was performed and the cellular morphological study of non-malignant Chang liver cells revealed that at 5 and 10 µg/ml, concentrations of DOX SNEDDS did not alter the cell morphology and suggests non-toxic to normal cells . The MTT data showed that percent cell viability reduction of normal liver cells was about 98.07, 93.69, 89.48, 85.16 and 80.84% at 0.5, 1, 2, 5 and 10 µg/ml concentrations of DOX SNEDDS , suggested non/least toxic and selective anti-hepatic carcinoma efficacy of synthesized compound.
Figure 4. Photomicrograph showing effect to DOX SNEDDS against Chang liver cells of non-malignant human liver cells. (A) Showing morphological changes in Chang liver cells at higher doses i.e. 5 and 10 µg/ml concentrations of DOX SNEDDS. (B) Graph representing the % cell viability of Chang liver cells after treated with various concentrations (0.5, 1, 2, 5 and 10 µg/ml) of DOX SNEDDS.
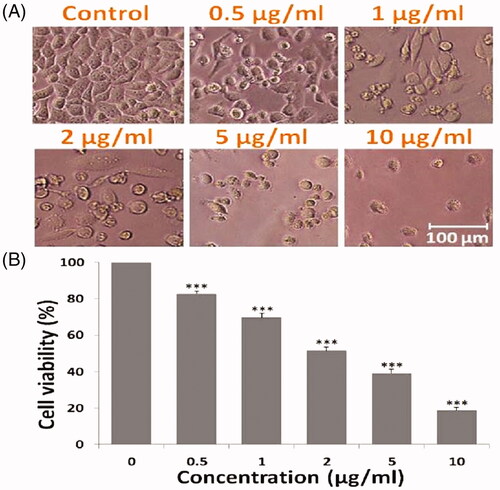
The MTT assay revealed that DOX SNEDDS after 24 h exposure significantly decreases the HepG2 cells viability in a concentration-dependent manner (). DOX SNEDDS at 0.5, 1, 2, 5 and 10 µg/ml concentrations reduces the cell viability approximately 82.68, 69.82, 51.49, 39.16 and 18.79% (***p≤.05), respectively as compared to untreated control cells ().
Figure 5. Anti-proliferative effect to DOX SNEDDS against HepG2 cells of human liver carcinoma. (A) Showing morphological changes in HepG2 cells after treated with 0.5, 1, 2, 5 and 10 µg/ml concentrations of DOX SNEDDS. (B) Graph representing the % cell viability of HepG2 cells after treated with various concentrations (0.5, 1, 2, 5 and 10 µg/ml) of DOX SNEDDS.
The morphology changes indicates a significant rise in apoptotic cells, which might be possible due to activation of p53 expression, it may also alter the ratio of pro-apoptotic/anti-apoptotic proteins, as described by Di et al. [Citation6].
A concentration-dependent cellular alteration in HepG2 cell morphology also justifies that DOX SNEDDS inhibits proliferation of hepatic carcinoma cells. As depicted from , DOX SNEDDS at various concentrations induces apoptotic morphological features in HepG2 cells viz. cellular shrinkage, decreased number of cells and rounding off, with respect to the untreated HepG2 cells signifying apoptotic cell death [Citation48].
Nuclear fragmentation in HepG2 cells
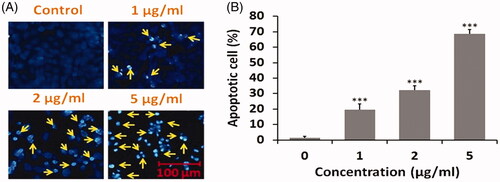
As depicted from (), DOX SNEDDS at 1, 2 and 5 µg/ml concentrations significantly encourage nuclear fragmentation in HepG2 cells of liver carcinoma. Typical apoptotic features in nuclear morphology such as fragmented and condensed nuclei were seen in DOX SNEDDS treated HepG2 cells. The quantitative % apoptotic cells exposed that DOX SNEDDS at 1, 2 and 5 µg/ml concentrations persuades around 19, 32 and 68% (***p ≤ .05) apoptotic cells as compared to untreated HepG2 cells. The finding of nuclear fragmentation assay reveals that DOX SNEDDS induces nuclear apoptosis in HepG2 cells of human hepatic carcinoma.
Figure 6. Nuclear fragmentation and apoptosis caused by DOX SNEDDS against human hepatic carcinoma HepG2 cells after 24 h. (A) Photomicrograph showing nuclear apoptosis in HepG2 cells after treated with 1, 2 and 5 µg/ml concentrations of DOX SNEDDS. (B) Graph representing the % apoptotic HepG2 cells after treated with selected effective concentrations (1, 2 and 5 µg/ml) of DOX SNEDDS.
Intracellular ROS production in HepG2 cells
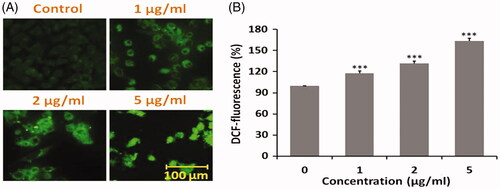
As shown in ), fluorescence microscopic images of HepG2 cells after treating with DOX SNEDDS suggested ROS-mediated apoptosis in human liver carcinoma cells. DOX SNEDDS at 1, 2 and 5 µg/ml concentrations induces dose-dependent ROS fluorescence with respect to untreated HepG2 cells. The quantitative % DCF fluorescence revealed that DOX SNEDDS at 1, 2 and 5 µg/ml concentrations encouraged around 117.80, 132.02 and 163.41% (***p≤.05), with respect to untreated HepG2 cells. The findings of intracellular ROS production revealed that DOX SNEDDS induced ROS mediated apoptosis in HepG-2 cells of human hepatic carcinoma.
Figure 7. Intracellular ROS production in HepG2 cells after treated with DOX SNEDDS. (A) Photomicrograph showing intracellular ROS production in HepG2 cells after treated with 1, 2 and 5 µg/ml concentrations of DOX SNEDDS. (B) Graph representing the % ROS production in HepG2 cells after treated with selected effective concentrations (1, 2 and 5 µg/ml) of DOX SNEDDS.
Conclusions
Nigella sativa oil and doxorubicin co-delivery self-nano emulsifying drug system was prepared by phase titration method with optimized composition of oil phase (5% w/w, (3:2) Nigella oil and Labrafil), 20% w/w of Smix (Kolliphor RH 40 and glycerol, 3:1 as surfactant and co-surfactant, respectively) and 75% w/w of deionized water as an aqueous phase. All formulations were evaluated on the basis of particle size, zeta potential, viscosity, refractive index, thermodynamic stabilities and % transmittance studies. The F6 formulation showed maximum drug release in less amount of oil as compared to doxorubicin suspension. Data obtained from the stability studies also showed that the selected SNEDDS (F6) remains stable over 6 months of storage period at 25 ± °C/60 ± 5% RH, as there was no phase separation or creaming seen in the F6 SNEDDS (p>.05), respectively. The in vitro cell viability analysis and morphological alterations in HepG2 cells suggested that DOX SNEDDS potentially inhibits the HepG2 cells proliferation in a concentration-dependent manner. The augmentation in fragmentation of apoptotic nuclei and their accumulation in intracellular ROS production showing that co-delivery of DOX and Nigella in SNEDDS have potent efficacy against (HepG2 cells) human hepatic cancer, without damaging normal cells. The result confirms SNEDDS contains low dose of DOX as compared to traditional formulation, the incorporation of N. sativa oil into SNEDDS might be responsible for reducing the profound cardiotoxicity of DOX, though the nanosized particles of Nigella oil and doxorubicin followed higher surface area may promote quicker rate of drug release with enhanced absorption to improve bioactivity in lower concentration of drugs as compared to definite dose, which in turn may reduce the high-risk cardiotoxicity, one of the compelling side effects of doxorubicin. Thus, SNEDDs delivery systems could be further utilized for the treatment of critical illness like cancer, neurological, gastrointestinal and other physiological disorders.
Acknowledgement
We are thankful to the Director and Pharmaceutics Division, Central Drug Research Institute (CSIR-CDRI), Lucknow (U.P.), India for providing the facilities of the Malvern-Zetasizer to access it. Thanks to Ms. Sharda Gurram for providing Labrafil, Capryol 90 as gift samples from Gattefosse Indian Pvt. Ltd., Mumbai. The authors also offer their sincere thanks to Dean Research and Development, Integral University for providing technical support and assigning Communication no IU/R&D/2018-MCN000457.
Disclosure statement
All authors have approved the final manuscript and no potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–1314.
- Usmani A, Mishra A, Ahmad M. Nanomedicines: a theranostic approach for hepatocellular carcinoma. Artif Cells Nanomed Biotechnol. 2018;46:680–690.
- Usmani A, Mishra A. Current updates on risk factors of hepatocellular carcinoma. Res Rev. 2017;8:23–31.
- Tam K. The roles of doxorubicin in hepatocellular carcinoma. ADMET and DMPK. 2013;1:29–44.
- Frederick CA, Williams LD, Ughetto G, et al. Structural comparison of anticancer drug-DNA complexes: adriamycin and daunomycin. Biochemistry. 1990;29:2538–2549.
- Di CX, Han L, Zhang H, et al. Diallyl disulfide attenuated carbon ion irradiation-induced apoptosis in mouse testis through changing the ratio of Tap73/ΔNp73 via mitochondrial pathway. Sci Rep. 2015;5:16020.
- Subedi RK, Kang KW, Choi HK. Preparation and characterization of solid lipid nanoparticles loaded with doxorubicin. Eur J Pharm Sci. 2009;37:508–513.
- Pereverzeva E, Treschalin I, Bodyagin D, et al. Influence of the formulation on the tolerance profile of nanoparticle-bound doxorubicin in healthy rats: focus on cardio-and testicular toxicity. Int J Pharm. 2007;337:346–356.
- Chen L, Ye HL, Zhang G, et al. Autophagy inhibition contributes to the synergistic interaction between EGCG and doxorubicin to kill the hepatoma Hep3B cells. PloS One. 2014;9:e85771.
- Chang JE, Yoon IS, Sun PL, et al. Anticancer efficacy of photodynamic therapy with hematoporphyrin-modified, doxorubicin-loaded nanoparticles in liver cancer. J Photochem Photobiol B Biol. 2014;140:49–56.
- Ohkawa K, Hatano T, Yamada K, et al. Bovine serum albumin-doxorubicin conjugate overcomes multidrug resistance in a rat hepatoma. Cancer Res. 1993;53:4238–4242.
- Jiang SP, He SN, Li YL, et al. Preparation and characteristics of lipid nanoemulsion formulations loaded with doxorubicin. Int J Nanomed. 2013;8:31–41.
- Sabeti B, Noordin MI, Mohd S, et al. Development and characterization of liposomal doxorubicin hydrochloride with palm oil. BioMed Res Int. 2014;2014:1–6.
- Yin H, Bae YH. Physicochemical aspects of doxorubicin-loaded pH-sensitive polymeric micelle formulations from a mixture of poly (L-histidine)-b-poly (L-lactide)-b-poly (ethylene glycol). Eur J Pharm Biopharm. 2009;71:223–230.
- Rapoport N, Nam KH, Gupta R, et al. Ultrasound-mediated tumor imaging and nanotherapy using drug loaded, block copolymer stabilized perfluorocarbon nanoemulsions. J Control Release. 2011;153:4–15.
- Sharma PC, Yelne MB, and Dennis TJ. Database on medicinal plants used in Ayurveda; Central Council for research on ayurveda and Siddha, Gvernment of India, New Delhi. 2005;6:420–440.
- Sultan MT, Butt MS, Anjum FM, et al. Nutritional profile of indigenous cultivar of black cumin seeds and antioxidant potential of its fixed and essential oil. Pak J Bot. 2009;41(3):1321–1330.
- Al-Oqail MM, Al-Sheddi ES, Al-Massarani SM, et al. Nigella sativa seed oil suppresses cell proliferation and induces ROS dependent mitochondrial apoptosis through p53 pathway in hepatocellular carcinoma cells. S Afr J Bot. 2017;112:70–78.
- Salomi MJ, Nair SC, Panikkar KR. Inhibitory effects of Nigella sativa and saffron (Crocus sativus) on chemical carcinogenesis in mice. Nutr Cancer. 1991;16:67–72.
- Swamy SM, Tan BK. Cytotoxic and immunopotentiating effects of ethanolic extract of Nigella sativa L. seeds. J Ethnopharmacol. 2000;70:1–7.
- Farah IO, Begum RA. Cellular responses to oxidative stress using MCF-7 breast carcinoma, black seed (N. Sativa L) extracts and H2O2. Proc Amer Assoc Cancer Res. 2004;45:452–453.
- Mahmoud SS, Torchilin VP. Hormetic/cytotoxic effects of Nigella sativa seed alcoholic and aqueous extracts on MCF-7 breast cancer cells alone or in combination with doxorubicin. Cell Biochem Biophys. 2013;66:451–460.
- Hosseini A, Shafiee-Nick R., Mousavi SH Combination of Nigella sativa with Glycyrrhiza glabra and Zingiber officinale augments their protective effects on doxorubicin-induced toxicity in h9c2 cells. Iran J Basic Med Sci. 2014;17:993.
- Ahmed MA, Hassanein KM. Cardio protective effects of Nigella sativa oil on lead induced cardio toxicity: anti inflammatory and antioxidant mechanism. J Physiol Pathophysiol. 2013;4:72–80.
- Ebru U, Burak U, Yusuf S, et al. Cardioprotective effects of Nigella sativa oil on cyclosporine A-induced cardiotoxicity in rats. Basic Clin Pharmacol Toxicol. 2008;103:574–580.
- Effenberger-Neidnicht K, Schobert R. Combinatorial effects of thymoquinone on the anti-cancer activity of doxorubicin. Cancer Chemother Pharmacol. 2011;67:867–874.
- Singh B, Beg S, Khurana RK, et al. Recent advances in self-emulsifying drug delivery systems (SEDDS). Crit Rev Ther Drug Carrier Syst. 2014;31(2):121–185.
- Seo YG, Kim DH, Ramasamy T, et al. Development of docetaxel-loaded solid self-nanoemulsifying drug delivery system (SNEDDS) for enhanced chemotherapeutic effect. Int J Pharm. 2013;452:412–420.
- Aboul Fotouh K, Allam AA, El-Badry M, et al. Development and in vitro/in vivo performance of self-nanoemulsifying drug delivery systems loaded with candesartan cilexetil. Eur J Pharm Sci. 2017;109:503–513.
- AOAC (Association of Official Analytical Chemists). Official methods of analysis. Gaithersburg MD. AOAC (Association of Official Analytical Chemists). Official methods of analysis. Washington, USA; 2000; 26–49.
- Janakiraman N, Johnson M, Sahaya SS. GC–MS analysis of bioactive constituents of Peristrophe bicalyculata (Retz.) Nees.(Acanthaceae). Asian Pac J Trop Biomed. 2012;2:S46–S49.
- Kalam MA, Raish M, Ahmed A, et al. Oral bioavailability enhancement and hepatoprotective effects of thymoquinone by self-nanoemulsifying drug delivery system. Mater Sci Eng C. 2017;76:319–329.
- Sandhu PS, Kumar R, Katare OP, et al. Surface-tailored nanomixed micelles containing quercetin–salicylic acid physical complex for enhanced cellular and in vivo activities: a quality by design perspective. Nanomedicine. 2017;11:1281–1303.
- Elnaggar YS, El-Massik MA, Abdallah OY. Self-nanoemulsifying drug delivery systems of tamoxifen citrate: design and optimization. Int J Pharm. 2009;380:133–141.
- Shakeel F, Haq N, El-Badry M, et al. Ultra fine super self-nanoemulsifying drug delivery system (SNEDDS) enhanced solubility and dissolution of indomethacin. J Mol Liq. 2013;180:89–94.
- Ahmad M, Sahabjada JA, Hussain A, et al. Development of a new rutin nanoemulsion and its application on prostate carcinoma PC3 cell line. Excli J. 2017;16:810.
- Balakumar K, Raghavan CV, selvan NT, et al. Self nanoemulsifying drug delivery system (SNEDDS) of rosuvastatin calcium: design, formulation, bioavailability and pharmacokinetic evaluation. Colloids Surf B Biointerfaces. 2013;112:337–343.
- Di C, Sun C, Li H, et al. Diallyl disulfide enhances carbon ion beams-induced apoptotic cell death in cervical cancer cells through regulating Tap73/DeltaNp73. Cell Cycle. 2015;14:3725–3733.
- Siddiqui S, Ahamad MS, Jafri A, et al. Piperine triggers apoptosis of human oral squamous carcinoma through cell cycle arrest and mitochondrial oxidative stress. Nutr Cancer. 2017;69(5):791–799.
- Sandhu PS, Kumar R, Beg S, et al. Natural lipids enriched self-nano-emulsifying systems for effective co-delivery of tamoxifen and naringenin: systematic approach for improved breast cancer therapeutics. Nanomedicine. 2017;13:170313.
- Ahamad MS, Siddiqui S, Jafri A, et al. Induction of apoptosis and antiproliferative activity of naringenin in human epidermoid carcinoma cell through ROS generation and cell cycle arrest. PloS One. 2014;9:e110003.
- Manocha B, Margaritis A. Controlled release of doxorubicin from doxorubicin/γ-polyglutamic acid ionic complex. J Nanomat. 2010; 2010:1–12.
- Patel J, Patel A, Raval M, et al. Formulation and development of a self-nanoemulsifying drug delivery system of irbesartan. J Adv Pharm Tech Res. 2011;2:9.
- Azeem A, Rizwan M, Ahmad FJ, et al. Nanoemulsion components screening and selection: a technical note. Aaps Pharmscitech. 2009;10:69–76.
- Nasr A, Gardouh A, Ghorab M. Novel solid self-nanoemulsifying drug delivery system (S-SNEDDS) for oral delivery of olmesartan medoxomil: design, formulation, pharmacokinetic and bioavailability evaluation. Pharmaceutics. 2016;8:20.
- Bansal T, Mustafa G, Khan ZI, et al. Solid self-nanoemulsifying delivery systems as a platform technology for formulation of poorly soluble drugs. Crit Rev Ther Drug Carrier Syst. 2008;25:63–116.
- Yoo JH, Shanmugam S, Thapa P, et al. Novel self-nanoemulsifying drug delivery system for enhanced solubility and dissolution of lutein. Arch Pharm Res. 2010;33:417–426.
- Srivastava S, Gupta P, Singh RP, et al. Synthesis, spectroscopic characterization, theoretical study and anti-hepatic cancer activity study of 4-(1E, 3Z, 6E)-3-hydroxy-7-(4-hydroxy-3-methoxyphenyl)-5-oxohepta-1, 3, 6-trien-1-yl)-2-methoxyphenyl 4-nitrobenzoate, a novel curcumin congener. J Mol Struct. 2017;1141:678–686.