Abstract
Differentiation of stem cells to Schwann is considered efficient way for nerve regeneration since the sources of human Schwann cells are limited for clinical application. It is demonstrated that mimicking micromechanical forces or micro/nanotopographical environments that stem cells are experienced in vivo could control their fate. Here, the potency of substrates with imprinted cell-like topographies for direct differentiation of adipose-derived mesenchymal stem cells (ADSCs) into Schwann cells (SCs) is reported. For the preparation of substrates with imprinted SC-Like topographies, SCs are isolated from the sciatic nerve, grown, fixed, and then SC morphologies are transferred to polydimethylsiloxane (PDMS) substrates by mold casting. Subsequently, mesenchymal stem cells (MSCs) are seeded on the SC-imprinted substrates and their differentiation to SCs is evaluated by immunocytochemistry, real-time PCR, and western blotting. Analysis of morphology and expression of SC-specific markers show that MSCs cultured on the imprinted substrates have the typical SC-like morphology and express SC-specific markers including S100b, p75NTR, and Sox10. It is believed that specific cell-like topographies and related micromechanical cues can be sufficient for direct differentiation of ADSCs into Schwann cells by cell-imprinting method as a physical technique.
Graphical Abstract
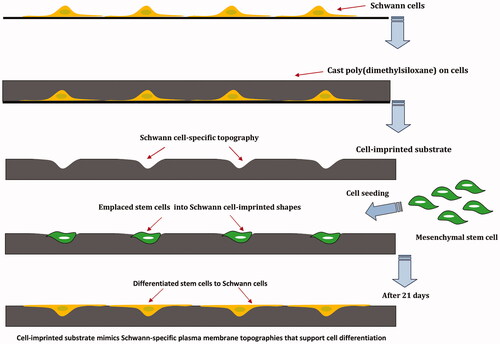
Introduction
Stem cells are unspecialized cells that have the remarkable potential to differentiate into many cell types during embryo development and growth. Under certain physiological or experimental conditions, stem cells can be induced to become tissue or organ-specific cells having special functions, thus offer a source of precursor cells for treatment of degenerative, malignant and genetic diseases. However, the regulation of stem cells differentiation has been a challenge in regenerative medicine. Generally, committing a stem cell to become differentiated cell is highly context dependent which requires multiple targets in different pathways in order to generate a cellular response that controls growth, apoptosis or differentiation [Citation1,Citation2]. Many studies have shown that soluble factors such as growth factors, hormones and small molecule can induce stem cells differentiation; however, some of the differentiating pathways can be activated regardless of these factors [Citation3]. There are numerous grounds that demonstrate physical cues could play a main role in controlling the fate of stem cells [Citation4]. Generally, cells in vivo are exposed to a broad variety of physical cues depending on their functions and locations. Based on the nature of physical cues in the ECM, these physical aspects are divided into three categories consist of matrix stiffness, mechanical force, and topography [Citation5]. Micromechanical forces are increasingly recognized as key regulators of cell structure and function. The ability of cells to sense micromechanical forces and transduce them into biochemical signals is essential for their motility, proliferation and differentiation [Citation6]. The mechanical stimuli that influence the differentiation of stem cells are categorized into internal and external forces. Internal forces are defined as contractile force arising from the cellular cytoskeleton that includes actomyosin contraction, microtubule polymerization/depolymerization and osmotic forces. These stimuli could regulate stem cell responses during cell development which can be altered by the cell density, cell–cell interactions, and ECM composition. On the contrary, external forces such as compressive, tensile and shear stress refer to the forces operating from outside of the cells [Citation7–9]. Although internal forces are important for cell functions, external forces are more significant for engineering of the cell microenvironment [Citation10]. For example, shear stress has been found to drive differentiation of embryonic stem cells (ESCs) toward vascular endothelial cells whereas stretching of mesenchymal stem cells resulted in up-regulation of specific markers related to smooth muscle cells [Citation11,Citation12]. Therefore, micromechanical forces that stem cells experience in vivo could be mimicked to control the fate of stem cells. Moreover, native ECM presents various geometrically defined physical boundaries through composition and structure (i.e. topographies). The components of the ECM can be arranged into different structures such as fibers and sheets with nano- or micrometer scales which affects the cells and regulate their function [Citation13,Citation14]. Based on various studies, nanoscale structures interact with cell receptors (β-integrins) and affect protein clustering and organization whereas microscale structures change the curvature of the cell membrane [Citation15]. It has also been shown that the surface topography in the groove and ridge patterns having microscale widths and nanoscale depths induced osteogenesis while nanotopographies in groove and ridge patterns induced neuronal differentiation of MSCs [Citation16]. Furthermore, the geometry of printed patterns such as triangle, square, pentagon, hexagon, or circular shapes could play a crucial role in cytoskeletal tension and differentiation of cultured stem cells [Citation17]. It is well documented that alteration in cell shape through micromechanical cues or binding of specific growth factors/ECM proteins to their respective cell surface receptors could drive cells between discrete fates of growth, differentiation, apoptosis and migration [Citation18]. For instance, a number of studies have shown that differentiation of adult or embryonic stem cells into a chondrocytic phenotype would require a rounded cell shape [Citation19]. Some in vitro studies have shown that stem cell fate can be influenced through control of their shape using artificial extracellular matrices [Citation20,Citation21]. Cell shape is therefore considered a potent regulator of cell growth and physiology which could influence many events related to embryonic development and differentiation [Citation22]. For this reason, many investigations have been performed on the effect of physical factors on stem cell differentiation in recent years [Citation8]. While growth factors have been extensively used as the conventional method for controlling stem cell fate, disappointing clinical results caused by growth factors (e.g. angiogenic factors) demonstrate the emerging need for the development of alternative strategies for inducing stem cells differentiation [Citation20]. Accordingly, many attempts have been done by patterned substrates in order to precisely control stem cell fates. Schwann cells (SCs) are categorized as peripheral nervous system cells which have an active and effective role in myelin formation, peripheral nerves and axonal regeneration of injured spinal cord [Citation23]. The isolation of the adequate number of Schwann cells for clinical practice is, however, confronted with donor morbidity and low cell yield. Therefore, many studies have been conducted to evaluate the capability of different types of stem cells to differentiate into SCs [Citation24,Citation25]. In this regard, the MSCs have been shown to transdifferentiate into neuronal-like cells in neuronal induction media [Citation26,Citation27]. In the current study, direct differentiation of mesenchymal stem cells to Schwann cells is reported using substrates with imprinted SC-like topographies and geometry. In this method, Schwann cells are isolated from sciatic nerve and grown in order to impart their specific morphology and cell-like topographies to a PDMS substrate by mold casting. The imprinted substrates could mimic the Schwann cell-specific shape and plasma membrane topographies. MSCs are seeded on the cell-imprinted substrates and differentiation of MSCs into SCs is then evaluated by immunocytochemistry (ICC), real-time PCR, and western blotting (.
Figure 1. Schwann cells (SCs) were isolated from sciatic nerve and grown on a polystyrene culture plate. After about 10 days cells were characterized by Schwann cell-specific markers, S100β and p75NTR. After fixation, SC morphologies were transferred to PDMS by mold casting. This replica containing SC-like shape and topography and was used as a substrate. MSCs were seeded on the substrate for 3 weeks and differentiation of MSCs to SCs was evaluated.
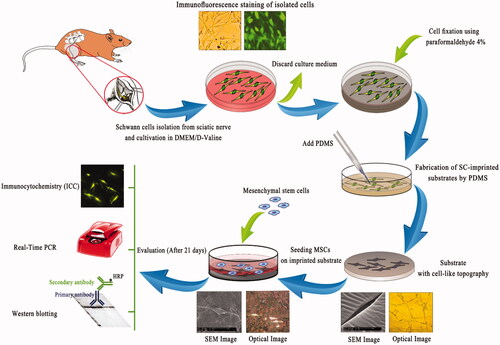
Methods and materials
Isolation and culture of primary Schwann cells
All animal experiments were approved by the Institutional Animal Care and Use Committee of the National Institute of Genetic Engineering and Biotechnology, Iran. Also, all experimental procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health. A simple, rapid and highly selective protocol for the primary culture of Schwann cells was used, which described by Kaewkhaw et al. [Citation28]. This protocol is based on a selective culture medium consists of forskolin as a mitogen and an inhibitory substrate to simultaneously restrict fibroblast overgrowth (D-valine substituted DMEM). The protocol permits derivation of highly pure Schwann cells directly from fresh sciatic nerve so that the purity of the Schwann cells could reach 95%. However, in the current study, β-heregulin, which effectively reduces the number of proliferating fibroblasts relative to Schwann cells, platelet-derived growth factor (PDGF) and neonatal rats from postnatal day 0 (P0) to P4 were used. Neonatal rats from postnatal day 0 (P0) to P4 (Pasteur Institute of Iran) were euthanized by diethyl ether and sciatic tissue was carefully dissected under aseptic conditions. The tissue was 3 times washed with phosphate-buffered saline (PBS) containing 100 U/ml penicillin and 100 μg/ml streptomycin (Invitrogen, USA) to remove contaminated debris and red blood cells. The tissue was enzymatically digested by collagenase type I (0.26 U) (Sigma, USA) which dissolved in DMEM at a concentration of 0.2% (w/v). The enzymatic solution was added to tissue (50 μl per segment) and incubated at 37 °C for 60 min. The tissue was subsequently treated with DMEM/F12 (3:1 Mixture) (Gibco, Switzerland) including 10% (v/v) fetal bovine serum (Gibco, Switzerland) and centrifuged at 1500 rpm/min for 5 min. The supernatant was carefully discarded and the residue was washed with DMEM/F12 (3:1 Mixture) twice and filtered through a 40 μm strainer to remove debris. The filtrate was centrifuged and the pellet was re-suspended in DMEM/D-valine medium (USBiologycal, USA) comprising 10% FBS, 14 µM forskolin, 200 ng/ml β-heregulin and 5 ng/ml PDGF (Sigma-Aldrich, USA). Cell culture was plated onto poly L-lysine coated flasks (SPL, Korea) and incubated at 37 °C for 10 days. Two-thirds (2/3) of the medium was refreshed every 3 days and after 10 days, the confluence of the cells was reached to 90% [Citation29].
Characterization of primary Schwann cells by immunocytochemistry
To confirm Schwann cells characteristic and culture purity, primary cells were cultured on poly L-lysine coated cell culture slides (SPL, Korea). After 10 days, SCs were fixed with 4% (w/v) paraformaldehyde in phosphate buffer (pH 7.4) at room temperature for 20 min. Consequently, cells were permeabilized with Triton X-100 (Sigma, USA) in PBS (0.2%) for 10 min at room temperature. Unspecific binding of the antibodies was blocked by incubating cells in PBS containing 0.05% Tween 20 and 1% (w/v) bovine serum albumin (BSA) for 30 min at room temperature. Detection and characterization of SCs were performed by two common primary monoclonal antibodies including S100β (rabbit monoclonal; 1:200; Abcam, USA) and p75 neurotrophin receptor (p75NTR) (rabbit monoclonal; 1:200; Abcam, USA) [Citation30]. After cell fixation and washing steps with ice-cold phosphate buffer, cells were incubated with primary antibodies overnight at 4 °C. The following day, slides were incubated for 1 h with fluorescein isothiocyanate (FITC)-conjugated secondary antibody (goat anti-rabbit; 1:500; Abcam, USA) at 25 °C. Before imaging, the cell nuclei were stained with 1 μg/ml Hoechst dye (Invitrogen, USA) for 5 min. Finally, fluorescent signals were observed under fluorescent microscopy (Nikon, Japan). L-929 fibroblast cells (Cell Bank, Pasteur Institute of Iran) were used as negative control.
Fabrication of Schwann cell-imprinted substrates
Polydimethylsiloxane (PDMS; SYLGARD 184, RTV, Dow Corning, USA) was used to fabricate the cell-imprinted substrates according to the manufacturer’s protocol. The two parts of the PDMS kit including the PDMS resin and curing agent were mixed with a 10:1 ratio. The Schwann cells were fixed with 4% glutaraldehyde (GLA) in phosphate buffer (pH 7.4) before treatment with PDMS to maintain their shape during the printing process. After fixation, the cured PDMS was poured on the fixed cell samples and incubated at 37 °C for 24−48 h. The cured PDMS was peeled off from the cells and washed two times with NaOH solution (1 M) at 100 °C and then autoclaved. The total mass of imprinted PDMS, temperature and curing time were similar for all the samples. The cell-imprinted substrates were prepared in a way to fit in 6-well culture plates. The imprinted substrates were examined with optical (Nikon, Japan) and scanning electron microscopy (SEM) (Pillips XL30, Netherlands).
Adipose-derived mesenchymal stem cells isolation
Adipose-derived mesenchymal stem cells (ADSCs) were isolated from adipose tissue of adult male Wistar rats (weight 250–300 g, n = 4) euthanized by diethyl ether. Epididymal fat was enzymatically digested for 60 min at 37 °C by 0.2% (w/v) collagenase type I (Sigma, USA). After centrifugation, the stromal cell pellet was resuspended in Dulbecco’s Modified Eagle’s Medium (DMEM/F12) (Gibco, Switzerland) containing 10% (v/v) FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin. Cultures were maintained at subconfluent levels in a 37 °C incubator with 5% CO2 and passaged with trypsin/EDTA (Sigma, USA) when required. Cells at passage 3 were used for experimentation. Mesenchymal stem cell markers were measured by flow cytometry. For this purpose, Phycoerythrin (PE) antibodies against rat CD44, CD90, CD105, CD33, and CD45 (eBioscience, UK) were used. Analyses were performed by flow cytometer (Becton Dickinson, USA) [Citation23].
Culture of MSCs on SC-imprinted substrates
The MSCs with a density of 1 × 104/100 μl of medium were seeded on each imprinted substrate and incubated at 37 °C. After 3 h, 1 ml of fresh DMEM/Ham’s F12 (3:1 Mixture) containing 10% (v/v) FBS was added on each substrate and incubated for 3 weeks (21 days) with medium exchange every 4 days. After the desired time, scanning electron microscopy (SEM), immunostaining, gene expression analysis, and western blot were used to evaluate differentiation of MSCs into Schwann cells. MSCs were also cultured on plain substrates (PDMS without cell imprinted patterns) and used as a control.
Characterizations of MSCs cultured on SC-imprinted substrates
Morphological characterization of emplaced MSCs
Morphological changes of MSCs to SCs on imprinted substrate were observed by optical and scanning electron microscopy. For SEM imaging, substrate was washed three times with phosphate buffer (pH 7.4) and fixed with 4% GLA in overnight at 4 °C. Then, it was washed three times with cold PBS and placed in osmium tetroxide for 2 h, dried on air, coated with gold and observed by SEM (Pillips XL30, Netherlands). It should be noted, in order to compare the morphology of Schwann and stem cells, after extracting the cells from the sciatic nerve and adipose tissue of rat, they were cultured on the slides and used for morphological characterization by SEM.
Immunocytochemistry
To investigate the capability of MSCs to differentiate on SC-imprinted substrates, cell morphology and expression of Schwann cell-specific markers (S100β and p75NTR) were also assessed by optical and fluorescent microscopy (Nikon, Japan). Prior to imaging, the cells were fixed with 4% GLA in phosphate buffer (pH 7.4) for 20 min and permeabilized with a solution containing Triton X-100 in PBS (0.2%) for 10 min at room temperature. Similar to immunostaining of primary SCs as described above, differentiation of MSCs on imprinted substrates was characterized by ICC using S100β and p75NTR monoclonal antibodies and FITC-conjugated secondary antibody. Before imaging, cell nuclei were stained with Hoechst dye.
Gene expression
RNA isolation and cDNA synthesis
Total RNA was extracted from the primary SCs (as a positive control) and MSCs cultured on plain (as a negative control) and imprinted PDMS substrates using Total RNA Miniprep Kit (Bio Basic, Canada) according to the manufacturer’s instructions. DNA was eliminated during RNA extraction utilizing DNase I. The concentration of cellular RNA was quantified by measuring maximum absorbance at 260 nm with a spectrophotometer (NanoDrop, Eppendorf, Germany). Finally, the complementary DNA (cDNA) was synthesized using the One-Step RT-PCR Kit (Takara, Japan).
Qualitative PCR assay
In the first step, cDNA of MSCs cultured on imprinted substrates and controls was used for qualitative assessment of S100β, p75NTR and Sox10 expression as SC-specific markers. The primers which were used synthesized by Sinaclon, Iran and listed in . An Eppendorf thermocycler (Germany) was used for all reactions.
Table 1. Sequences of primers used in qualitative and quantitative PCR.
Quantitative real-time PCR assay
The qPCR assay was performed by the StepOne Plus instrument (Applied Biosystems, USA). The primary SCs and MSCs which were cultured on a plain PDMS substrate were used as positive and negative controls, respectively. Each reaction was contained 20 μl of mixture solution consisting of 10 μl of SYBR PCR Master Mix (ABI, United States), 1 nM concentration of each primer (), 3.5 μl of sterilized deionized water and 4.5 μl of cDNA. S100β, p75NTR and Sox10, which are known as expressed markers during Schwann cell development were used as differentiation-specific markers [Citation30]. β-actin was used as calibrator control. The cycling conditions were as follows: one cycle of 95 °C for 10 min as the holding time and then 95 °C for 30 s (denaturation) and 60 °C for 1 min (annealing/extension). For gene expression, 40 cycles as a cycle cut-off point for no detection was set. During the annealing/extension time amplification data were collected. Melting curve analysis was performed as follows: reactions were heated to 95 °C for 15 s, followed by cooling to 60 °C and stepwise heating to 95 °C with a ramp rate of 0.3 °C. Finally, the comparative threshold cycle (CT) method (using the formula 2−ΔΔ(CT)) was used to analyze the data.
Western blotting
Lysates were prepared from primary SCs and MSCs cultured on plain and imprinted PDMS substrate. Cells were treated with lysis buffer containing 100 mM piperazine-N,N′-bis (2-ethane sulfonic acid) or PIPES, 5 mM MgCl, 20% (v/v) glycerol, 0.5% (v/v) Triton X-100, 5 mM EGTA, and protease inhibitor (Sigma, USA). After cell lysis, supernatants were incubated for 15 min on ice followed by centrifuging at 13,000 g for 5 min. Proteins were denatured by heating at 95 °C in Laemmli buffer for 5 min. The protein lysates were fractionated on a 15% SDS-PAGE gel for S100β and 10% SDS-PAGE gel for p75NTR and transferred to polyvinylidene difluoride (PVDF) membrane (Millipore, USA) using a semi-dry transfer system (BioRad, USA). For S100β, the membrane was blocked for 2 h with 5% (w/v) BSA in TBS-Tween (10 mM Tris pH 7.5, 100 mM NaCl, 0.1% Tween). After washing with TBS-Tween 20, the membrane was incubated overnight at 4 °C with anti-S100β (rabbit monoclonal; 1:500; Abcam, USA) antibody which was diluted in TBST containing 3% nonfat milk. For p75NTR, the membrane was blocked overnight in 5% casein (Sigma), 5% BSA (Fluka) in TBS-Tween and similar to anti-S100β, it was washed and incubated with anti-p75NTR (rabbit monoclonal; 1:500; Abcam, USA) at 4 °C for 24 h. After 3 washes in TBST, the blots were reacted with horseradish peroxidase-conjugated secondary antibody (Abcam, USA) for 1 h at room temperature followed by several washes with TBS-Tween. The bound antibodies were revealed by the addition of 3,3'-diaminobenzidine (DAB) substrate [Citation31].
Statistical analysis
Statistical analysis was carried out using SPSS software (v 17.0; IBM New York, NY, USA) when statistical differences were detected, a Student’s t-test was performed. Data are reported as mean ± SD at a significance level of p < .05.
Results
Isolation and characterization of primary SCs
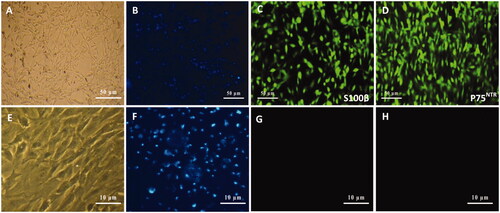
After isolation of SCs from sciatic nerves of neonatal rats and culturing in DMEM/D-valine medium for 10 days, cells were visualized by optical and fluorescence microscopy. Optical images () confirmed the bipolar or tripolar spindle morphology and elongated shape of SCs with a small cytoplasm/nuclear ratio. Nuclei were also stained by Hoechst (). Cultured cells were also assessed by immunocytochemistry for expression of SC-specific markers, S100β and P75NTR, to determine evidence of phenotypic SCs. According to the results, cell cultures were immunoreactive for S100β () and P75NTR () markers with high purity for SCs. In addition, fibroblast cells as negative control () were also visualized by nuclei staining () and characterized by ICC using S100β () and p75NTR () antibodies which were non-immunonreactive for these markers.
Figure 2. Schwann cells (SCs) isolated from sciatic nerve of neonatal rats using DMEM/D valine medium (A). SCs were visualized by nuclei staining with Hoechst (B), and characterized by immunocytochemistry using S100β (C) and p75NTR (D) monoclonal antibodies which were immunoreactive for markers. Similar to SCs, fibroblast cells as a negative control (E) were also visualized by nuclei staining (F) and characterized with S100β (G) and p75NTR (H) antibodies which were non-immunoreactive for these markers.
Characterization of SCs-imprinted substrates
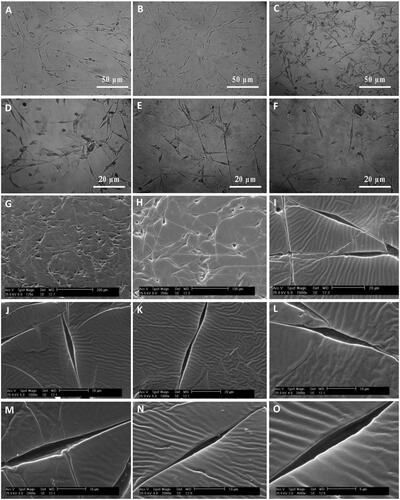
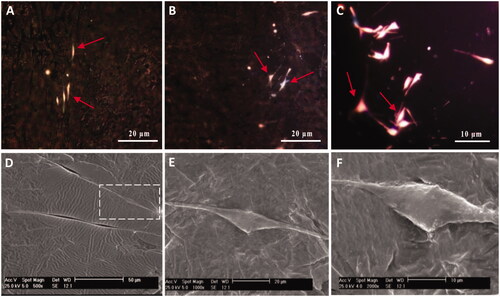
After fixation of cultivated SCs and fabrication of SC-imprinted substrates by PDMS, substrates were characterized by optical microscopy followed by scanning electron microscopy for identification of SC-imprinted patterns on substrates. is an optical image of fixed SCs while are optical images of SCs-imprinted substrates which indicated morphological similarity between SCs-imprinted shapes and patterned SCs. also represent similar features using SEM microscopy. It can be seen that cell shape which was imprinted on substrates stimulated the specific topography of the SC shapes. These images show that the bipolar or tripolar spindle morphology of SCs can be successfully transferred to PDMS by imprinting method.
Figure 3. Optical microscopy images and SEM micrographs of SCs-imprinted PDMS substrates which biomimic SC morphology. SCs (A), optical imaging of SCs-imprinted patterns on PDMS substrate (B–F), SEM imaging of SCs-imprinted patterns on PDMS substrate (G–O). As seen in the SEM micrographs, cell templates obtained on the imprinted substrate simulate the specific topography of the SC shape.
Isolation and characterization of MSCs
The MSCs were identified by their fibroblast-like morphology and phenotype characteristics using light microscopy and flow cytometry, respectively. After three passages, adipose-derived mesenchymal stem cells formed a confluent fibroblast-like monolayer. For flow cytometric analysis, mesenchymal stem cell markers were examined for characterization and identification. The results revealed a negative response for hematopoietic stem cell markers, CD33 and CD45. On the other hand, the isolated stromal cells strongly expressed mesenchymal stem cell markers consist of CD44, CD90 and CD105. According to the results, more than 95% of the isolated cells were identified as mesenchymal stem cells.
Characterization of MSCs cultured on SC-imprinted substrates
Morphological characterization
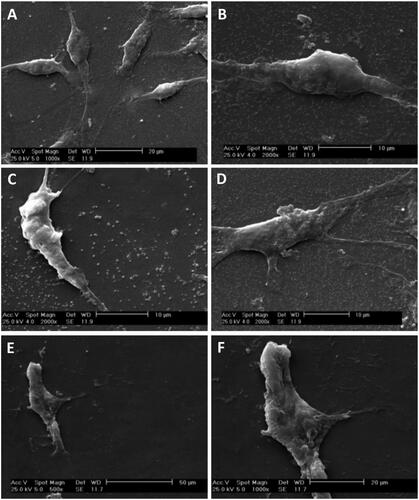
The morphological changes of the SCs and MSCs were characterized by SEM. show micrographs of SCs and MSCs cultured on glass slides. In the SEM micrographs, bipolar spindle morphology and elongated shape of SCs were identified while MSCs showed irregular shape.
Figure 4. SEM micrographs of Schwann and adipose-derived mesenchymal stem cells cultured on culture slide. SEM micrographs showed bipolar or tripolar spindle morphology and elongated shape of SCs (A–D) compared to the flattened fibroblast-like morphology of the MSCs (E and F).
In order to evaluate morphological changes of emplaced MSCs to SCs, cells were fixed and their emplacements in the SC-imprinted substrates, as well as their morphology, were monitored. The optical and electron microscopy images of MSCs cultured on imprinted substrates were revealed a similar morphology to Schwann cells. In , as optical images, bipolar and tripolar morphologies were clearly visible. Also, SEM micrographs () showed emplaced MSCs into SC-imprinted pattern with similar bipolar spindle morphology of SCs. This is a confirmation for morphological conversion of the irregular shape of MSCs into the elongated and bipolar shape of SCs which is represented in . It can be seen that emplacement of MSCs into the imprinted patterns can lead to a shape deformation related to the cellular morphology of patterned cells.
Figure 5. Optical microscopy images (A–C) and SEM micrographs (D–F) of cultivated MSCs (red arrows) on SCs-imprinted substrates after 3 weeks. During 21 days MSCs which were emplaced into the SC-imprinted patterns showed bipolar or tripolar morphology similar to Schwann cells (A–C). SEM micrographs show an emplaced MSC into SC-imprinted pattern which clearly indicates bipolar spindle morphology of SC (D–F).
Immunocytochemistry analysis
At the molecular level, the expression of S100β and p75NTR proteins, the most widely used SC-specific markers in neuron development and regeneration, was examined. As shown in and , which represent ICC staining for S100β and p75NTR markers, respectively, after 21 days a significant number of cultured MSCs on imprinted substrates was immunoreactive for these markers while MSCs which were cultured on plain PDMS substrates was non-immunoreactive (images not shown). Accordingly, represent optical imaging, fluorescence microscopy of nuclei staining, and ICC staining, respectively, of some differentiated MSCs which emplaced into SC-imprinted patterns. On the other hand, as shown in and , in a specific area of the substrate there are differences in the number of visible cells after nuclei staining with Hoechst and SC-specific antibody. In fact, the number of cells labeled with Hoechst is more than cells that are labeled with antibodies. This indicates that a number of cells are undifferentiated stem cells which have not been emplaced into patterned areas. In addition, and represent some SCs differentiated from MSCs with higher magnification. In it seems that some early differentiated Schwann cells are dividing.
Figure 6. Characterization of cultivated MSCs on SCs-imprinted substrate by anti-S100β antibody after 3 weeks. Optical imaging (A) and fluorescence microscopy of nuclei staining (B), ICC staining (C) of some differentiated MSCs emplaced into SC-imprinted patterns. MSCs on imprinted substrates with the lower magnification were characterized by nuclei Hoechst staining (D–F) and anti-S100β antibody (G–I). Non-immunoreactive cells (undifferentiated MSCs) shown with white arrows (D-I). High magnification of differentiated SCs from MSCs (J–N). Cultivated MSCs on the plain substrate were negative for this marker (not shown).
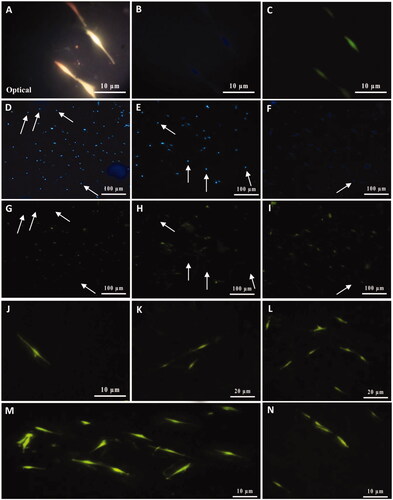
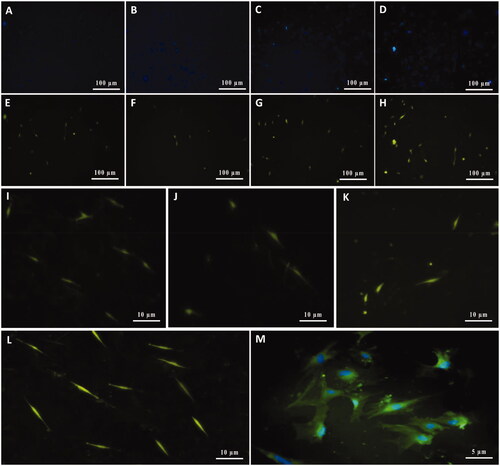
Figure 7. Characterization of cultivated MSCs on SCs-imprinted substrate by anti-p75NTR antibody after 3 weeks. Cell nuclei were stained with Hoechst (A–D) and then characterized by the anti-p75NTR antibody (E–H). Differentiated MSCs show immunoreactive for the p75NTR marker (E–H). Higher magnification of some SCs differentiated from MSCs (I–M). Similar to S100β, MSCs which cultured on the plain substrate were negative for the p75NTR marker (not shown).
SC-specific gene expression
Qualitative PCR assay
Qualitative analysis was also performed by PCR to confirm the expression of some Galileo genes including S100β, p75NTR, and Sox10 (). The qualitative analysis was confirmed that after 21 days induction, cultivated MSCs on the imprinted substrates have been differentiated to SCs.
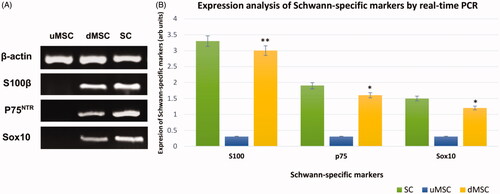
Figure 8. Qualitative (A) and quantitative (B) expression status of the SC-specific S100β, p75NTR, and Sox10 genes in cultivated MSCs on imprinted substrates (differentiated MSCs or dMSCs) after 3 weeks. The primary SCs and cultivated MSCs on plain substrates (undifferentiated MSCs or uMSC) were used as positive and negative controls, respectively. qPCR assay showed that the expression level of the Schwann markers in differentiated MSCs (dMSCs) is not significantly different from the primary SCs (*P < .01 & **P < .001). For qPCR assay, housekeeping β-actin gene was used as calibrator control. In addition, the DNA bands related to the qualitative PCR have been cropped from different gels.
Quantitative real-time PCR assay
In addition, qPCR for detection of SCs-specific markers (S100β, p75NTR, Sox10) relative to the housekeeping gene β-actin was utilized. The mRNA levels of SC-specific markers in total RNAs, which were extracted from primary SCs, undifferentiated MSCs (uMSCs) and differentiated MSCs (dMSCs), were compared (). As it can be clearly seen in , expression of S100β in differentiated MSCs showed a 5-fold increase compared to undifferentiated MSCs (P < .001). In addition, expression of p75NTR and Sox10 in dMSCs was increased 4-times in comparison to uMSCs (P < .01). The level of expression for three markers in dMSCs was not significantly different from that of primary SCs, which were isolated from the sciatic nerve.
Western blotting analysis
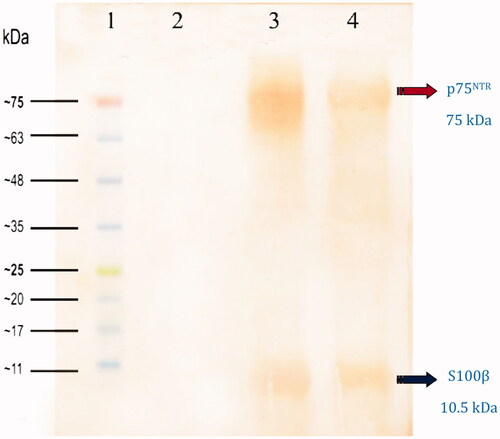
Western blot analysis of the dMSCs by S100β and p75NTR antibodies supported the results of immunocytochemical staining (ICC) and qPCR analysis. Bands at 10.5 and 75 kDa were detected in dMSCs lysates exposed to S100β and P75NTR antibodies respectively (.
Figure 9. Western blot analysis of cultivated dMSCs on the SCs-imprinted substrates after 3 weeks. Lane 1: protein marker; Lane 2: extracted proteins from MSCs which were seeded on a plain substrate (without SC-imprinting); Lane 3: extracted proteins from primary SCs; Lanes 4: extracted proteins from dMSCs which were seeded on SCs-imprinted substrate.
Discussion
Recent findings underscore that mammalian cells could respond to topographic features at micro- and nanoscales of synthetic surfaces [Citation32,Citation33]. Microtopography and geometry such as micropits, micropillars and microgrooves can frequently guide the cell body through physical confinement or alignment. These substrata can induce changes in cell spreading, nuclear shape, nuclear orientation, cytoskeletal architecture, and make alterations in transcript levels and protein expression. While differentiation may cause changes in cell shape, changes in cell shape could interestingly alter the differentiation of stem cells [Citation34]. In 2004, McBeath et al. demonstrated that cell shape can regulate the commitment of human mesenchymal stem cells (hMSCs) to adipocyte or osteoblast fate [Citation35]. According to their report, hMSCs confined to large ECM islands that allowed adhering, spreading and flattening of the cells which resulted in differentiation to osteocytes while unspread and round cells in small ECM islands became adipocytes. Similarly, Kilian et al. have shown that cell shape independent from soluble factors would have a strong influence on the differentiation of MSCs [Citation36]. Other studies have been reported in this area are considered the geometry of single cells or cell clusters on a 2D surface through micro-contact printing, microwells, and direct cell printing [Citation37,Citation38]. While many reports are available on microwell printing, little is known about shape-dependent differentiation using the direct cell-imprinting method. In this study, shape-dependent differentiation of MSCs to Schwann cells through 2D PDMS substrate fabricated by the direct cell-imprinting method is reported. Accordingly, it is demonstrated that cell-imprinted substrates and stimulating cell-like topographies and geometry can be used to promote differentiation of MSCs to SCs. It seems that emplacing MSCs into the SC-imprinted patterns on the PDMS substrates can be considered as potent micromechanical and biophysical stimulators which rearranged the cytoskeleton structure of MSCs. Immunocytochemistry, real-time PCR and western blot data revealed that the MSCs cultured on Schwann cell-imprinted patterns expressed the SC-specific markers S100β, P75NTR and Sox10 while stem cells cultured on the plain substrates without SC-imprinted patterns did not express any SC markers and exhibited their native gene expression profile. SC has various developmental stages and each stage has distinct specific markers. During embryonic development, SCs are originated from neural crest cells and differentiate into Schwann cell precursors (SCPs), immature Schwann cells (iSCs), and pro-myelin Schwann cell (pro-mSC), subsequently. Following the pro-mSC stage, two types of matured SCs are eventually formed which are the myelinating Schwann cell (mSC) and the non-myelinating Schwann cell (nmSC) [Citation30]. The SC specific markers in each stage are different but partially overlapping. According to the reports, Sox10 protein, a key transcription factor which is required for the generation of SCPs from the neural crest and development of SCs, is the only marker that expressed at all stages of SCs development. Therefore, Sox10 alone cannot be used to distinguish SCs in different developmental stages or to differentiate between other cell types derived from neural crest cells [Citation39]. The S100 is expressed in iSC, pro-mSC, mSC and nmSC, but not in SCPs. Some studies have shown that S100 is closely related to the maturation of SC. Accordingly, studies have demonstrated that S100 immunoreactivity is predominantly relevant to myelin-forming Schwann cells, the thickness of the myelin sheath or degree of myelination [Citation40,Citation41]. On the other hand, P75NTR protein is a marker for SCs but commonly expressed in iSCs and nmSCs. In the present study, during 21 days and compared to stem cells that were cultured on a plain PDMS substrate, the increase in the expression level of these markers was significantly enhanced which indicates the differentiation of MSCs into Schwann cells. The increase in the expression level of SC-specific markers in differentiated cells is comparable to that of Schwann cells extracted from rat sciatic nerve. It is therefore believed that geometric patterns could induce stem cell differentiation to a targeted lineage via the shape-dependent mechanism as indicated by both morphological and genotypic changes of MSCs toward SCs.
For proper interpretation of the results of this study one may emphasize that during the separation of PDMS from fixed Schwann cells, some of the cell surface proteins could adhere to the PDMS and possibly affect the process of physical differentiation of stem cells. Therefore, in order to annihilate any possible interfering factor, the imprinted substrates were washed twice with NaOH (1 M) at 100 °C and autoclaved which resulted in removal or inactivation of any possible biological agent adhered to the substrates. Furthermore, it has been shown that the application of a gold layer after cell imprinting to cover the surface of the imprinted substrate could prevent any possible cross-contamination between the cells. This could be another indication that only physical parameters affect the differentiation of stem cells into target cells on the cell-imprinted substrate [Citation20].
In a similar study by Bonakdar et al. chondrocytes, tenocytes and semi-fibroblasts were employed as templates for differentiation, redifferentiation and transdifferentiation. It has been shown that adipose-derived stem cells, semi-fibroblasts, and tenocytes can acquire the chondrocyte phenotype after 14-day culture on chondrocyte-imprinted substrates [Citation42]. Machinchian et al. have demonstrated that cell-imprinted substrates which were fabricated based on mature human keratinocyte shape could act as an artificial niche for skin regeneration [Citation43]. They showed that the keratinocyte-imprinted PDMS casting could imitate the surface morphology of plasma membrane having micro to nanoscale topographical cell fingerprints to induce differentiation.
Generally, the mechanism of stem cell differentiation induced by physical stimuli is not understood, however, several key mechanotransduction components including focal adhesions, cytoskeletal contractility, Rho GTPase signaling and nuclear regulation have been demonstrated to be involved in the force-mediated differentiation [Citation44,Citation45]. Cytoskeleton of the cells is a dynamic structure, hence, the cellular response to the physical cues is not passive and cells are able to adjust their mechanical properties through the dynamic remodeling of cytoskeletal components. The cellular contractility and dynamic changes of cytoskeleton can induce downstream molecular signaling pathways which trigger the Rho GTPases and consequently modulate the intracellular pathways and the gene on/off switching [Citation46]. McBeath et al. have reported that high RhoA activity associated with high actomyosin contractility would induce MSC differentiation to osteoblast while low RhoA activity leads to adipogenesis [Citation35]. Gao et al. have also demonstrated that cell shape confers a switch between chondrogenic and smooth muscle cell fates [Citation47]. According to their findings, adherent and well-spread hMSCs stimulated with TGF-β upregulated smooth muscle cell genes whereas attached cells to micropatterned substrates that were prevented from spreading upregulated chondrogenic genes. Woods et al. investigated the role of RhoA/ROCK pathway as the regulator of cytoskeletal organization in chondrogenesis [Citation48]. Generally, chondrogenesis is as the initial stage of endochondral ossification that occurs by differentiation of mesenchymal precursor cells to chondrocytes. This process is strongly dependent on the cell shape, cytoskeletal organization, and subsequently the onset of chondrogenic gene expression. Woods and his colleagues showed that the inhibition of RhoA/ROCK signaling could promote chondrogenic differentiation of murine embryonic mesenchymal cells by increasing the level of Sox9 expression [Citation48]. Their results confirmed that cytoskeletal tension and RhoA activity could regulate lineage commitment in human MSCs. The critical role of the cell shape and RhoA in stem cell differentiation not only demonstrated in mesodermal stem cells but also in other germ layers such as the ectoderm layer. Keung et al. illustrated that ECM-derived mechanical signals act as a key factor during neural stem cell (NSC) differentiation that operates through Rho GTPases for activation of cellular contractility and regulation of NSC lineage commitment. It is very interesting that Rho-dependent signals can be activated or inhibited by micromechanical cues, therefore RhoA activity appears to be a potential convergence point of soluble factors and mechanical signaling [Citation49]. In addition to the above-mentioned factors, the interaction between physical cues, cytoskeleton and nuclear membrane is another effective factor which affects the differentiation of stem cells through shape- and physical-dependent mechanisms [Citation50]. Li and colleagues reported the effect of micropatterned PDMS substrates on nuclear morphology changes and alteration of gene expression profile in hMSCs [Citation51]. They observed a decrease in histone deacetylase activity accompanied by an increase in histone acetylation in hMSCs cultured on micropatterns which suggests a direct mechanical coupling of chromatin with the ECM through mechanotransduction signaling networks. Other studies have shown that mechanosensing of ECM rigidity can be transmitted to the nucleus by regulation of other factors including transcriptional factors Yorkie-homologs, Yes-associated protein and transcriptional co-activator with the PDZ-binding motif. Rho GTPase activity and tension in the actomyosin cytoskeleton are also required for regulation of these factors. According to the reports, Yes-associated protein/transcriptional coactivator with PDZ-binding motif are functionally required for differentiation of MSCs provoked by ECM stiffness [Citation52,Citation53]. The survival or apoptosis of endothelial cells is also under the influence of ECM geometry and mechanical forces. Based on these reports, biophysical signals can regulate stem cell differentiation by externally applied mechanical forces and/or the manipulation of the substrate rigidity, topography or geometry of ECM [Citation54]. It is apparent that the biophysical signals are sufficient to direct the stem cell fate however; induction can also be in synergy with biochemical cues. The present study intended to induce direct differentiation of mesenchymal stem cells to Schwann cell using SC-imprinted substrates. It was observed that MSCs adopted Schwann cell morphology and expressed SC-specific markers.
In summary, the results confirmed that by the imprinting method, reverse engineering of cell-specific surface patterns on PDMS polymer can be used for substrate fabrication which mimicks the shape of target cells and their surface-specific topographies. These cell imprinted topographies could be employed to control cell differentiation and fate through the shape-dependent mechanism. This method is 100% efficient in the induction of differentiation if and only if a stem cell falls into a pattern of a target cell. The only decisive parameter is time. In the current study, we usually consider 21 days for our differentiation evaluation, while longer periods may lead to a higher number of differentiated cells with a profile similar to the native cells. Although, by modification of surface substrate using cell adhesive molecules, especially in patterned microgrooves, can increase the likelihood of cell adhesive and emplacement of the stem cells into cellular patterns [Citation55]. On the other hand, even though the exact mechanism is not clear yet, it seems that biophysical forces and mechanotransduction have fundamental roles in the regulation of cell fate. In practical, there are some limitations for using growth factors for differentiation including unsafety, their high price and stability while cell imprinting is a safe and economical method for differentiation and can be easily utilized in every laboratory for clinical uses. In fact, conventional tissue culture plates can be easily replaced by a cell imprinted substrate in every laboratory for efficient cell culture.
Acknowledgements
This research was supported by National Institute of Genetic Engineering and Biotechnology (NIGEB) and Pasteur Institute of Iran. The authors would like to thank National Institute of Genetic Engineering and Biotechnology (NIGEB) and Pasteur Institute of Iran as well as Facility for Electron Microscopy Research, McGill University. In addition, the authors declare that there are no conflicts of interest.
Disclosure statement
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
References
- Mizuno H, Tobita M, Orbay H, et al. Adipose-derived stem cells as a novel tool for future regenerative medicine. In: Stem cells and cancer stem cells, Volume 12. Springer; 2014. p. 165–174.
- Lin B, Srikanth P, Castle AC, et al. Modulating cell fate as a therapeutic strategy. Cell Stem Cell. 2018;23:329–341.
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676.
- Kumari S, Vermeulen S, van der Veer B, et al. Shaping cell fate: influence of topographical substratum properties on embryonic stem cells. Tissue Eng Part B: Rev. 2018;24:
- Sun M, Chi G, Li P, et al. Effects of matrix stiffness on the morphology, adhesion, proliferation and osteogenic differentiation of mesenchymal stem cells. Int J Med Sci. 2018;15:257.
- Vining KH, Mooney DJ. Mechanical forces direct stem cell behaviour in development and regeneration. Nat Rev Mol Cell Biol. 2017;18:728.
- Li D, Zhou J, Chowdhury F, et al. Role of mechanical factors in fate decisions of stem cells. Regen Med. 2011;6:229–240.
- Clause KC, Liu LJ, Tobita K. Directed stem cell differentiation: the role of physical forces. Cell Commun Adhes. 2010;17:48–54.
- Kumar A, Placone JK, Engler AJ. Understanding the extracellular forces that determine cell fate and maintenance. Development. 2017;144:4261–4270.
- Lee J-H, Park H-K, Kim KS. Intrinsic and extrinsic mechanical properties related to the differentiation of mesenchymal stem cells. Biochem Biophys Res Communic. 2016;473:752–757.
- Yamamoto K, Sokabe T, Watabe T, et al. Fluid shear stress induces differentiation of Flk-1-positive embryonic stem cells into vascular endothelial cells in vitro. Am J Physiology-Heart and Circulatory Physiol. 2005;288:H1915–H1924.
- Kurpinski K, Chu J, Hashi C, et al. Anisotropic mechanosensing by mesenchymal stem cells. Proc of the Nat Acad Sci. 2006;103:16095–16100.
- Bettinger CJ, Langer R, Borenstein JT. Engineering substrate topography at the micro‐and nanoscale to control cell function. Angew Chem Int Ed. 2009;48:5406–5415.
- Turner L-A, Dalby MJ. Nanotopography–potential relevance in the stem cell niche. Biomater Sci. 2014;2:1574–1594.
- Kolind K, Leong KW, Besenbacher F, et al. Guidance of stem cell fate on 2D patterned surfaces. Biomaterials. 2012;33:6626–6633.
- Dalby MJ, McCloy D, Robertson M, et al. Osteoprogenitor response to defined topographies with nanoscale depths. Biomaterials. 2006;27:1306–1315.
- Song W, Lu H, Kawazoe N, et al. Adipogenic differentiation of individual mesenchymal stem cell on different geometric micropatterns. Langmuir. 2011;27:6155–6162.
- Dike LE, Chen CS, Mrksich M, et al. Geometric control of switching between growth, apoptosis, and differentiation during angiogenesis using micropatterned substrates. In Vitro Cell Dev Biol Anim. 1999;35:441–448.
- Ingber D. Extracellular matrix and cell shape: potential control points for inhibition of angiogenesis. J Cell Biochem. 1991;47:236–241.
- Mahmoudi M, Bonakdar S, Shokrgozar MA, et al. Cell-imprinted substrates direct the fate of stem cells. ACS Nano. 2013;7:8379–8384.
- Guilak F, Cohen DM, Estes BT, et al. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell. 2009;5:17–26.
- Folkman J, Moscona A. Role of cell shape in growth control. Nature. 1978;273:345–349.
- Assinck P, Duncan GJ, Hilton BJ, et al. Cell transplantation therapy for spinal cord injury. Nat Neurosci. 2017;20:637–647.
- Zaminy A, Shokrgozar MA, Sadeghi Y, et al. Transplantation of Schwann cells differentiated from adipose stem cells improves functional recovery in rat spinal cord injury. Arch Iranian Med (AIM). 2013;16:533–541.
- Rodrigues MCO, Rodrigues AA, Glover LE, et al. Peripheral nerve repair with cultured Schwann cells: getting closer to the clinics. The Scientific World Journal. 2012;2012:1.
- Woodbury D, Schwarz EJ, Prockop DJ, et al. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:364–370.
- Moosazadeh Moghaddam M, Bonakdar S, Shokrgozar MA, et al. Repair of spinal cord injury; mesenchymal stem cells as an alternative for Schwann cells. Jabr. 2018;5:42–47.
- Kaewkhaw R, Scutt AM, Haycock JW. Integrated culture and purification of rat Schwann cells from freshly isolated adult tissue. Nat Protoc. 2012;7:1996.
- Caddick J, Kingham PJ, Gardiner NJ, et al. Phenotypic and functional characteristics of mesenchymal stem cells differentiated along a Schwann cell lineage. Glia. 2006;54:840–849.
- Liu Z, Jin Y-Q, Chen L, et al. Specific marker expression and cell state of Schwann cells during culture in vitro. PLoS One. 2015;10:e0123278.
- Moghaddam MM, Mousavi L, Shokrgozar MA, et al. Cloning and expression of a region of vesicle associated membrane protein2 (VAMP2) gene and its use as a recombinant peptide substrate for assaying clostridial neurotoxins in contaminated biologicals. Biologicals. 2010;38:113–119.
- Worley K, Certo A, Wan LQ. Geometry–force control of stem cell fate. Bionanosci. 2013;3:43–51.
- Moghaddam M, Bonakdar S, Shariatpanahi M, et al. The effect of physical cues on the stem cell differentiation. Curr Stem Cell Res & Ther. 2018;
- Sevcik EN, Szymanski JM, Jallerat Q, et al. Patterning on topography for generation of cell culture substrates with independent nanoscale control of chemical and topographical extracellular matrix cues. Curr Prot Cell Biol. 2017;75:10.23.1–10.23.25.
- McBeath R, Pirone DM, Nelson CM, et al. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Developmental Cell. 2004;6:483–495.
- Kilian KA, Bugarija B, Lahn BT, et al. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc Natl Acad Sci USA. 2010;107:4872–4877.
- Kim BS, Lee J-S, Gao G, et al. Direct 3D cell-printing of human skin with functional transwell system. Biofabrication. 2017;9:025034.
- Abagnale G, Sechi A, Steger M, et al. Surface topography guides morphology and spatial patterning of induced pluripotent stem cell colonies. Stem Cell Reports. 2017;9:654–666.
- Britsch S, Goerich DE, Riethmacher D, et al. The transcription factor Sox10 is a key regulator of peripheral glial development. Genes Dev. 2001;15:66–78.
- Mata M, Alessi D, Fink DJ. S100 is preferentially distributed in myelin-forming Schwann cells. J Neurocytol. 1990;19:432–442.
- Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6:671.
- Bonakdar S, Mahmoudi M, Montazeri L, et al. Cell-imprinted substrates modulate differentiation, redifferentiation, and transdifferentiation. ACS Appl Mater Interfaces. 2016;8:13777–13784.
- Mashinchian O, Bonakdar S, Taghinejad H, et al. Cell-imprinted substrates act as an artificial niche for skin regeneration. ACS Appl Mater Interfaces. 2014;6:13280–13292.
- Ohashi K, Fujiwara S, Mizuno K. Roles of the cytoskeleton, cell adhesion and rho signalling in mechanosensing and mechanotransduction. J Biochem. 2017;161:245–254.
- Martino F, Perestrelo AR, Vinarský V, et al. Cellular mechanotransduction: from tension to function. Front Physiol. 2018;9:824.
- Harris AR, Jreij P, Fletcher DA. Mechanotransduction by the actin cytoskeleton: converting mechanical stimuli into biochemical signals. Annu Rev Biophys. 2018;47:617–631.
- Gao L, McBeath R, Chen CS . Stem cell shape regulates a chondrogenic versus myogenic fate through Rac1 and N-cadherin. Stem Cells. 2010;28:564–572.
- Woods A, Wang G, Beier F. RhoA/ROCK signaling regulates Sox9 expression and actin organization during chondrogenesis. J Biol Chem. 2005;280:11626–11634.
- Konstantinidis G, Moustakas A, Stournaras C. Regulation of myosin light chain function by BMP signaling controls actin cytoskeleton remodeling. Cell Physiol Biochem. 2011;28:1031–1044.
- Chen B, Co C, Ho C-C. Cell shape dependent regulation of nuclear morphology. Biomaterials. 2015;67:129–136.
- Li Y, Chu JS, Kurpinski K, et al. Biophysical regulation of histone acetylation in mesenchymal stem cells. Biophys J. 2011;100:1902–1909.
- Zhu C, Li L, Zhao B. The regulation and function of YAP transcription co-activator. Acta Biochim Biophys Sin (Shanghai). 2015;47:16–28.
- Dobrokhotov O, Samsonov M, Sokabe M, et al. Mechanoregulation and pathology of YAP/TAZ via Hippo and non-Hippo mechanisms. Clin Transl Med. 2018;7:23.
- Dupont S, Morsut L, Aragona M, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179.
- Kim Y, Kwon C, Jeon H. Genetically engineered phage induced selective H9c2 cardiomyocytes patterning in PDMS microgrooves. Materials. 2017;10:973.
