Abstract
The purpose of this research is to assess the feasibility of poly(lactic-co-glycolic) acid (PLGA) incorporating gelatin microspheres (PLGA/GMs scaffold) for enhancing osteogenesis in vitro and at a radius defect of rabbits after X-ray radiation in vivo. After incorporating gelatin microspheres, PLGA scaffold demonstrated improved mechanical properties. Moreover, a sustained release property of recombinant human bone morphogenetic protein-2 (BMP-2) was achieved in BMP-2-releasing PLGA/GMs scaffold. BMP-2-releasing PLGA/GMs scaffold also enhanced proliferation and osteogenesis of rabbit bone mesenchymal stem cells (BMSCs) in vitro, indicating the bioactivity of BMP-2. After finishing X-ray radiation of the radius bone, 20-mm radius bone defects were generated, followed by being implanted with BMP-2-releasing PLGA/GMs scaffolds with or without bone marrow. Both PLGA/GMs scaffolds containing bone marrow or BMP-2 showed more obvious enhancement for bone regeneration than the empty scaffolds (control) at the radius defect. In the X-ray radiated groups, however, the bone regeneration was inhibited either with bone marrow or BMP-2. When combined with bone marrow, the BMP-2 showed significantly high osteogenic effect, regardless of X-ray radiation. It is considered that it is a promising way to repair bone defects even after X-ray radiation by a combination of bone marrow with the BMP-2-releasing PLGA/GMs scaffold.
Introduction
After surgical resection of a large bone to remove the tumor, to avoid recurrence or metastasis of the tumor, doctors often carry out radiation therapy for the surrounding tissue. This radiation therapy has a certain effect on surgical treatment for tumors. However, X-ray radiation ultimately leading to osteoporosis has been reported, which involves inhibition of cell proliferation, alkaline phosphatase activity, cross-linking or denaturation of collagen phase and neovascularization [Citation1]. Therefore, it is often difficult for the site of tumor resection to achieve bone regeneration, which has been a serious problem clinically to be solved [Citation2]. So it is actually necessary for researchers to develop a technique capable of inducing bone regeneration even under the X-ray radiation conditions. Traditionally, autologous bone grafting has been applied to repair this type of bone defect. However, this method has significant drawbacks such as increasing the incidence of donor sites, and a limited amount of available donor bone [Citation3]. At present, tissue engineering technology [Citation4] has been expected to surmount these shortcomings. Howard et al. [Citation5] used hydroxyapatite (HA) disks combining with BMP-2 to repair bone defects of rabbit nasal bone after X-ray radiation. They found that when combined with BMP-2, the bone regeneration was more obvious than that without it. Between the non-radiated and radiated groups both with BMP-2; however, no significant difference was observed in bone regeneration. Würzler et al. [Citation6] investigated bone regeneration of rat calvarial non-critical size defects. Before the operation, they carried out radiation in the cranium with the dose of 1200 rad for 2 or 7 d. The results showed that type I collagen carrier alone led to only 7 ± 2% healing, but when it contained BMP-2, the bone regeneration was significantly improved. However, in this experiment, the application of BMP-2 was performed only 2 d after radiation, an interval considered too short. Moreover, the dose of radiation was low and the defect was non-critical size.
PLGA is often used as substrates for bone tissue engineering, which has demonstrated to be non-inflammatory and biocompatible [Citation7]. However, due to its poor osteogenic bioactivity and low mechanical strength, their biological applications have been limited to some extent [Citation8]. Therefore, to achieve some of the desired properties, researchers often combine the advantages of two biomaterials. PLGA is often used in combination with other materials such as ceramics, bioactive glass, gelatin, or it is modified to be more biomimetic [Citation9]. Researchers have found that polycaprolactone (PCL)/PLGA scaffold blended with tri-calcium phosphate (TCP) by solid freeform fabrication technology can enhance the mechanical strength of the scaffold [Citation10]. These scaffolds, however, are lack of carriers to load and sustained-release osteoinductive growth factor [Citation11]. Due to their biocompatibility and osteoconductive properties, apatite cement (such as calcium phosphate cement, CPC) represent a promising candidate material for bone substitution. Many animal studies showed that PLGA microspheres embedded within CPC increases cement degradation and enhances osteogenesis [Citation11,Citation12]. However, the osteoconductive properties of CPC/PLGA are not sufficient to achieve complete defect filling [Citation13]. Consequently, the enrichment of CPC/PLGA with osteoinductive factors is necessary to improve their biological performance. PLGA/HA scaffolds combined with osteoinductive growth factor via polydopamine were fabricated by some studies, but based on its high porosity, the low mechanical strength, which can influence osteogenesis, has yet to be solved [Citation14]. A wide number of PLGA composite in combination with gelatin (PLGA/gelatin) are being studied as a new type of alternatives for bone tissue regeneration [Citation15]. Gelatin is a kind of biodegradable polymer originating from denatured collagen, which has been commercially available. By long-term clinical applications, its biosafety has been confirmed [Citation16]. And based on its degradation, researchers have found that gelatin can be applied as delivery systems for drugs and therapeutic biomolecules [Citation17]. In addition, gelatin is present in the structural sequence of arginine–glycine–aspartate (RGD), which was associated with the regulation of interactions between cells, as well as between extracellular matrix (ECM) and cells. Also, this kind of biomimetic peptide has the potential of enhancing cell adhesion and promoting tissue regeneration [Citation18]. Pure scaffold has limited osteoinductive ability, but it showed a suitable environment for osteoinduction when combined with osteoinductive growth factor [Citation19]. And among these, BMP-2 has been commonly used to guide bone regeneration in those defects that cannot spontaneously heal [Citation20]. Yet, due to the short half-life of BMP-2 in solution form, its therapeutic efficacy was always low in vivo. To address this problem, gelatin microspheres had been applied widely to load and release BMP-2 in a controlled way, which may better induce repairment of bone defect [Citation21] and ectopic bone formation [Citation22].
To investigate the osteogenic capability of the scaffold, normal animals in healthy conditions have been widely used. However, it is necessary to make a thorough inquiry about the osteogenic capability of the PLGA/GMs scaffold even in an unhealthy condition such as X-ray radiation from the clinical viewpoint. So in this research, we aimed to assess the feasibility of BMP-2-releasing PLGA/GMs scaffold to induce osteogenesis in vitro and at radius defects after X-ray radiation in vivo. The effect of bone repairment was assessed by the Digital Radiograph and histological examinations after implanting the BMP-2-releasing PLGA/GMs scaffold with or without bone marrow into the radius defect site.
Materials and methods
Ethics statement
All the protocols for handling and treating animals were reviewed and finally approved by the Animal Care and Use Committee of Jilin University, China.
Materials
PLGA (LA:GA = 50:50, 14,000 kDa, Jinan Daigang Biomaterial Co., Ltd., Jinan, China), N-methyl-2-pyrrolidone (NMP) (Sigma-Aldrich Co., Shanghai, China) and gelatin (Sigma-Aldrich Co., Shanghai, China).
Fabrication of gelatin microspheres and BMP-2-loaded gelatin microspheres
Gelatin microspheres (GMs) were obtained using the water-in-oil emulsion method [Citation23]. The details are as follows. First, gelatin was dissolved in water to achieve a gelatin solution of 25% concentration (g/mL). Next, add three drops (about 50 μL) of Span 80–60 ml to liquid paraffin. After heating the liquid paraffin in a 37 °C water bath and being stirred continuously at 200 rpm, the gelatin solution was added to it and stirred for 5 min, then immediately transferred to an ice bath at 4 °C at the rotation speed of 200 rpm for 1 h. Glutaraldehyde was then added to the gelatin solution above for cross-linking for 5 min, and the gelatin microspheres were finally obtained by suction filtration and dried. BMP-2-loaded gelatin microspheres were prepared by adding BMP-2 to gelatin solution to obtain a concentration of 2 ng/mg (i.e. 1 mg gelatin containing 2 ng BMP-2).
Fabrication of PLGA scaffold via phase inversion method
A PLGA scaffold was fabricated via combining particulate leaching and phase inversion [Citation24]. First, 1 g of PLGA was dissolved into 5 ml of NMP for 8 h to obtain PLGA/NMP mixture. Then add sodium chloride particulates which were sieved by 30–150 µm in diameter into the mixture. The weight ratio of the salt particulates to PLGA was 6:1. Next, the PLGA/NMP mixture was immersed in distilled water for 2 d with the water exchanged every 8 h so as to remove NMP and salt particulates. Then, after 3 d of lyophilization, the porous PLGA scaffold was finally obtained.
Fabrication of PLGA/GMs and PLGA/GMs/BMP-2 scaffolds via phase inversion method
PLGA/GMs scaffold was fabricated via phase inversion. First, 1 g of PLGA was dissolved into 5 ml of NMP for 8 h to obtain PLGA/NMP mixture. Then add GMs which were sieved by 30–150 µm in diameter into the mixture. The ratio of the GMs to PLGA was 40 wt%. Next, the PLGA/GMs/NMP mixture was immersed in distilled water for 2 d with the water exchanged every 8 h so as to remove NMP. Then after 3 d of lyophilization, the PLGA/GMs scaffold was finally obtained. PLGA/GMs/BMP-2 scaffold was fabricated by adding BMP-2-loaded GMs into the PLGA/NMP mixture above.
Characterizations of GMs and scaffolds
Snap-freezed scaffolds were fractured to obtain a section. Then the surface of GMs and scaffolds were sputter-coating with gold and observed while accelerating voltage of 15 kV by scanning electron microscopy (SEM; XL30, Philips, Amsterdam, The Netherlands). For mechanical properties measurement of the scaffolds, cylindrical rods of 30 mm × 5 mm × 5 mm were used by a universal testing machine (Instron 1121, Norwood, MA, USA). And the crosshead speed was 2 mm/min. Three replicates were tested (n = 3).
Release kinetics of BMP-2
PLGA scaffold was directly immersed in BMP-2 solution (5.0 μg/mL) to get PLGA/BMP-2. The PLGA/BMP-2 and PLGA/GMs/BMP-2 were employed to investigate BMP-2 release properties in vitro. Each BMP-2-containing scaffold (5 × 5 × 5 mm3) was incubated in of phosphate-buffered saline (PBS, 5.0 ml) at 37 °C. Then the supernatant (0.2 ml) was collected at specific time intervals, and the same volume of fresh PBS was supplemented. The content of BMP-2 was tested by the enzyme-linked immunosorbent assay (ELISA) kit (R&D System, Minneapolis, MN). Then by plotting the percentage of cumulative content of released BMP-2 as time, release profile was finally obtained. Tests were carried out in triplicate.
Cell culture
Isolation of rabbit bone marrow mesenchymal stem cells (BMSCs)
New Zealand white rabbits (three months old, provided by Jilin University, Changchun, China) were selected to isolate BMSCs. Animals were treated based on the NIH Guide for the Care and Use of Laboratory Animals (NIH Publication no. 85–23). Bone marrow aspirates of 5 ml were acquired from the tibia and then cultured. Briefly, resuspend the isolated cell pellets in 5.0 ml of culture medium (DMEM; Dulbecco’s Modified Eagle Medium (Gibco, Carlsbad, CA) complemented with 10% (V/V) foetal calf serum (Gibco, Carlsbad, CA) and 100 IU/mL of penicillin-streptomycin (Sigma, Shanghai, China). The isolated cells were seeded in culture dishes (Corning Costar Co., Cambridge, MA) and then cultured in an incubator (37 °C, 5% carbon dioxide). Non-adherent cells were removed when the medium was changed after 24 h. Thereafter, the medium was changed every 3 days until the cells reached 80% confluence. Then, the cells were washed twice with PBS, treated with 0.25% trypsin-ethylenediaminetetraacetic acid (EDTA, Sigma, Shanghai, China) and subcultured until the third passage.
Cell proliferation
BMSCs (1 × 105) were seeded into scaffolds (5 × 5 × 3 mm3) to investigate the cell proliferation. It was tested at day 1, 3 and 7 by a standard 3-(4,5-dimethylthiazoyl-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Briefly, an MTT solution (5.0 mg/mL) was used to incubate the scaffolds for 4 h. After removing the MTT solution, acidified isopropanol (0.2 ml of 0.04 M HCl in 10 ml of isopropanol) was added to it, so as to solubilize resultant formazan product. The absorbance of the extractant was tested at 492 nm by a multifunction microplate scanner (Thermo Scientific, Hudson, NH) and the number of cells was proportional to the optical density (OD). At least, three measurements were made for the averaging.
Cell proliferation
For the proliferation study, 1 × 105 BMSCs were seeded on the scaffolds (5 × 5 × 3 mm3). The proliferation of the cells for each sample was determined by a standard 3-(4,5-dimethylthiazoyl-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Cell proliferation was determined on Day 1, 3, and 7. The scaffolds were incubated in an MTT solution (5.0 mg/mL in PBS) for 4 h. After the removal of the MTT solution, the acidified isopropanol (0.2 ml of 0.04 M HCl in 10 ml of isopropanol) was added to solubilize the resultant formazan product. The absorbance of the extractant at 492 nm was recorded on a Thermo Electron MK3 spectrophotometer (Thermo Scientific, Hudson, NH). The relative cell number (%) was determined by comparing the absorbance to that for each scaffold. The mean value of nine readings for each sample was used as the final result.
Cell differentiation
At 7 and 14 d after cells seeding, alkaline phosphatase (ALP) activity on different samples was tested. Briefly, after removing the medium of each well, the cells were then carefully washed twice using PBS. The cells were then lysed in 200 μl Radio-Immunoprecipitation Assay (RIPA) buffer for 3 min before freezing at 80 °C and then thawed at 37 °C. ALP quantitative was evaluated by mixing 200 μl p-nitrophenyl phosphate (p-NPP, Sigma, St. Louis, MO) in the supernatant. Then end the reaction by adding sodium hydroxide (3.0 M). It was incubated (37 °C) in the dark for 30 min and then tested OD value at 405 nm.
Calcium deposition was tested by alizarin red S (ARS, Sigma-Aldrich, St. Louis, MO) at days 14 and 21. At 37 °C, the scaffolds were incubated in ARS stain solution (0.1% ARS in Tris-HCl buffer, pH 8.0) for 30 min. Then wash the scaffolds thrice by water for 5 min. Cetylpyridinium chloride (CPC, Sigma-Aldrich, St. Louis, MO) was used to treat the stained scaffolds for 15 min at room temperature. Then add 1 ml of 10% (w/v) CPC solution to ARS-stained samples for desorbing calcium ions for 1 h. The absorbance was tested at 540 nm as described above.
The difference of the gene expression of BMSCs cells on the different scaffolds was quantitatively analyzed by real-time RT-PCR. According to the manufacturer's protocol, total RNA was extracted from the cells after culturing for two weeks by TRIzol Reagent (Invitrogen, Carlsbad, CA). The total RNA concentration and purity were detected by Nanodrop Assay (Tecan M200), and the first strand cDNA synthesis was carried out by the reverse transcriptase in accordance with the MMLV manual (Promega, Madison, WI). The expression level of osteogenic markers was quantified by qPCR SYBR Green Mix Kit (Takara, Berkeley, CA). The genes and related specific primer sequences for the target gene were used for qRT-PCR, including osteopontin (OPN), Collagen I (Col-I) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were listed in . The results were analyzed by iCycleriQ Detection System software with GAPDH as the reference gene. All relative results were quantified by the △△Ct relative quantification method.
Table 1. Sequences of primers for quantitative reverse transcription-polymerase chain reaction (qRT-PCR).
In vivo animal study
Establishment of radiated bone model
Totally, 12 male New Zealand white rabbits (20 weeks age, 3.5 kg) were used for in vivo study. According to the previously reported method [Citation25], the right forelimb was X-ray radiated and the contralateral left forelimb was not radiated. Briefly, the right radius, ulna, and the surrounding tissues were radiated by an X-ray source (250 kVP, 16 mA, 5-Gy, MI-201, Shimadzu, Kyoto, Japan). It was performed twice a week for 4 weeks. And the total dose is 40 Gy.
Surgical process and experimental grouping
At the supermedial port of tibia, a 2-mm-diameter hole was drilled to harvest bone marrow. The bone marrow aspirate (500 μL) was then carefully inoculated into the pores formed by phase inversion of PLGA/GMs and PLGA/GMs/BMP-2 scaffolds as homogeneously as possible. Then they were incubated for 1 h to get the scaffolds containing bone marrow. A 20-mm defect of rabbit radius was fabricated based on the previously reported method [Citation26]. Briefly, surrounding periosteum and radius segment was removed, while periosteum in the residual radius and ulna remains intact. The scaffolds including BMP-2-releasing PLGA/GMs scaffold (BMP), BMP-2-releasing PLGA/GMs scaffold with bone marrow (BM/BMP) and BMP-2-free PLGA/GMs scaffolds with (BM) and without (control) bone marrow were implanted into radiated and non-radiated defects, separately. After surgery, all rabbits were injected intramuscularly daily with a dose of 200,000 units of penicillin for 3 d. After 12 weeks post-surgery, all rabbits were euthanized by the overdose administration of anaesthetic agents. The specimens containing the defect as well as radius-ulna were removed from the experimental animals, followed by being fixed in neutral phosphate-buffered formalin solution (10 wt%).
Assessment of bone regeneration
Bone regeneration in vivo was evaluated by X-ray films using Digital Radiograph (DR, Philips, Eindhoven, The Netherlands) and histological assessments at 12 weeks post-surgery. All the X-rays films were scored by the Lane–Sandhu scoring system [Citation27]. Histological sections of bone specimens were fabricated and stained by haematoxylin and eosin (HE) as well as Masson Trichrome for histological assessments by a light microscope (AX-80T, Olympus, Tokyo, Japan).
Statistical analysis
All quantitative data were analyzed by OriginPro 8.0 (Origin Lab Corporation, Northampton, MA) and presented as the mean ± standard deviation. The independent and replicated experiments were used to analyze the statistical variability of the data analyzed using Student’s t-test, and Value of p < .05 was considered to be statistically significant.
Results and discussion
Characterization of GMs, BMP-2-loaded GMs, PLGA scaffold and PLGA/GMs scaffolds
The fabrication of gelatin microspheres has been studied for many years, most of which are used for drug carriers and cell microcarriers. Kinds of methods have been used to fabricate GMs for tissue engineering in recent years such as solvent evaporation, self-assembly method, and two-step desolvation [Citation28]. And the widest method used has been to date water-in-oil emulsion method, as far as production GMs concerned, followed by a cross-linking process. shows the scanning electron micrographs of gelatin microspheres and BMP-2-loaded gelatin microspheres. Irrespective of BMP-2 loading, the similar spherical structure with the smoother surface was observed, with a diameter of 30–150 µm. shows the internal structures of PLGA and PLGA/GMs scaffolds. In the PLGA scaffold, dense micropores in the matrix with the pore size range of 0.2–5 μm by phase inversion and macropores after removing sodium chloride particulates were observed. Gelatin microspheres were homogeneously localized in the PLGA/GMs scaffold, whose matrix was also distributed with dense micropores by phase inversion.
Figure 1. SEM microimages of gelatin microspheres (A, B) and BMP-2-loaded gelatin microspheres (C, D).
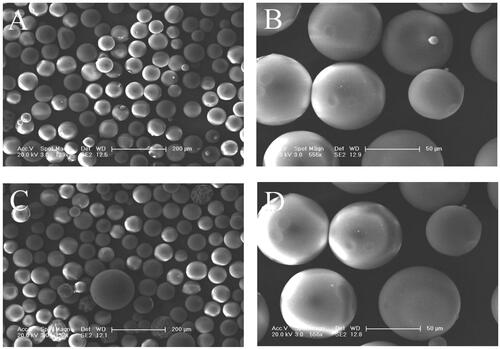
Figure 2. SEM microimages of internal structures for PLGA scaffold (A, B and C) and PLGA/GMs scaffold (D, E and F). The arrows indicate pores after removing sodium chloride particulates, pores by phase inversion and gelatin microspheres.
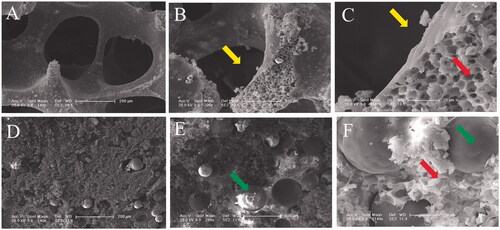
Three-dimensional scaffolds can be fabricated by kinds of techniques, such as solvent casting/particle leaching, melts processing, electrospinning, phase inversion, freeze-drying, foam templating and rapid prototyping [Citation29]. The phase inversion technique, which was first reported in 1995, can be used to fabricate porous scaffolds [Citation30]. It is based on the fact that a homogeneous polymer solution tends to separate into a multiphase system including a polymer-rich phase and a polymer-lean phase after inducing thermal treatment [Citation31]. After removing the solvent, the polymer-rich phase solidifies to become matrix, while the polymer-lean phase forms micropores [Citation32]. In the PLGA/GMs scaffold, the micropores by phase inversion in the matrix were very favourable for the nutrients infiltration [Citation33] and can also carry autologous bone marrow for bone defect repair in vivo in our study below.
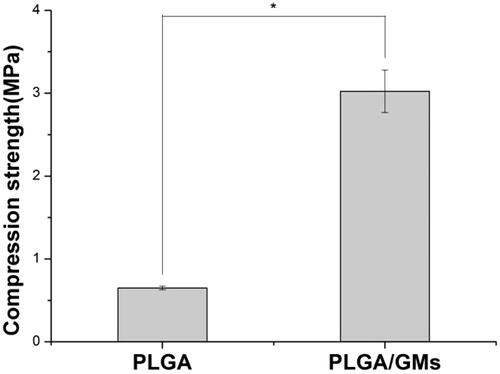
Maintaining the mechanical strength of PLGA scaffold is of great importance for bone tissue engineering. Previous study has confirmed that gelatin can enhance the mechanical strength of polymer materials to a certain degree [Citation34]. However, no research about the use of gelatin microspheres in scaffold fabricated via phase inversion was reported before. shows the compression modules of the PLGA and PLGA/GMs scaffolds fabricated via phase inversion. The compression modules of the PLGA/HA and PLGA/GMs scaffolds are 0.63 MPa and 3.16 MPa, respectively. The PLGA/GMs scaffolds exhibit higher compressive strength, by almost 5.02-fold than that of PLGA scaffolds. These results demonstrate that mixing PLGA matrices with GMs did have shown obvious enhancement on the mechanical strength of the polymer materials, which makes them more suitable for bone tissue regeneration.
Figure 3. Compressive strength of PLGA and PLGA/GMs scaffolds. The data were represented as mean ± standard deviation (SD; n = 3; *p < .05).
Pores and mechanical strength are contradictory in traditional porous scaffolds and often require compromise [Citation35]. The cross-linking gelatin microspheres do not degrade rapidly in the body, and the degradation time can be adjusted by controlling the degree of cross-linking. The undegraded gelatin microspheres in scaffold may provide mechanical support. As the degradation of gelatin microspheres, we expected pores may form gradually, providing space for the surrounding bone tissue growing into the scaffold. The advantages of gelatin microspheres in osteogenic differentiation have been investigated in recent years [Citation36]. Owing to the degradation of gelatin, these particles increase the pore size of the scaffold, which improved the cellular infiltration into the internal space [Citation37]. In our future work, the pore formation in the PLGA/GMs scaffold resulted from degradation of gelatin microspheres will be further investigated with.
BMP-2 release profiles of PLGA scaffold and PLGA/GMs scaffolds
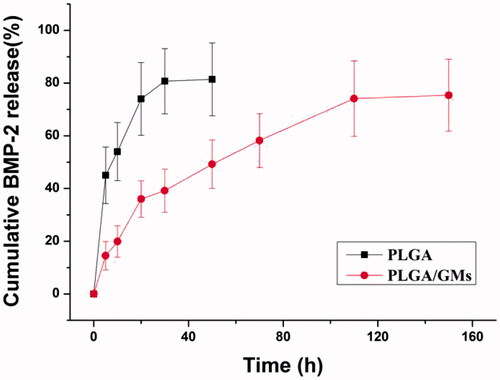
As shown in , without GMs encapsulated, BMP-2 was released at a high burst rate and the cumulative released BMP-2 exceeded 80% within the first 24 h. While in the PLGA/GMs, a sustained release characteristic of BMP-2 was noted. It showed a slight initial burst release within 24 h, but a slower release over 7 d was followed by, which was consistent with previous studies [Citation38]. Without GMs encapsulated, BMP-2 attached on the surface of pores in the PLGA scaffold and when it touched PBS, the BMP-2 can be released at a high burst rate from the pores. In the PLGA/GMs, a slight initial burst release was resulted from the released BMP-2 attaching on the pores and then as the degradation of GMs, the BMP-2 in the GMs was released gradually. In recent years, gelatin has been widely applied for protein delivery [Citation39]. Employing gelatin to separately encapsulate small-molecule drugs and proteins can lead to their efficient release in a controlled way [Citation36]. Yamamoto et al. [Citation22] reported that, within 40 min, BMP-2 from glutaraldehyde-cross-linked gelatin microspheres was initial burst released, followed by a sustained release. Clearly, the efficacy of BMP-2 in bone tissue depends on the administered dose and the mode of administration. Sustained release has been shown to be sufficient to induce bone regeneration [Citation40].
Cell proliferation and differentiation in PLGA, PLGA/GMs and PLGA/GMs/BMP-2 scaffolds
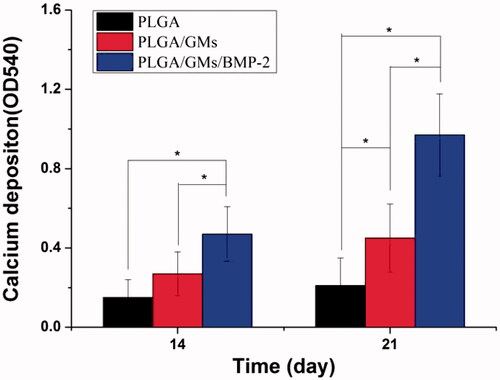
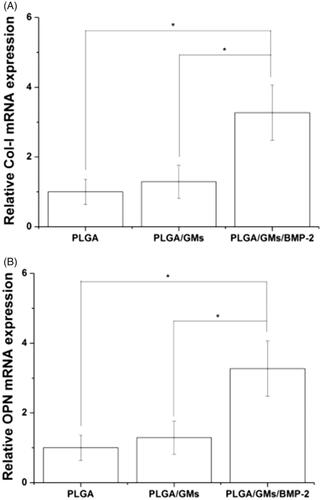
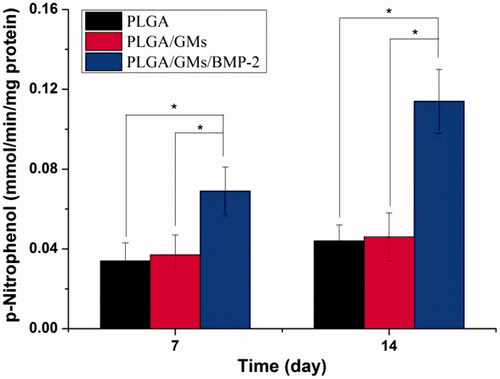
To evaluate the metabolic activity of the BMSCs at days 1, 3, and 7, the proliferation was tested quantitatively by the MTT assay. As shown in , within 7 d, each scaffold displayed the proliferation of BMSCs at different levels. The PLGA exhibited the lowest cellular activity, while the PLGA/GMs/BMP-2 showed the highest proliferation at days 3 and 7, suggesting that the gelatin and BMP-2 encapsulated promoted the cell proliferation. BMP-2 has been confirmed to enhance bone regeneration by inducing osteoblastic and chondrogenic differentiation of BMSCs [Citation41], promote the expression of integrin β1, which is needed for improving cell adhesion, spreading and proliferation [Citation42]. Moreover, since the gelatin molecule has cell recognition sites, which is favourable of cell adhesion and growth [Citation17], the PLGA/GMs group exhibited higher cellular activity than the PLGA group. Interactions between cells and biomaterials have been shown to have an important impact on the differentiation of BMSCs [Citation43]. And to assess the osteogenic differentiation of BMSCs, ALP activity and calcium deposition were tested. The high reactivity of ALP was usually considered to be a marker of osteogenic differentiation of the cells. As shown in , the intracellular ALP activity from cells cultured after 7 and 14 d on the PLGA/GMs/BMP-2 scaffold was significantly higher than that on the PLGA and PLGA/GMs scaffolds. By alizarin red staining, calcium deposition was tested. As shows, the deposition amount of calcium in the PLGA/GMs/BMP-2 was markedly greater than that in the other ones. In addition, the deposition amount of calcium in the PLGA/GMs was higher than in the PLGA at 21 d. And these indicated that the PLGA/GMs and PLGA/GMs/BMP-2 promoted the osteogenic differentiation of BMSCs. Differentiation is accompanied by a series of intracellular regulatory gene expression. For instance, OPN is a middle stage differentiation marker of differentiation. Col-I is one of the major components of the natural bone extracellular matrix (ECM), so Col-I gene expression indicates ECM secretion from BMSCs [Citation43]. shows that the expression levels of COL-I and OPN were greater in PLGA/GMs/BMP-2 than those in PLGA and PLGA/GMs on day 7. This result indicated that the PLGA/GMs/BMP-2 scaffold can enhance the osteogenic differentiation of BMSCs, which was ascribed to the gelatin bioactivity as well as the BMP-2. All the above data demonstrated that the bioactivity of BMP-2 was well retained during the encapsulated process, and the sustained release of BMP-2 can improve the differentiation of BMSCs. These findings indicate that the PLGA/GMs/BMP-2 scaffold can function as a cell scaffold for BMSCs and as a release carrier for BMP-2.
Figure 5. Cell proliferation on PLGA, PLGA/GMs and PLGA/GMs/BMP-2 scaffolds on days 1, 3 and 7. The data were represented as mean ± standard deviation (SD; n = 3; *p < .05).
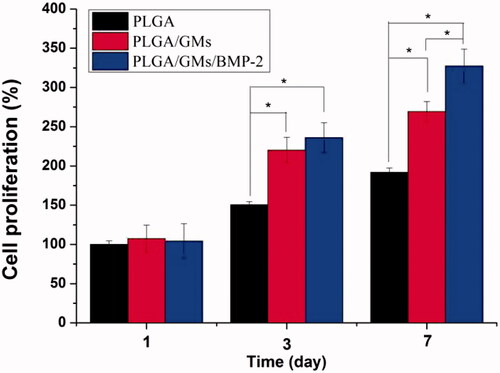
Figure 6. ALP activities of BMSCs in PLGA, PLGA/GMs and PLGA/GMs/BMP-2 scaffolds during 14-d in vitro culture. The data were represented as mean ± standard deviation (SD; n = 3; *p < .05).
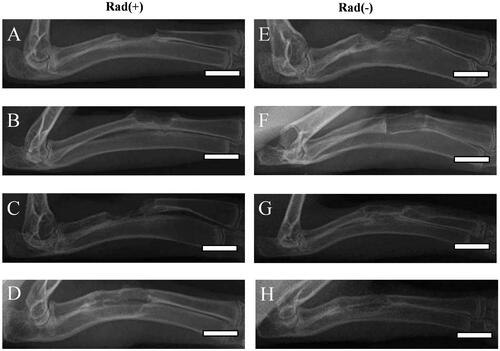
DR measurement and Lane–Sandhu X-ray scores of radius defect repair
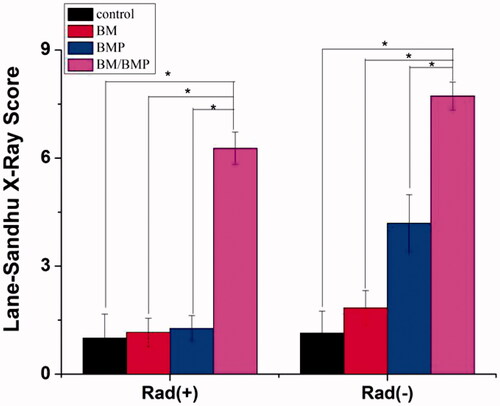
shows the DR images of rabbit radius defects with [Rad(+)] or without [Rad(−)] X-ray radiation at 12 weeks post-surgery after using different kinds of PLGA/GMs scaffolds. Regardless of X-ray radiation, in the BM/BMP group, new bone formation and bone bridge from the proximal to the distal host tissue were observed (). The BM and BMP groups displayed new bone formation and bone bridge in the entire area of the radius defect without X-ray radiation (), while the bone regeneration was inhibited by X-ray radiation (). In contrast, regardless of X-ray radiation, no new bone formation was detected in the control group (). To evaluate quantificationally the bone formation, osseous bridging and bone shaping, all the DR images were scored by the Lane–Sandhu X-ray scoring system [Citation27]. At 12 weeks post-surgery, the Lane–Sandhu X-ray scores () revealed that the control, BM, BMP with X-ray radiation groups and the control without X-ray radiation group had the lowest score and the BM/BMP with or without X-ray radiation groups had the highest one.
Figure 9. DR images of longitudinal sections of rabbit radius defects with (A–D) and without (E–H) X-ray irradiation 12 weeks after the application of empty PLGA/GMs scaffolds (control) (A and E), PLGA/GMs scaffolds containing bone marrow (BM) (B and F), BMP-2-releasing PLGA/GMs scaffolds (BMP) (C and G), and BMP-2-releasing PLGA/GMs scaffolds with bone marrow (BM/BMP) (D and H). Scale bars correspond to the length of 20 mm.
Histological evaluation of radius defect repair
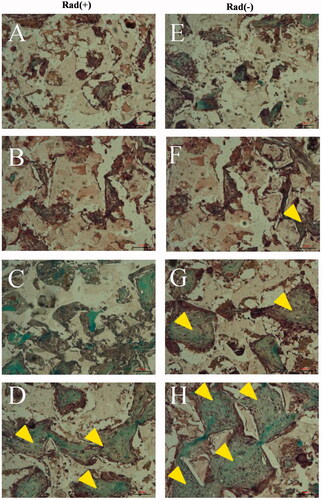
and show the histological results at 12 weeks post-surgery. The BM/BMP group showed the new bone formation at the radius defect and bone bridge between proximal and distal host bone tissue, irrespective of X-ray irradiation ( and ). Especially, the BM/BMP group exhibited cortex-like bone formation. In both BM and BMP groups without X-ray radiation, the entire area of the radius defect was detected infiltrated with new bone tissue ( and ). Whereas, the radius defect was infiltrated with soft connective tissues after X-ray radiation (), and bone bridge between the proximal and distal host bone tissue was not detected (). In contrast, there was no bone regeneration in the PLGA/GMs (control) and the defect was infiltrated with soft connective tissues (), regardless of X-ray radiation (). Moreover, the scaffold does not retain its original structure, while the residue of the scaffold is detected to be present in the bone tissues or fibrous tissues.
Figure 11. Histological evaluation with haematoxylin and eosin staining. Histological sections of rabbit radius defects with (A–D) and without (E–H) X-ray irradiation 12 weeks after the application of control (A and E), BM (B and F), BMP (C and G) and BM/BMP (D and H). The yellow triangle indicates the new bone formation. Scale bars are 100 μm.
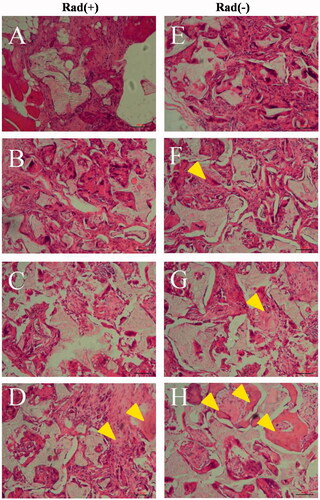
Figure 12. Histological evaluation with Masson Trichrome staining. Histological sections of rabbit radius defects with (A–D) and without (E–H) X-ray irradiation 12 weeks after the application of control (A and E), BM (B and F), BMP (C and G) and BM/BMP (D and H). The yellow triangle indicates the new bone formation. Scale bars are 100 μm.
The function of BMP-2 for osteogenesis and promoting osteoblastic differentiation during bone repair processes are well known [Citation44]. Sustained release of BMP-2 from the scaffolds is critical to guarantee the permanence of the substances in the defect and, as a result, the maintenance of therapeutic efficacy might be achieved. In the previous study, the pluronic hydrogel was able to sustain BMP-2 release pre-encapsulated in microspheres of PLGA [Citation45]. Although it might avoid the excessive burst release of BMP-2 in this biodegradable delivery system, gelatin microspheres in our study could degrade earlier than PLGA microspheres, and porosity created by degradation of gelatin microspheres in the PLGA matrix would be useful for bone healing [Citation38]. By surface binding technology such as polydopamine-assisted immobilization of BMP-2, some researchers found it can effectively enhance BMP-2 binding on the surface of the PLGA scaffold, and the BMP-2 can be slowly released over three weeks in vitro [Citation14]. However, poor biocompatibility in vitro and insufficient release time in vivo were observed, which was not sufficient to achieve complete defect filling [Citation46]. In our research, different kinds of PLGA/GMs scaffolds were applied to induce osteogenesis. The DR and histological results showed no bone regeneration in the empty PLGA/GMs (control), which demonstrated that the PLGA/GMs scaffold itself had the low ability for inducing osteogenesis. The capacity of the BMP-2-releasing PLGA/GMs scaffold (BMP group) to induce osteogenesis was inhibited to some extent by X-ray radiation (). The histological assessment demonstrated that the radius defect was partly infiltrated with soft connective tissue (). This result can be explained by poor vascularity and cellularity of X-ray radiated bone tissue. The degeneration of stem cells and progenitor cells in bone marrow, osteoblasts in bone and endothelial cells in blood vessel walls resulted in the failure of osteogenesis [Citation48]. First, X-ray radiation can reduce the number of osteoblasts [Citation49]. Next, X-ray treatment can inhibit osteoblast differentiation as well as osteogenic activity [Citation50]. Third, it has been reported that after X-ray radiation, progenitor cells exposed to radiation area cannot respond to BMP signals normally [Citation51]. Furthermore, angiogenesis can be suppressed by X-ray radiation, leading to impaired osteogenesis [Citation52]. For the BM/BMP group in the X-ray radiated radius defect (), the obvious bone regeneration indicated that the osteogenesis in the radiated area was benefited from the bone marrow, which was consistent with previous study [Citation31]. It is possible that the bone marrow proliferated and differentiated to osteogenic lineages, leading to enhanced osteogenesis (). Owing to MSC and hematopoietic stem cells in bone marrow, bone formation and vascularization can be promoted [Citation53]. Moreover, due to the release of BMP-2, cellular activity was also enhanced. Thus, the combination of bone marrow with BMP-2 is necessary for osteogenesis at X-ray radiated defects, where growth factors and host cells are damaged. Indeed, we lack direct experimental data for explaining the relative functions of transplanted bone marrow in the defect. However, based on these results, it is highly imaginable that bone marrow can improve the microenvironment of bone defects by providing osteoblasts and angiogenesis to induce bone regeneration. For understanding the direct function of bone marrow cells in osteogenesis combined with BMP-2, cell tracking, such as BMP-2-induced green fluorescent protein (GFP)-labelled bone marrow cells [Citation54] may be used in our further research. Taken together, by combining autologous bone marrow with BMP-2-releasing GMs, it is possible that the bone regeneration could be achieved even in the radiated tissue, while its specific mechanism should be investigated in our further research.
Conclusions
In the present study, after incorporating gelatin microspheres, PLGA scaffold demonstrated improved mechanical properties. Furthermore, in BMP-2-releasing PLGA/GMs scaffold, a sustained release property of BMP-2 was detected. It also enhanced the proliferation and osteogenesis of BMSCs in vitro, indicating the bioactivity of BMP-2. For the bone repairment experiment in vivo, in the X-ray radiated groups, the bone regeneration was inhibited either with bone marrow or BMP-2. However, when combined with bone marrow, the BMP-2 showed significantly high osteogenic effect, irrespective of X-ray radiation. Taken together, it is a promising way to repair bone defects after X-ray radiation by a combination of bone marrow with the BMP-2-releasing PLGA/GMs scaffold.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Marx RE. Osteoradionecrosis: a new concept of its pathophysiology. J Oral Maxillofac Surg. 1983;41:283–288.
- Valentin-Opran A, Wozney J, Csimma C, et al. Clinical evaluation of recombinant human bone morphogenetic protein-2. Clin Orthop Relat R. 2002;395:110–120.
- Bak M, Jacobson AS, Buchbinder D, et al. Contemporary reconstruction of the mandible. Oral Oncol. 2010;46:71–76.
- Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926.
- Howard BK, Brown KR, Leach JL, et al. Osteoinduction using bone morphogenic protein in irradiated tissue. Arch Otolaryngol Head Neck Surg. 1998;124:985–988.
- Wurzler KK, DeWeese TL, Sebald W, et al. Radiation-induced impairment of bone healing can be overcome by recombinant human bone morphogenetic protein-2. J Craniofac Surg. 1998;9:131–137.
- Gentile P, Chiono V, Carmagnola I, et al. An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int J Mol Sci. 2014;15:3640–3659.
- Wang NX, Zheng WF, Cheng SY, et al. In vitro evaluation of essential mechanical properties and cell behaviors of a novel polylactic-co-glycolic acid (PLGA)-based tubular scaffold for small-diameter vascular tissue engineering. Polym-Basel. 2017;9:318.
- Pan Z, Ding JD. Poly(lactide-co-glycolide) porous scaffolds for tissue engineering and regenerative medicine. Interface Focus. 2012;2:366–377.
- Shim J-H, Moon T-S, Yun M-J, et al. Stimulation of healing within a rabbit calvarial defect by a Pcl/Plga scaffold blended with Tcp using solid freeform fabrication technology. J Mater Sci: Mater Med. 2012;23:2993–3002.
- Damia C, Marchat D, Lemoine C, et al. Functionalization of phosphocalcic bioceramics for bone repair applications. Mater Sci Eng C-Mater Biol Appl. 2019;95:343–354.
- Renno ACM, van de Watering FCJ, Nejadnik MR, et al. Incorporation of bioactive glass in calcium phosphate cement: an evaluation. Acta Biomater. 2013;9:5728–5739.
- Brown ME, Zou Y, Peyyala R, et al. Testing of a bioactive, moldable bone graft substitute in an infected, critically sized segmental defect model. J Biomed Mater Res. 2018;106:1878–1886.
- Zhang J, Li J, Jia G, et al. Improving osteogenesis of Plga/Ha porous scaffolds based on dual delivery of Bmp-2 and Igf-1 via a polydopamine coating. RSC Adv. 2017;7:56732–56742.
- An G, Zhang WB, Ma DK, et al. Influence of VEGF/BMP-2 on the proliferation and osteogenetic differentiation of rat bone mesenchymal stem cells on PLGA/gelatin composite scaffold. Eur Rev Med Pharmacol. 2017;21:2316–2328.
- Zekorn D. Intravascular retention, dispersal, excretion and break-down of gelatin plasma substitutes. Bibl Haematol. 1969;33:131.
- Echave MC, Sanchez P, Pedraz JL, et al. Progress of gelatin-based 3D approaches for bone regeneration. J Drug Deliv Sci Technol. 2017;42:63–74.
- Zheng JF, Zhao FJ, Zhang W, et al. Sequentially-crosslinked biomimetic bioactive glass/gelatin methacryloyl composites hydrogels for bone regeneration. Mater Sci Eng C-Mater. 2018;89:119–127.
- Petite H, Viateau V, Bensaid W, et al. Tissue-engineered bone regeneration. Nat Biotechnol. 2000;18:959–963.
- Lieberman JR, Daluiski A, Einhorn TA. The role of growth factors in the repair of bone – biology and clinical applications. J Bone Joint Surg-Am. 2002;84A:1032–1044.
- Kawakatsu N, Oda S, Kinoshita A, et al. Effect of rhBMP-2 with PLGA/gelatin sponge type (PGS) carrier on alveolar ridge augmentation in dogs. J Oral Rehabil. 2008;35:647–655.
- Takahashi Y, Yamamoto M, Tabata Y. Enhanced osteoinduction by controlled release of bone morphogenetic protein-2 from biodegradable sponge composed of gelatin and beta-tricalcium phosphate. Biomaterials. 2005;26:4856–4865.
- Kawai K, Suzuki S, Tabata Y, et al. Accelerated tissue regeneration through incorporation of basic fibroblast growth factor-impregnated gelatin microspheres into artificial dermis. Biomaterials. 2000;21:489–499.
- Young TH, Chen LW. Pore formation mechanism of membranes from phase inversion process. Desalination. 1995;103:233–247.
- Yamamoto M, Hokugo A, Takahashi Y, et al. Combination of BMP-2-releasing gelatin/beta-TCP sponges with autologous bone marrow for bone regeneration of X-ray-irradiated rabbit ulnar defects. Biomaterials. 2015;56:18–25.
- Muschler GF, Raut VP, Patterson TE, et al. The design and use of animal models for translational research in bone tissue engineering and regenerative medicine. Tissue Eng Part B-Rev. 2010;16:123–145.
- Lane JM, Sandhu HS. Current approaches to experimental bone grafting. Orthop Clin North Am. 1987;18:213–225.
- Sahoo N, Sahoo RK, Biswas N, et al. Recent advancement of gelatin nanoparticles in drug and vaccine delivery. Int J Biol Macromol. 2015;81:317–331.
- Mano JF, Silva GA, Azevedo HS, et al. Natural origin biodegradable systems in tissue engineering and regenerative medicine: present status and some moving trends. J R Soc Interface. 2007;4:999–1030.
- Yang YF, Zhao J, Zhao YH, et al. Formation of porous PLGA scaffolds by a combining method of thermally induced phase separation and porogen leaching. J Appl Polym Sci. 2008;109:1232–1241.
- Whang K, Thomas CH, Healy KE, et al. A novel method to fabricate bioabsorbable scaffolds. Polymer. 1995;36:837–842.
- Wei GB, Ma PX. Nanostructured biomaterials for regeneration. Adv Funct Mater. 2008;18:3568–3582.
- Zhang N, Wang Y, Xu WP, et al. Poly(lactide-co-glycolide)/hydroxyapatite porous scaffold with microchannels for bone regeneration. Polym-Basel. 2016;8:216.
- Wang J, Li DS, Li TY, et al. Gelatin tight-coated poly(lactide-co-glycolide) scaffold incorporating rhBMP-2 for bone tissue engineering. Materials. 2015;8:1009–1026.
- Schloegl W, Marschall V, Witting MY, et al. Porosity and mechanically optimized PLGA based in situ hardening systems. Eur J Pharm Biopharm. 2012;82:554–562.
- Binulal NS, Natarajan A, Menon D, et al. Gelatin nanoparticles loaded poly(epsilon-caprolactone) nanofibrous semi-synthetic scaffolds for bone tissue engineering. Biomed Mater. 2012;7:065001.
- Nguyen AH, Wang Y, White DE, et al. MMP-mediated mesenchymal morphogenesis of pluripotent stem cell aggregates stimulated by gelatin methacrylate microparticle incorporation. Biomaterials. 2016;76:66–75.
- Tang GW, Zhang H, Zhao YH, et al. Prolonged release from PLGA/HAp scaffolds containing drug-loaded PLGA/gelatin composite microspheres. J Mater Sci: Mater Med. 2012;23:419–429.
- Jaipan P, Nguyen A, Narayan RJ. Gelatin-based hydrogels for biomedical applications. MRC. 2017;7:416–426.
- Haidar ZS, Hamdy RC, Tabrizian M. Delivery of recombinant bone morphogenetic proteins for bone regeneration and repair. Part B: delivery systems for BMPs in orthopaedic and craniofacial tissue engineering. Biotechnol Lett. 2009;31:1825–1835.
- Urist MR. Bone: formation by autoinduction. Science. 1965;150:893.
- Mckay WF, Peckham SM, Badura JM. A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE (R) Bone Graft). Int Orthop (Sico). 2007;31:729–734.
- Hanson S, D'Souza RN, Hematti P. Biomaterial-mesenchymal stem cell constructs for immunomodulation in composite tissue engineering. Tissue Eng Part A. 2014;20:2162–2168.
- Kim K, Park J, Kim S. Bone morphogenetic protein-2 associated multiple growth factor delivery for bone tissue regeneration. J Pharm Investig. 2018;48:187–197.
- Reyes R, Antonio Rodriguez J, Orbe J, et al. Combined sustained release of Bmp2 and Mmp10 accelerates bone formation and mineralization of Calvaria critical size defect in mice. Drug Deliv. 2018;25:750–756.
- Kirby GTS, White LJ, Steck R, et al. Microparticles for sustained growth factor delivery in the regeneration of critically-sized segmental tibial bone defects. Materials. 2016;9:E259.
- Al-Jarsha M, Moulisova V, Leal-Egana A, et al. Engineered coatings for titanium implants to present ultralow doses of BMP-7. ACS Biomater Sci Eng. 2018;4:1812–1819.
- Jegoux F, Malard O, Goyenvalle E, et al. Radiation effects on bone healing and reconstruction: interpretation of the literature. Oral Surg Oral Med O. 2010;109:173–184.
- Dudziak ME, Saadeh PB, Mehrara BJ, et al. The effects of ionizing radiation on osteoblast-like cells in vitro. Plast Reconstr Surg. 2000;106:1049–1061.
- Matsumura S, Jikko A, Hiranuma H, et al. Effect of X-ray irradiation on proliferation and differentiation of osteoblast. Calcif Tissue Int. 1996;59:307–308.
- Arnold M, Stas P, Kummermehr J, et al. Radiation-induced impairment of bone healing in the rat femur: effects of radiation dose, sequence and interval between surgery and irradiation. Radiother Oncol. 1998;48:259–265.
- Gerber HP, Vu TH, Ryan AM, et al. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;5:623–628.
- Espitalier F, Vinatier C, Lerouxel E, et al. A comparison between bone reconstruction following the use of mesenchymal stem cells and total bone marrow in association with calcium phosphate scaffold in irradiated bone. Biomaterials. 2009;30:763–769.
- Ratanavaraporn J, Furuya H, Kohara H, et al. Synergistic effects of the dual release of stromal cell-derived factor-1 and bone morphogenetic protein-2 from hydrogels on bone regeneration. Biomaterials. 2011;32:2797–2811.