Abstract
Osteoarthritis (OA) is a common degenerative joint disease worldwide. Long non-coding RNAs (lncRNAs) have been widely confirmed to involve in the modulation of OA progression. However, the underlying mechanisms of lncRNA FOXD2-AS1 in OA remain unclear. In the present study, we showed FOXD2-AS1 expression was upregulated and positively associated with the severity of OA patients. IL-1β and/or TNF-α treatment could increase FOXD2-AS1 expression in chondrocytes. FOXD2-AS1 overexpression induced cell proliferation, inflammation and extracellular matrix (ECM) degradation in chondrocytes. Mechanistically, we found that FOXD2-AS1 upregulated the expression level of TLR4 by sponging miR-27a-3p. In addition, we revealed that miR-27a-3p mimics could abolish the effects of FOXD2-AS1 overexpression on cell proliferation, inflammation, and ECM degradation in chondrocytes. Therefore, we demonstrated that FOXD2-AS1 could play a crucial role in the progression of OA, at least partially, by regulating miR-27a-3p/TLR4 axis.
Keywords:
Introduction
Osteoarthritis (OA) is a degenerative joint disease, which is characterized by progressive degenerative alterations in the articular cartilage and other joint tissues [Citation1]. Increasing evidence showed that articular chondrocyte hypertrophy and apoptosis appear to participate in initiation and development of OA [Citation2,Citation3]. Although current treatments could light the pain and inflammation, they cannot modify the disease progression [Citation4]. Therefore, it is important to elucidate the underlying molecular mechanisms in OA progression.
Long non-coding RNAs (lncRNAs) are longer than 200 nucleotides non-protein coding RNA, which modulate gene expression at the level of post-transcriptional and chromatin modification [Citation5,Citation6]. Recently, increasing evidence showed that lncRNAs could play critical roles in a variety of biology functions, such as proliferation, invasion, differentiation and metabolize [Citation7,Citation8]. Growing studies revealed that lncRNAs could play critical roles in OA progression. For example, Tang and coworkers showed that lncRNA ROR regulated cell apoptosis and autophagy in chondrocytes [Citation9]. Zhu et al found that lncRNA FAS-AS1 promoted the degradation of extracellular matrix (ECM) of cartilage in OA [Citation10]. Fan et al. suggested that lncRNA DANCR functioned as a competing endogenous RNA to regulate OA progression via miR-577/SphK2 axis [Citation11]. However, the roles and underlying mechanisms of lncRNAs in OA remain unclear.
In the present study, we revealed that FOXD2-AS1 expression was increased in OA samples, IL-1β and TNF-α treatment upregulated FOXD2-AS1 expression in chondrocytes. Moreover, the results indicated that lncRNA FOXD2-AS1 could induce cell proliferation, inflammation and ECM degradation by regulating miR-27a-3p/TLR4 axis.
Materials and methods
Sample collection
The degenerated cartilage samples from the OA patients and normal cartilage samples from the non-OA cases (patients with the femoral neck fracture with no history of rheumatoid arthritis or OA) (NC) were collected from Shanghai General Hospital, Shanghai Jiaotong University. These cartilage samples were immediately snap-frozen and stored in the liquid nitrogen until protein or RNA extraction. The present study was approved by the Clinical Research Ethics of Shanghai Jiaotong University.
Cell cultures and transfection
Human cartilage C28/I2 cells were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). The cells were grown in RPMI-1640 medium containing 10% fetal bovine serum (Gibco) at 37 °C in a humidified atmosphere containing 5% CO2. FOXD2-AS1 vector and control vector, miR-27a-3p mimics and miR-NC were constructed from Genechem (Shanghai, China) and were transfected into cells by Lipofectamine2000 (Invitrogen, Carlsbad, CA, USA) according to manufacturer’s instructions.
RNA extraction and qRT-PCR
Total RNA was extracted from tissue samples using TRIzol reagents (Invitrogen). RNA was reversely transcribed into cDNAs by using a Reverse Transcription Kit of the Prime-ScriptTM one step (Takara, Dalian, China). Quantitative reverse transcriptase polymerase chain reactions (qRT-PCR) were performed using the ABI7500 System (Applied Biosystems, CA, USA) and the SYBR Green PCR Master Mix kit (TaKaRa) according to the supplied protocol of the manufacturer’s instructions. Each experiment was repeated in triplicate at least. GAPDH was used as the internal control. Fold change of was calculated by 2–△△Ct methods.
Western blot
Total protein in cells was extracted by RIPA (Beyotime, Shanghai) reagent supplemented with a protease inhibitor cocktail (Roche, Basel, Switzerland) and PMSF (Roche). Protein was then transferred to PVDF membrane with 10% SDS-PAGE. After blocked with 5% skimmed milk for 1 h, primary antibodies (Abcam, Cambridge, MA, USA) were added and incubated at 4 °C overnight. In the following day, membrane was washed with TBST and incubated with HRP-labeled secondary antibodies for 1 h. Signals visualization was conducted by ECL Substrates (Millipore, MA, USA) using GAPDH as an endogenous protein for normalization.
Cell proliferation assay
Cell Counting Kit-8 (CCK-8, Dojindo, Japan) kit was used to detect cell viability. Briefly, 1 × 104 cells were seeded into 96-well plates. After incubation at the indicated time points, 10 μl of CCK-8 solution was added to each well and then incubated at 37 °C for 90 min. Then the absorbance at 450 nm was measured using a spectrophotometer (Thermo, Waltham, MA, USA).
Luciferase reporter assay
The fragment of FOXD2-AS1 3′-UTR or TLR4 was amplified by PCR and cloned into the downstream of the Renilla psiCHECK2 vector (Promega, Madison, WI, USA), named FOXD2-AS1-Wt 3′-UTR or TLR4-Wt. Cells were co-transfected with the indicated vectors and miR-27a-3p mimics, respectively. Luciferase assays were performed 48 h after transfection using the Dual Luciferase Reporter Assay System (Promega).
RNA immunoprecipitation assay
RNA immunoprecipitation was performed using Magna RIP RNA-Binding Protein Immunoprecipitation Kit (Millipore) with AGO2 antibody according to manufacturer’s instructions. RNA for in vitro experiments was transcribed using T7 High YieldRNA Synthesis Kit according to manufacturer’s instructions. IgG, FOXD2-AS1 and miR-27a-3p levels in the immunoprecipitates were measured by qRT-PCR.
Statistical analysis
Data are processed using SPSS17.0 statistical software and presented as mean ± standard deviation of results from at least three independent experiments. A Student t test or One-way analysis of variance was carried out to estimate the significant group differences. p < .05 was considered as statistically significant.
Results
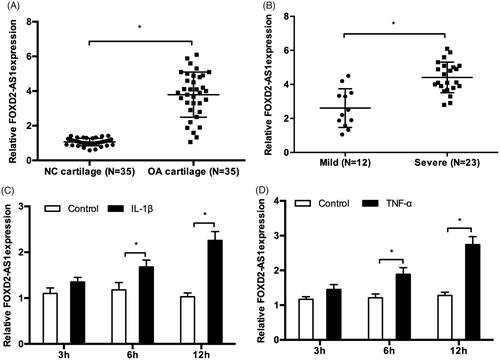
FOXD2-AS1 expression was increased in OA patients
In the present study, we explored FOXD2-AS1 expression in OA tissues by qRT-PCR. The results showed that FOXD2-AS1 expression was significantly upregulated and positively associated with the severity of OA patients (). Next, to verify whether inflammation could promote cartilage damage by inducing FOXD2-AS1 expression. We determined effects of IL-1β and TNF-α on FOXD2-AS1 expression in chondrocytes. QRT-PCR results showed that IL-1β or TNF-α treatment could markedly increase FOXD2-AS1 expression in C28/I2 cells (). Those data indicated that FOXD2-AS1 might play critical roles in OA progression.
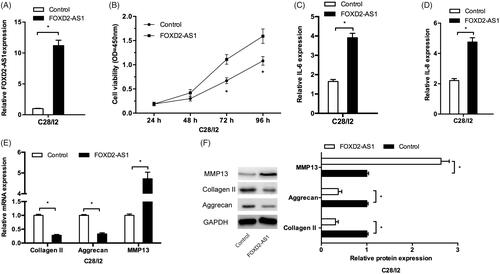
FOXD2-AS1 induced cell proliferation, inflammation and ECM degradation in chondrocytes
Next, we explored the functions of FOXD2-AS1 in OA processes. We firstly upregulated FOXD2-AS1 expression in C28/I2 cells (). CCK-8 assay showed that FOXD2-AS1 overexpression significantly promoted the viability of C28/I2 cells (). Consistently, the results showed that FOXD2-AS1 overexpression induced inflammatory factors (IL-6, IL-8) expression levels in C28/I2 cells (). Moreover, FOXD2-AS1 upregulation significantly reduced collagen II and aggrecan expression, while increased MMP13 expression in C28/I2 cells both in mRNA and protein levels (). Those data indicated that FOXD2-AS1 might induce the cell proliferation, inflammation, and ECM degradation in chondrocytes.
Figure 2. FOXD2-AS1 promoted cell proliferation, inflammation and ECM degradation in chondrocytes. (A) FOXD2-AS1 expression in C28/I2 cells transfected with FOXD2-AS1 overexpression plasmids. (B) FOXD2-AS1 overexpression promoted C28/I2 cells viability in vitro. (C, D) FOXD2-AS1 overexpression induced inflammatory factors expression (IL-6, IL-8) in C28/I2 cells. (E, F) FOXD2-AS1 upregulation reduced the expression of collage II, and aggrecan, while increased the expression of MMP13 in C28/I2 cells both in mRNA and protein levels. *p < .05.
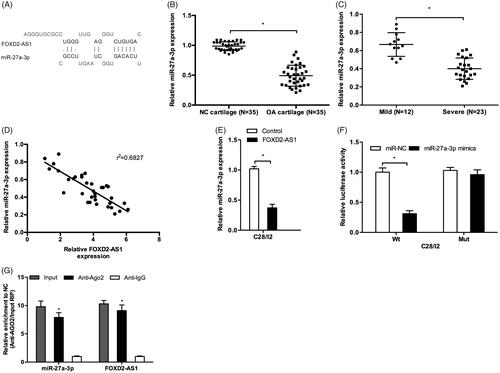
FOXD2-AS1 interacted with miR-27a-3p in OA
LncRNA could play their biological effects by serving as competing endogenous RNAs (ceRNAs) to inhibit miRNA expression, therefore rescuing the expression of miRNA downstream targets [Citation12,Citation13]. In the present study, bioinformatics analysis revealed that miR-27a-3p harbored FOXD2-AS1 with the complementary binding at 3′-UTR (). miR-27a-3p expression was significantly decreased in OA tissues compared to NC tissues (). Low miR-27a-3p expression was associated with the severity and negatively correlated with FOXD2-AS1 expression in OA tissues (). Ectopic expression of FOXD2-AS1 decreased miR-27a-3p expression in C28/I2 cells (). Luciferase reporter assay showed that miR-27a-3p mimics significantly reduced the luciferase activity of FOXD2-AS1-Wt group (). Moreover, RIP assay showed that FOXD2-AS1 and miR-27a-3p expression were both enriched in Ago2 pellet compared to Anti-IgG (). These data suggested that FOXD2-AS1 could negatively regulate miR-27a-3p expression in OA.
Figure 3. MiR-27a-3p served as a target of FOXD2-AS1. (A) The binding site between FOXD2-AS1 and miR-27a-3p. (B, C) MiR-27a-3p expression was decreased and negatively associated with the severity of OA patients. (D) MiR-27a-3p expression was negatively correlated with FOXD2-AS1 expression in OA tissues. (E) FOXD2-AS1 overexpression reduced miR-27a-3p expression in C28/I2 cells. (F) Luciferase reporter assay showed that miR-27a-3p mimics reduced the luciferase activity of FOXD2-AS1-Wt group. (G) RIP assay showed that FOXD2-AS 1 and miR-27a-3p expression was enriched in Ago2 pellet. *p < .05.
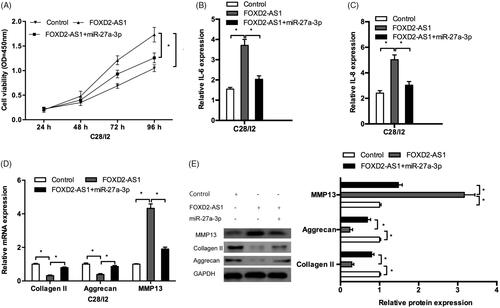
FOXD2-AS1 induced cell proliferation, inflammation and ECM degradation in chondrocytes via regulating miR-27a-3p
CCK-8 assay showed that FOXD2-AS1 overexpression promoted cell proliferation of C28/I2 cells, while miR-27a-3p mimics could abolish the effects of FOXD2-AS1 overexpression (). QRT-PCR results indicated that miR-27a-3p mimics decreased the inflammatory factors (IL-6, IL-8) levels in C28/I2 cells transfected with FOXD2-AS1 (). In addition, the results showed that miR-27a-3p mimics could promote the expression of collagen II, aggrecan and reduce the expression of MMP13 in FOXD2-AS1 overexpressed C28/I2 cells both in mRNA and protein levels (). Those data indicated that FOXD2-AS1 could function as an endogenous sponge for miR-27a-3p.
Figure 4. FOXD2-AS1 promoted OA progression via regulating miR-27a-3p. (A) FOXD2-AS1 upregulation promoted C28/I2 cells proliferation, while miR-27a-3p mimics abolished the effects. (B, C) miR-27a-3p mimics reduced inflammatory factors (IL-1β, IL-6) levels in C28/I2 cells transfected with FOXD2-AS1. (D, E) miR-27a-3p mimics promoted collagen II, aggrecan expression and reduced MMP13 expression in C28/I2 cells transfected with FOXD2-AS1 both in mRNA (D) and protein (E) levels. *p < .05.
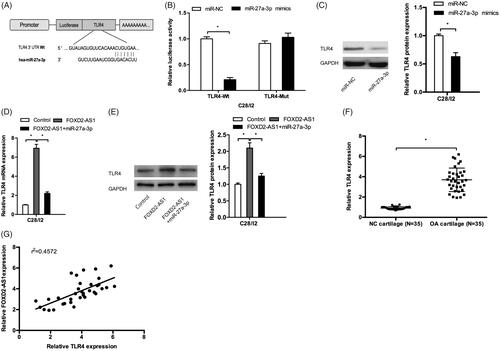
FOXD2-AS1/miR-27a-3p/TLR4 axis mediated the OA progression
MiRNAs could exert their functions by directly targeting their downstream genes [Citation14]. In the present study, TLR4 was found to possess a binding sequence with miR-27a-3p (). Luciferase reporter assay showed that miR-27a-3p mimics significantly reduced the luciferase activity of TLR4-Wt vectors (). Western blot showed that miR-27a-3p mimics decreased TLR4 protein levels in C28/I2 cells (). Moreover, we explored the effects of FOXD2-AS1 on TLR4 expression, the results showed that FOXD2-AS1 overexpression increased TLR4 expression both in mRNA and protein levels in C28/I2 cells, which could be abolished by miR-27a-3p mimics (). In addition, TLR4 mRNA expression was significantly increased and positively correlated with FOXD2-AS1 expression in OA tissues (). Taken together, these results indicated that FOXD2-AS1 could promote OA progression by modulating the miR-27a-3p/TLR4 axis.
Figure 5. FOXD2-AS1/miR-27a-3p/TLR4 axis mediated the OA progression. (A) The binding site between the miR-27a-3p and TLR4. (B) Luciferase reporter assay showed that miR-27a-3p mimics reduced the luciferase activity of TLR4-Wt group. (C) MiR-27a-3p mimics decreased TLR4 protein levels in C28/I2 cells. (D, E) FOXD2-AS1 overexpression increased TLR4 expression both in mRNA and protein levels in C28/I2 cells, which could be abolished by miR-27a-3p mimics. (F) TLR4 mRNA expression was significantly increased in OA patients. (G) TLR4 mRNA expression was positively correlated with FOXD2-AS1 expression in OA tissues. *p < .05.
Discussion
OA is one chronic joint disease characterized with articular cartilage degeneration and chondrocyte play important roles in OA progression [Citation12]. Recently, increasing evidence revealed that FOXD2-AS1 play critical roles in lots of biological processes such as tumorigenesis and OA progression. For example, Rong et al showed that FOXD2-AS1 promoted non-small cell lung cancer progression via Wnt/β-catenin signaling [Citation15]. Ren et al revealed that FOXD2‐AS1 expression was correlated with the malignant status in cutaneous melanoma and could regulate cell proliferation, migration and invasion ability [Citation16]. Zhu et al suggested that FOXD2‐AS1 contributed to colorectal cancer proliferation through interaction with miR-185-5p [Citation17]. In the present study, we found that FOXD2-AS1 expression was significantly upregulated and associated with the severity of OA patients. IL-1β and TNF-α treatment markedly induced FOXD2-AS1 expression in chondrocytes. In addition, function assays revealed that FOXD2-AS1 overexpression promoted the proliferation, inflammation, and ECM degradation in chondrocytes. These data indicated that FOXD2-AS1 was involved in OA progression.
A growing body of evidence has demonstrated that lncRNAs can serve as a natural miRNA sponge and regulate their functions [Citation13]. For example, Su et al. found that lncRNA H19 could function as a ceRNA to regulate AQP3 expression by sponging miR-874 in the intestinal barrier [Citation18]. Li et al. showed that lncRNA ADNCR suppressed adipogenic differentiation by targeting miR-204 [Citation19]. An et al. suggested that lncRNA NEAT1 contributed to paclitaxel resistance of ovarian cancer cells by regulating ZEB1 expression via miR-194 [Citation20]. In the present study, FOXD2-AS1 interacted with miR-27a-3p in chondrocytes. The expression of miR-27a-3p was decreased and negatively associated with the severity in OA patients. Correlation analysis found that FOXD2-AS1 expression was negatively correlated with miR-27a-3p expression in OA tissues. Moreover, we found that FOXD2-AS1 promoted cell proliferation, inflammation, and ECM degradation in chondrocytes via regulating miR-27a-3p. Thus, we suggested that FOXD2-AS1 could act as a molecular sponge of miR-27a-3p in OA progression.
Toll-like receptors (TLRs) are pattern recognition receptors, and their signaling pathways are particularly relevant in inflammatory processes [Citation21,Citation22]. Previous studies showed that TLR4 could play critical roles in OA progression [Citation23]. For example, Jin et al found that isofraxidin targeted the TLR4/MD-2 axis to prevent OA development [Citation24]. Ding et al found that miR-93 inhibited chondrocyte apoptosis and inflammation in OA by targeting the TLR4/NF-κB signaling pathway [Citation25]. Zhang et al suggested that curcumin reduced inflammation in knee OA rats through blocking [Citation26]. In the present study, we found that TLR4 acted as a target of miR-27a-3p. FOXD2-AS1 overexpression increased TLR4 expression both in mRNA and protein levels, which could be abolished by miR-27a-3p mimics. In addition, TLR4 expression was increased and positively correlated with FOXD2-AS1 expression in OA tissues. Thus, those data indicated that lncRNA FOXD2-AS1 promoted OA progression by miR-27a-3p/TLR4 axis.
In conclusions, our study showed that FOXD2-AS1 contributed OA progression by sponging miR-27a-3p and inducing the expression of TLR4, indicating FOXD2-AS1/miR-27a-3p/TLR4 axis could serve as a novel therapeutic target for OA patients’ treatment.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Arden N, Nevitt MC. Osteoarthritis: epidemiology. Best Pract Res Clin Rheumatol. 2006;20:3–25.
- Glyn-Jones S, Palmer AJR, Agricola R, et al. Osteoarthritis. Lancet. 2015;386:376–387.
- Wang X, Hunter D, Xu J, et al. Metabolic triggered inflammation in osteoarthritis. Osteoarthr Cartil. 2015;23:22–30.
- Singh JA, Noorbaloochi S, MacDonald R, et al. Chondroitin for osteoarthritis. Cochrane Database Syst Rev. 2015;1:CD005614.
- Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10:155–159.
- Quinn JJ, Chang HY. Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet. 2016;17:47–62.
- Engreitz JM, Ollikainen N, Guttman M. Long non-coding RNAs: spatial amplifiers that control nuclear structure and gene expression. Nat Rev Mol Cell Biol. 2016;17:756–770.
- Fatica A, Bozzoni I. Long non-coding RNAs: new players in cell differentiation and development. Nat Rev Genet. 2014;15:7–21.
- Yang Z, Tang Y, Lu H, et al. Long non‐coding RNA reprogramming (lncRNA‐ROR) regulates cell apoptosis and autophagy in chondrocytes. J Cell Biochem. 2018;119(10):8432–8440.
- Zhu JK, He TD, Wei ZX, et al. LncRNA FAS-AS1 promotes the degradation of extracellular matrix of cartilage in osteoarthritis. Eur Rev Med Pharmacol Sci. 2018;22:2966–2972.
- Fan X, Yuan J, Xie J, et al. Long non-protein coding RNA DANCR functions as a competing endogenous RNA to regulate osteoarthritis progression via miR-577/SphK2 axis. Biochem Biophys Res Commun. 2018;500:658–664.
- Kartha RV, Subramanian S. Competing endogenous RNAs (ceRNAs): new entrants to the intricacies of gene regulation. Front Genet. 2014;5:8.
- Xia T, Liao Q, Jiang X, et al. Long noncoding RNA associated-competing endogenous RNAs in gastric cancer. Sci Rep. 2014;4:6088.
- Croce CM, Calin GA. miRNAs, cancer, and stem cell division. Cell 2005;122:6–7.
- Rong L, Zhao R, Lu J. Highly expressed long non-coding RNA FOXD2-AS1 promotes non-small cell lung cancer progression via Wnt/β-catenin signaling. Biochem Biophys Res Commun. 2017;484:586–591.
- Ren W, Zhu Z, Wu L. FOXD2‐AS1 correlates with the malignant status and regulates cell proliferation, migration, and invasion in cutaneous melanoma. J Cell Biochem. 2019;120(4):5417–5423.
- Zhu Y, Qiao L, Zhou Y, et al. Long non-coding RNA FOXD2-AS1 contributes to colorectal cancer proliferation through its interaction with microRNA-185-5p. Cancer Sci. 2018;109:2235–2242.
- Su Z, Zhi X, Zhang Q, et al. LncRNA H19 functions as a competing endogenous RNA to regulate AQP3 expression by sponging miR-874 in the intestinal barrier. FEBS Lett. 2016;590:1354–1364.
- Li M, Sun X, Cai H, et al. Long non-coding RNA ADNCR suppresses adipogenic differentiation by targeting miR-204. Biochim Biophys Acta. 2016;1859:871–882.
- An J, Lv W, Zhang Y. LncRNA NEAT1 contributes to paclitaxel resistance of ovarian cancer cells by regulating ZEB1 expression via miR-194. Onco Targets Ther. 2017;10:5377–5390.
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21(1):335–376.
- Hausmann M, Kiessling S, Mestermann S, et al. Toll-like receptors 2 and 4 are up-regulated during intestinal inflammation. Gastroenterology. 2002;122:1987–2000.
- Gómez R, Villalvilla A, Largo R, et al. TLR4 signalling in osteoarthritis-finding targets for candidate DMOADs. Nat Rev Rheumatol. 2015;11:159–170.
- Jin J, Yu X, Hu Z, et al. Isofraxidin targets the TLR4/MD-2 axis to prevent osteoarthritis development. Food Funct. 2018;9:5641–5652.
- Ding Y, Wang L, Zhao Q, et al. MicroRNA-93 inhibits chondrocyte apoptosis and inflammation in osteoarthritis by targeting the TLR4/NF-κB signaling pathway. Int J Mol Med. 2019;43:779–790.
- Zhang Y, Zeng Y. Curcumin reduces inflammation in knee osteoarthritis rats through blocking TLR4/MyD88/NF‐κB signal pathway. Drug Dev Res. 2019.