?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective
To study the effect of miR-16-5p on lung cancer cell injury and apoptosis, and its mechanism.
Methods
LPS induced lung cancer cell A549 injury; qRT-PCR method was applied to detect the expression of miR-16-5p and CXCR3 in A549 cells. Con (without LPS treatment), LPS + miR-NC group (transfected negative control samples), LPS + miR-16-5p group (transfected miR-16-5p mimics); LPS + si-NC group (transfected negative control samples), LPS + si-CXCR3 group (transfected si-CXCR3); LPS + miR-16-5p + pcDNA3.1 group (co-transfected miR-16-5p mimics and pcDNA3.1), LPS + miR-16-5p + pcDNA3.1-CXCR3 group (co-transfected miR-16-5p mimics and pcDNA3.1-CXCR3) were transfected into A549 cells by liposome method. Western blot was used to detect protein expression of CXCR3, IL-6 and TNF-α in A549 cells; apoptosis of A549 cells was detected by flow cytometry.
Results
Compared with the control group, the expression of miR-16-5p mRNA was significantly decreased in A549 cells in LPS group, and the mRNA and protein expression of CXCR3 were significantly increased (p < .05). Overexpression of miR-16-5p and knockdown of CXCR3 both can down-regulated protein expression of IL-6 and TNF-α, and up-regulated apoptosis in LPS-induced A549 cell; CXCR3 is a target of miR-16-5p. Overexpression of CXCR3 rescued the protective effect of miR-16-5p on LPS-induced A549 cell injury.
Conclusion
miR-16-5p can protect LPS-induced A549 cell injury, and its mechanism may be related to the targeted regulation of CXCR3, which could provide a new target for targeted therapy of lung cancer.
Keywords:
Introduction
miRNAs are short-chain non-coding endogenous small RNAs that can participate in the development of cancers through regulating the translational synthesis of post-transcriptional regulatory proteins [Citation1]. miR-16-5p plays an important role in a variety of cancers [Citation2], but its role in lung cancer has not been reported. Chemokines can be divided into four families according to the number and spacing of N-terminal cysteine residues, C, CC, CXC and CX3C [Citation3]. In addition, CXC chemokines are mainly involved in tumor progression by binding to specific receptors. The CXC receptor that has been found so far was CXCR1-CXCR7. The affinities of different chemokines and their receptors are also different, and the biological effects they produce are also diverse [Citation4]. CXCR3 is a 7-transmembrane G-protein coupled receptor whose N-terminal region and C-terminal region are located in the transmembrane portion of the cell membrane. The N-terminal region outside the cell membrane can bind to cytokines, resulting in a series of cell signaling [Citation5]. CXCR3 is expressed in a variety of cancers, such as breast cancer, gastric cancer and prostate cancer [Citation6–8]. However, the role and mechanism of CXCR3 in lung cancer has not been clear yet. In this study, human lung adenocarcinoma cell line A549 was used to detect the expression of CXCR3 and miR-16-5p in LPS-induced A549 cell injury. The effects of overexpression of miR-16-5p, knockdown of CXCR3 and overexpression of CXCR3 on LPS were also observed. The induced apoptosis of A549 cells and the expression of IL-6 and TNF-α revealed that the mechanism of miR-16-5p may be related to the targeted regulation of CXCR3, which will provide a new target for the prevention and treatment of lung cancer.
Materials and methods
Materials
Human lung adenocarcinoma cell A549 was purchased from ATCC; DMEM medium, fetal bovine serum, MTT reagent, trypsin were purchased from GIBCO, USA; LipofectamineTM2000, reverse transcription kit were purchased from Invitrogen, USA. Annexin V-FITC/PI apoptosis detection kit was provided from Sigma, USA; dual luciferase reporter assay kit was purchased from Promega, USA.
Methods
Cell culture and grouping
Human lung adenocarcinoma cell line A549 was cultured in DMEM medium containing 10% fetal bovine serum in an incubator at 37 °C, 5% CO2. Con group: normal cultured A549 cells; LPS group: conventionally cultured A549 cells were treated with LPS (10 μg/mL) for 12 h. LPS + miR-16-5p/NC group: miR-16-5p/NC mimics were transfected into A549 cells and then treated with LPS; LPS + si-CXCR3/NC group, si-CXCR3/NC was transfected into A549 cells, and then treated with LPS; LPS + miR-16-5p + pcDNA3.1 group: miR-16-5p mimics and pcDNA3.1 were co-transfected into A549 cells and treated with LPS treatment; LPS + miR-16-5p + pcDNA3.1-CXCR3 group: miR-16-5p mimics and pcDNA3.1-CXCR3 were co-transfected into A549 cells and treated with LPS treatment. The above groups of cells were transfected for 30 h according to the procedure of the liposome LipofectamineTM 2000, then the subsequent experiments were carried out.
qRT-PCR experiment
Total RNA of the cell sample in the logarithmic growth phase was extracted using Trizol method according to the instructions of the RNA extraction kit. The first-strand cDNA was synthesized according to the instructions of the reverse transcription kit. Finally, the detection of miR-16-5p and CXCR3 were performed according to the instruction of the qRT-PCR kit. The expression of miR-16-5p and CXCR3 were calculated by 2−ΔΔCt.
Western blot
The cells of each group were collected and lysed on ice for 25 min. Centrifuge at 12,000 rpm for 10 min. The supernatant was placed in an EP tube, and 5 × SDS loading buffer was added and boiled for 10 min. After electrophoresis, the protein was transferred to PVDF membrane by a membrane transfer instrument; the membrane was blocked by 5% skim milk powder for 2 h. After the membrane was washed, I-antibody was added and incubated at 4 °C overnight; then, the II-antibody was added at 4 °C for 2 h. Add luminescent liquid and expose.
Annexin V-FITC/PI flow cytometry experiment
The cells in each group were collected, and the cells were suspended in 500 μl of binding buffer. Five microlitres of Annexin V-/FITC and PI were added, mixed, and stayed at room temperature for 15 min in the dark. The results of the assay were analyzed using flow cytometry. Apoptosis rate of cells (%) = early apoptotic rate + late apoptotic rate. Each sample was repeated three times.
Dual luciferase reporter gene assay
Each group of A549 cells was collected and operated according to the dual luciferase reporter assay kit technical manual. The psiCHECK2 vector expressed firefly and Renilla luciferase activity. The luciferase reporter vectors psiCHECK2-CXCR3-3′UTR WT and psiCHECK2-CXCR3-3′UTR MUT were constructed. After 24 h of transfection, the fluorescence intensity was measured. The ratio of the luminescence intensity of Renilla luciferase to the luminescence intensity of firefly luciferase showed the binding force of miR-16-5p and CXCR3.
Statistical processing
All data in the experiment were analyzed using SPSS 21.0 software. Measurement data were expressed as mean ± standard deviation ( ± s). Data between groups were compared by one-way analysis of variance. Pairwise comparisons were performed using SNK-q test. p < .05 was considered statistically significant.
Results
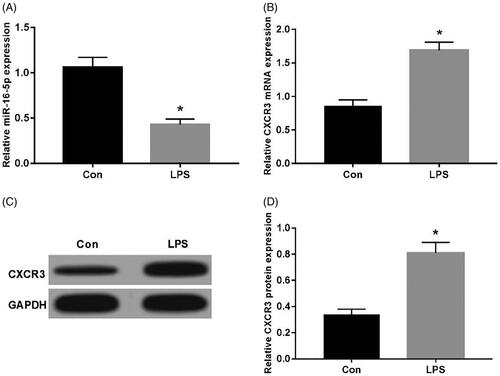
Expression of CXCR3 and miR-16-5p in LPS-induced A549 cell injury
The expression of CXCR3 and miR-16-5p in A549 cells induced using LPS was detected using qRT-PCR and Western blot. Compared with Con group, the expression of miR-16-5p was significantly decreased in LPS group (). CXCR3 mRNA was significantly elevated (); moreover, CXCR3 protein expression was significantly increased (), which were statistically significant (p < .05).
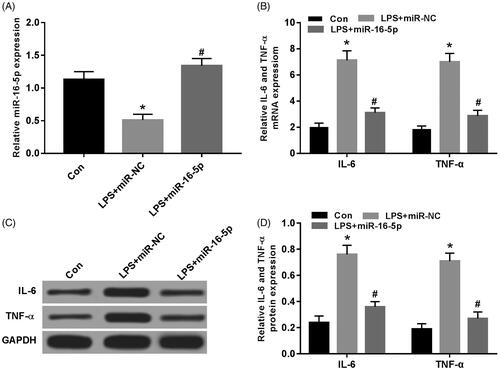
Effect of overexpression of miR-16-5p on LPS-induced expression of IL-6 and TNF-α in A549 cells
When miR-16-5p mimics was transfected into LPS-induced A549 cells, compared with Con group, miR-16-5p expression was significantly decreased in LPS group; compared with LPS + miR-NC group, the expression of miR-16-5p was significantly increased in LPS + miR-16-5p group (). Compared with the Con group, the mRNA expression of IL-6 and TNF-α were significantly increased in the LPS group; compared with the LPS + miR-NC group, the mRNA expression of IL-6 and TNF-α were significantly decreased in the LPS + miR-16-5p group (). Compared with the Con group, the protein expression of IL-6 and TNF-α was significantly higher in the LPS group. Compared with the LPS + miR-NC group, the protein expression of IL-6 and TNF-α were significantly decreased in the LPS + miR-16-5p group (), and both were statistically significant (p < .05).
Figure 2. Effects of miR-16-5p overexpression on the expression of IL-6 and TNF-α in A549 cells treated with LPS. (A) The expression level of miR-16-5p in A549 cells after overexpression miR-16-5p; (B) The effect of overexpression miR-16-5p on the mRNA expression of IL-6 and TNF-α in A549 cells treated with LPS; (C,D) Overexpression of miR-16-5p on the protein expression of IL-6 and TNF-α in A549 cells treated with LPS, compared with Con group, *p < .05; compared with LPS + miR-NC group, #p < .05.
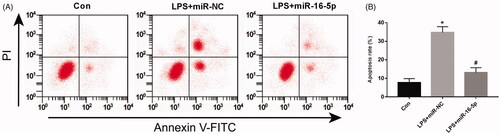
Effect of overexpression of miR-16-5p on apoptosis of LPS-treated A549 cells
Flow cytometry was used to detect apoptosis in cells. Compared with the Con group, the apoptosis rate was significantly increased in the LPS group. Compared with the LPS + miR-NC group, the apoptosis rate was significantly decreased in the LPS + miR-16-5p group (), and both were statistically significant (p < .05).
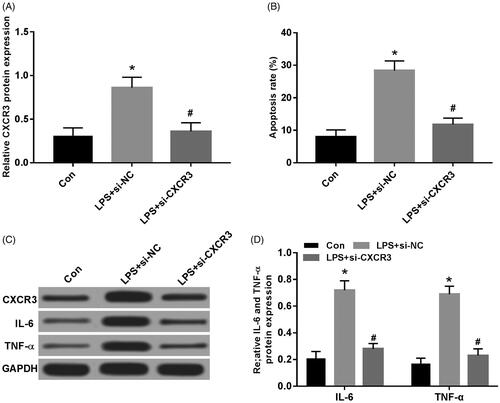
Effect of knockdown of CXCR3 expression on LPS-induced A549 cell injury
When si-CXCR3 was transfected into LPS-induced A549 cells, compared with the Con group, the protein expression of CXCR3 was significantly increased in the LPS group. Compared with the LPS + si-NC group, the protein expression of CXCR3 was significantly decreased in the LPS + si-CXCR3 group (); compared with the Con group, the apoptosis rate of the LPS group was significantly increased; compared with the LPS + si-NC group, the apoptosis rate was significantly decreased in the LPS + si-CXCR3 group (); compared with the Con group, the protein expression of IL-6 and TNF-α were significantly increased in the LPS group. Compared with LPS + si-NC group, the protein expression of IL-6 and TNF-α were significantly decreased in LPS + si-CXCR3 group (), and both were statistically significant (p < .05).
Figure 4. Effect of knockdown of CXCR3 expression on LPS-induced A549 cell injury. (A) Knockdown of CXCR3 on protein expression of CXCR3 in A549 cells; (B) knockdown of CXCR3 on apoptosis of LPS-induced A549 cells; (C,D) knockdown of CXCR3 on protein expression of CXCR3, IL-6, TNF-α in LPS-induced A549 cells, compared with Con group, *p < .05; compared with LPS + si-NC group, #p < .05.
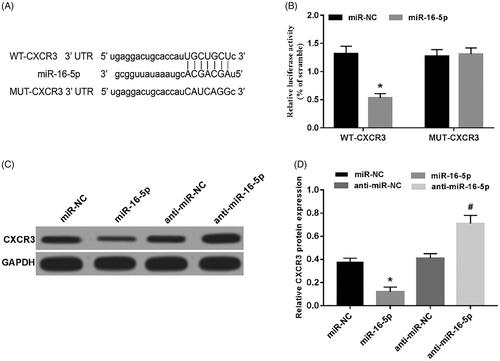
miR-16-5p targets CXCR3
Prediction of the potential binding between CXCR3 and miR-16-5p was performed using Target scan, and it was found that miR-16-5p is likely to target CXCR3 (). The luciferase reporter assay was used to detect the fluorescence activity of the cells. Compared with the miR-NC group, the fluorescence activity of the WT cells in the miR-16-5p group was significantly decreased, and the fluorescence activity of the MUT cells was not affected (). Compared with the miR-NC group, the protein expression of CXCR3 was significantly decreased in the miR-16-5p group; compared with the anti-miR-NC group, the protein expression of CXCR3 was significantly increased in the anti-miR-16-5p group () and both were statistically significant (p < .05). These results showed that miR-16-5p can target CXCR3in A549 cells.
Figure 5. miR-16-5p targets CXCR3. (A) The 3'UTR of CXCR3 contains a nucleotide sequence complementary to miR-16-5p; (B) the effect of miR-16-5p on the fluorescence activity of lung cancer cells; (C,D) miR-16-5p regulates the protein expression of CXCR3, compared with miR-NC group, *p < .05; compared with anti-miR-NC group, #p < .05.
Overexpression of CXCR3 rescues the protective effect of miR-16-5p on LPS-induced A549 cell injury
As shown in , the protein expression of IL-6 and TNF-α in LPS + miR-16-5p cells were significantly decreased compared with the LPS + miR-NC group. Compared with LPS + miR-16-5p + pcDNA3.1 group, the protein expression of IL-6 and TNF-α in LPS + miR-16-5p + pcDNA 3.1-CXCR3 group was significantly increased (); compared with LPS + miR-NC group, the apoptotic rate of the LPS + miR-16-5p group was significantly decreased. In comparison with the LPS + miR-16-5p + pcDNA3.1 group, the apoptosis rate of the LPS + miR-16-5p + pcDNA 3.1-CXCR3 group was significantly increased (), and both were statistically significant (p < .05). These data suggested that overexpression of CXCR3 rescues the protective effect of miR-16-5p on LPS-induced A549 cell injury.
Figure 6. Overexpression of CXCR3 reverses the protective effect of miR-16-5p on LPS-induced A549 cell. (A,B) Overexpression of CXCR3 on the protein expression of IL-6, TNF-α in LPS-induced A549 cells; (C) Overexpression of CXCR3 on the apoptosis of LPS-induced A549 cells compared with LPS + miR-NC group, *p < .05; compared with LPS + miR-16-5p + pcDNA3.1 group, #p < .05.

Discussion
As oncogenes or tumor suppressor genes, miRNAs play a regulatory role in a variety of cancers, including lung cancer. Recently, miR-1284, miR-17–92, miR-33a-5p and miR-128-3p have been found to take important part in lung cancer [Citation9–11]. miR-16-5p is involved in the development of gastric cancer and chordoma [Citation12,Citation13], and its research in lung cancer is still unclear. Gu et al. [Citation14] used qRT-PCR, flow cytometry, dual luciferase reporter gene assay and MTS assay to detect miR-16 expression, cell proliferation, apoptosis and miR-16 in A549 cells. miR-16-5p was down-regulated and exerted functions in A549 cells through targeting to wip1. In addition, overexpression of miR-16-5p inhibited proliferation and promoted apoptosis of A549 cells, suggesting that miR-16-5p may be beneficial to NSCLC patients. Ke et al. [Citation15] found that miR-16-5p is down-regulated in NSCLC tissues and cells in non-small cell lung cancer studies. Overexpression of miR-16-5p significantly suppressed cell proliferation and colony formation. Furthermore, liver cancer-derived growth factor (HDGF) was thought to be a direct target of miR-16, which inhibits NSCLC cell migration and invasion. The inhibitory effect of miR-16-5p on cell proliferation, colony formation, migration and invasion is partly mediated by inhibition of HDGF expression, suggesting that miR-16 plays an important role in NSCLC. This study examined the expression of miR-16-5p in LPS-induced A549 cell injury and considered that miR-16-5p was under-expressed; overexpression of miR-16-5p can significantly promote apoptosis and down-regulate expression of IL-6 and TNF-α. We as well verified that miR-16-5p negatively regulated CXCR3 expression via target binding as evidenced by dual luciferase reporter assay.
The role of chemokines and their receptors in tumors is gaining more and more attention. CXCR3 is a member of the CXC chemokine family, which contains CXCL9, CXCL10 and CXCL11 [Citation16,Citation17]. With collection of peripheral and tumor-drained blood samples from lung cancer patients, Spaks et al. [Citation18] measured CXCL1, CXCL4, CXCL5, CXCL6, CXCL7, CXCL8, CXCL9, CXCL10, CXCL11 and CXCL12 levels using ELISA and calculated chemokine concentration gradients. Immunization histochemical evaluation revealed the expression of CXCR1, CXCR2, CXCR3 and CXCR4 in tumor infiltrating immune cells (T helper cells, T cytotoxic cells, macrophages, B cells, plasma cells) and tumor tissues, among which CXCL4 and CXCL5 were significantly decreased, and CXCL7 was significantly increased. Moreover, high expression of CXCL9 and CXCL10 is associated with a good prognosis in patients, and chemokine levels and gradients are associated with both CXC receptor expression and the number of tumor infiltrating immune cell subsets. Åsa et al. [Citation19] found that IL-7 reduced tumor burden, accompanied by CD4 and CD8 T lymphocyte subsets, T cell activation markers CXCR3, CD69 and CD127, effector memory T cells and T cell lysing cells against parental tumor cells. The increased frequency of activity indicates that IL-7 induces CXCR3 ligand-dependent T cell anti-tumor reactivity in lung cancer. Andersson et al. [Citation20] studied the anti-tumor effect of IL-7/IL-7Rα-Fc in a lung cancer model and found that IL-7/IL-7Rα-Fc administration inhibits tumor growth and increases lung cancer progression. The survival rate of patients with tumor growth inhibition is duo to the increase of APC and T cell activity, levels of CXCL9, CXCL10, IFNγ, IL-12, the characteristics of tumor macrophage infiltration M1 phenotype, as well as upregulated IL-12, iNOS, T Increased activation of cells and NK cells, increased T cell activation markers CXCR3, increased CD69, and decreased CD127, in which CXCR3 ligand plays an important role in IL-7/IL-7Rα-Fc-mediated antitumor activity. CXCL9, CXCL10 or IFNγ activated T cell infiltration of tumors and abolished IL-7/IL-7Rα-Fc mediated tumor growth inhibition. In this study, qRT-PCR and Western blot were used to detect the expression of CXCR3 in LPS-induced lung cancer cell injury. CXCR3 was highly expressed in injured A549 cells, which is consistent with previous studies. Further research revealed that knockdown of CXCR3 can inhibit the apoptosis of A549 cells and the expression of IL-6 and TNF-α. Overexpression of CXCR3 could reverse the protective effect of miR-16-5p on LPS-induced A549 cell injury.
In summary, miR-16-5p can protect LPS-induced A549 cell injury, and its mechanism may be related to negative regulation of CXCR3 through target binding, providing a basis for accurate treatment of lung cancer.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- He Y, Lin J, Kong D, et al. Current state of circulating microRNAs as cancer biomarkers. Clin Chem. 2015;61:1138–1155.
- Zhang J, Song Y, Zhang C, et al. Circulating MiR-16-5p and MiR-19b-3p as two novel potential biomarkers to indicate progression of gastric cancer. Theranostics. 2015;5:733–745.
- Yoshie O. Chemokines and chemokine receptors. Nihon Rinsho. 2012;70:212–217.
- Loetscher M, Loetscher P, Brass N, et al. Lymphocyte-specific chemokine receptor CXCR3: regulation, chemokine binding and gene localization. Eur J Immunol. 1998;28:3696–3705.
- Qiu MY, Li JW, Zheng M. The chemokine receptors CXCR4 and CXCR7 in cancer. China Oncol. 2010;20:222–226.
- Wu Q, Dhir R, Wells A. Altered CXCR3 isoform expression regulates prostate cancer cell migration and invasion. Mol Cancer. 2012;11:74–80.
- Li L, Chen J, Lu ZH, et al. Significance of chemokine receptor CXCR3 expression in breast cancer. Zhonghua Bing li Xue za Zhi. 2011;40:85–88.
- Li K, Zhu Z, Luo J, et al. Impact of chemokine receptor CXCR3 on tumor-infiltrating lymphocyte recruitment associated with favorable prognosis in advanced gastric cancer. Intern J Clin Exp Path. 2015;8:14725–14732.
- Li J, Jin H, Yu H, et al. miRNA‑1284 inhibits cell growth and induces apoptosis of lung cancer cells. Mol Med Rep. 2017; 16:3049–3054.
- Zhang X, Li Y, Qi P, et al. Biology of MiR-17-92 cluster and its progress in lung cancer. Int J Med Sci. 2018;15:1443–1448.
- Pan J, Zhou C, Zhao X, et al. A two-miRNA signature (miR-33a-5p and miR-128-3p) in whole blood as potential biomarker for early diagnosis of lung cancer. Sci Rep. 2018;8:16699.
- Zhu C, Huang Q, Zhu H. Melatonin inhibits the proliferation of gastric cancer cells through regulating the miR-16-5p-Smad3 pathway. DNA Cell Biol. 2018;37:244–252.
- Zhang H, Yang K, Ren T, et al. miR-16-5p inhibits chordoma cell proliferation, invasion and metastasis by targeting Smad3. Cell Death Dis. 2018;9:680.
- Gu Y, Wang XD, Lu JJ, et al. Effect of mir-16 on proliferation and apoptosis in human A549 lung adenocarcinoma cells. Int J Clin Exp Med. 2015;8:3227–3233.
- Ke Y, Zhao W, Xiong J, et al. Downregulation of miR-16 promotes growth and motility by targeting HDGF in non-small cell lung cancer cells. FEBS Lett. 2013;587:3153–3157.
- Flier J, Boorsma DM, van Beek PJ, et al. Differential expression of CXCR3 targeting chemokines CXCL10, CXCL9, and CXCL11 in different types of skin inflammation. J Pathol. 2001;194:398–405.
- Groom JR, Luster AD. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol Cell Biol. 2011;89:207.
- Spaks A. Role of CXC group chemokines in lung cancer development and progression. J Thorac Dis. 2017;9:S164–S171.
- Åsa A, Seok CY, Min H, et al. IL-7 promotes CXCR3 ligand-dependent T cell antitumor reactivity in lung cancer. J Immunol. 2009;182:6951–6958.
- Andersson A, Srivastava MK, Harris WM, et al. Role of CXCR3 ligands in IL-7/IL-7R alpha-Fc-mediated antitumor activity in lung cancer. Clin Cancer Res. 2011;17:3660–3672.