Abstract
Objective
The objective of this study was aimed to investigate the interactions between Aurora kinase A (AURKA) gene polymorphisms (T91A and G169A) and smoking and their effects on the susceptibility of oral cancer.
Methods
One hundred five healthy controls were frequency-matched with 91 oral cancer patients by age, sex and nationality. Detection of AURKA T91A and G169A polymorphisms was performed using polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method. Differences in genotypes and alleles between case and control groups were compared using χ2 test. Relative risk of oral cancer was presented as odds ratios (ORs) and 95% confidence intervals (95% CIs).
Results
AA genotypes respectively of AURKA T91A and G169A polymorphisms had higher frequencies in case group than in control group (p = .023; p = .038). However, only 91AA genotype was significantly associated with the occurrence of oral cancer risk (OR = 3.113, 95% CI = 1.176–8.236). A alleles of the two polymorphisms respectively increased the risk of oral cancer (OR = 1.700, 95% CI = 1.113–2.596; OR = 1.978, 95% CI = 1.132–3.454). Interactions between smoking and AURKA polymorphisms significantly associated with the onset of oral cancer (p < .05).
Conclusions
AURKA polymorphisms are susceptible factors for oral cancer. Interactions between AURKA polymorphisms and smoking increase the risk of oral cancer.
Introduction
Oral cancer is a common highly malignant tumour in the head and neck region and accounts for 3% of the malignant tumours of the whole body. Squamous cell carcinoma is the most common kind of oral cancer. The incidence of oral cancer is increasing year by year, and the onset age is getting younger and younger [Citation1]. Unfortunately, with the significant development of tumour diagnosis and treatment technology, 5-year survival rate had no obvious rising [Citation2]. Because of lacking understanding of early oral cancer lesions, most of the patients ignore the early changes in the oral and always miss the proper moment of diagnosis and treatment. When it was diagnosed, these patients have advanced stage of this disease [Citation3]. If the oral cancer lesions can be found early, the treatment effect and quality of life for patients can be improved greatly.
Studies have proved that oral cancer is not induced by a single factor, but by the long-time interactions of internal factors of the susceptible population and external environment factors [Citation4]. Many risk factors are involved in oral cancer [Citation5], of which smoking is considered as the most important one [Citation6]. But not all smokers will suffer from oral cancer, which suggests that genetic factors play a crucial role in the onset process of oral cancer [Citation7–9]. Besides, chromosome instability is likely to be one of the reasons for the formation and development of malignant tumours [Citation10].
Aurora Kinase A (AURKA) is a member of serine/threonine kinase family and involved in maintaining the accurate separation process of cell centriole and chromosome [Citation11]. Excessive expression of AURKA will result in centriole abnormality, chromosome instability and tumour formation [Citation12]. AURKA encoding gene, AURKA is located on chromosome 20q13.2 and had been discovered a number of polymorphisms. Moreover, AURKA polymorphisms have been reported to be associated with the occurrence of many tumours [Citation13]. But, few studies refer to the association of AURKA polymorphisms with oral cancer risk, especially in Chinese Han population.
In this study, we explored the influence of AURKA T91A and G169A polymorphisms on the susceptibility of individuals to oral cancer in a Chinese Han population. Furthermore, the interaction of AURKA polymorphisms with smoking was also investigated in oral cancer risk.
Materials and methods
Cases and controls
Ninety-one cases included 52 males and 39 females, and their age was 41–75 with the mean age of 57.1 ± 3.5. These cases were oral cancer patients from Department of Oncology of The First Affiliated Hospital of Jinzhou Medical University. They were diagnosed by routine inspection and histopathological examinations. None of the cases received radiotherapy, chemotherapy and biological treatments before sample collection.
One hundred five healthy people with 59 males and 46 females aged from 40 to77 years (mean age 56.6 ± 4.5) were enrolled into the control group. Controls were from the physical examination center of the same hospital during the same period. Medical examination reports exhibited that controls had no oral inflammatory disease, genetic disease history, family history of cancer and system disease history.
Controls were matched with cases in age, sex, nationality and native place with the cases. Smoking status of the two groups was similar to each other. One cigarette per day and lasted a half year was considered as smoking. All the subjects were Chinese Han population, and they had no blood connection with each other. Sample collection process was conducted in accordance with the national human genome research ethics guidelines. This study was approved by the ethics committee of The First Affiliated Hospital of Jinzhou Medical University. Informed consent forms were obtained from all of the participants.
Clinical data and sample collection
Demographic data, smoking history, family tumour history of the subjects as well as their anamnesis (including histories of oral diseases, diabetes, dyslipidemia, malignant tumours and cerebrovascular diseases) were inspected by special employees.
We collected 4 ml fasting peripheral venous blood in the morning. The blood sample was put into EDTA-2Na anticoagulant tubes and saved in a cryogenic refrigerator at −20 °C until used. Phenol-chloroform method was conducted for the genomic DNA extraction.
Polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) amplification
PCR primers for the two AURKA polymorphisms were designed by Primer Premier 5.0 program and synthesized by Shanghai Sangon Biotech Co. Ltd. Primer sequences are shown in .
Table 1. Primers used for the detecting and genotyping of AURKA polymorphisms.
PCR-restriction fragment length polymorphism (PCR-RFLP) method was applied to analyze AURKA T91A and G169A polymorphisms. Total volume of PCR system was 25.0 µl, with 2.0 μL template DNA, 0.8 μl forward primer, 0.8 μl reverse primer, 2.0 μl dNTPs, 5.0 μl 10 × Buffer solution, 0.5 μl Taq DNA polymerase and the rest volume of sterile water. PCR conditions were 95 °C initial predenaturation for 3 min, followed by 35 cycles of 94 °C denaturation for 45 s, 56 °C annealing for 30 s, 72 °C extension for 50 s; 72 °C finally extension for 5 min.
The mixture of 2.0 µl 10 × buffer, 2.0 μl Apo I and 10.0 μl PCR products digested at 40 °C for 12 h. Digested products were examined using 2% agarose gel electrophoresis.
Statistical analysis
Hardy–Weinberg equilibrium (HWE) was inspected by PLINK1.07 software. χ2 test was used to compare sex and smoking status between the two groups as well as the differences of the genotype and allele distributions. Statistically significant differences of the age in the two groups were compared using t-test. We used crossover analysis to investigate the gene-environment interactions. Calculations were performed by PASW Statistics 18.0 software. p < .05 indicated statistical significance. Relative risk of oral cancer was exhibited as odds ratios (ORs) and 95% confidence intervals (95% CIs).
Results
General situation of research objects
This study recruited 91 cases and 105 healthy controls, and their basic information is listed in . Differences of the ratios of sex and age between the two groups were not statistically significant (p > .05). But, the ration of smoking status of the case group was significantly higher than that of the control group (p < .01). In case group, nearly half of oral cancer patients were in tumour size more than 3 cm (46.15%) and over one-third of cases existed lymph node metastasis (38.46%). 51.65% of oral cancer patients were in TNM I–II stages, and 48.35% cases were III–IV stages. Only nearly one-third of the cases (34.07%) was well-differentiated.
Table 2. Analysis of characteristics.
HWE inspection
Distributions of AURKA T91A and G169A polymorphisms in 105 controls satisfied HWE. Genotype frequencies of the polymorphisms in the control group had significant goodness of fit. Thus, we confirmed that the controls were in equilibrium and was typical enough to represent the local normal people.
Association of AURKA polymorphisms with oral cancer
Genotype and allele distributions of T91A and G169A polymorphisms are shown in and . High frequency of T91A polymorphism AA genotype was observed in case group, and the difference was statistically significant (p = .023). This result indicated that 91AA was the risk genotype of oral cancer (OR = 3.113, 95% CI = 1.176–8.236). Frequency of 91A allele between the two groups was significantly different (p = .015), and the occurrence of oral cancer was increased by A allele (OR = 1.700, 95% CI = 1.113–2.596). Frequency of 169AA genotype was obviously higher in cases than that in controls (p = .038), but it was not related to the onset of oral cancer. Meantime, G169A polymorphism A allele was more frequently observed in case group than in control group, and A allele was closely related to the onset of oral cancer (OR = 2.515, 95% CI = 1.448–4.367).
Figure 1. The distributions of AURKA T91A (A) and G169A (B) polymorphisms in case and control groups.
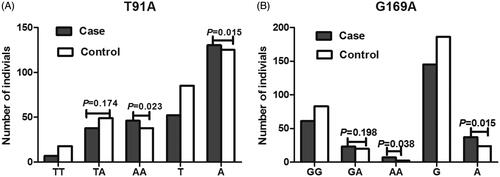
Table 3. Distributions of the genotypes and alleles in AURKA T91A and G169A polymorphisms.
Effects of the interactions between AURKA polymorphisms and smoking on oral cancer
Interaction existed between AURKA gene T91A polymorphism and smoking status (). Smokers who were TA or AA carries had increased risk of oral cancer compared with non-smokers who carried TT genotype (OR = 3.733, 95% CI = 1.136–12.272; OR = 10.267, 95% CI = 2.914–36.170). Interactions of G169A polymorphism with smoking status in oral cancer are shown in . Smokers with all of the genotypes of G169A polymorphism had high risk of oral cancer compared to non-smokers with TT genotype (GG: OR = 2.713, 95% CI = 1.359–5.414; GA: OR = 7.121, 95% CI = 2.593–19.557; AA: OR = 12.207, 95% CI = 1.403–106.183), suggesting that interactions emerged between smoking and G169A polymorphism. The interactions could increase the risk of oral cancer.
Table 4. Interaction of AURKA T91A polymorphism and smoking on oral cancer.
Table 5. Joint effects of AURKA G169A polymorphisms and smoking on oral cancer.
Discussion
Oral cancer belongs to malignant tumour and occurs in oral and adjacent anatomic structures. Tongue carcinoma, gingiva carcinoma, carcinoma of the buccal mucosa and carcinoma of the palate all belong to oral cancer. Surgical operation is an efficient therapy method for oral cancer. Treatment of oral cancer always makes the patients lose their physiological functions, such as swallowing difficulty and changed facial appearance. Incidence of oral cancer in China is very high, with tongue carcinoma more common in the south [Citation14]. Oral cancer rate among young people presents a generally upward trend in the global scope. For the above reasons, prevention and treatment of such disease is very important, especially the screening of susceptibility genes of oral cancer, which makes it more targeted to take prevention measures for vulnerable groups.
Relationship between centrosome abnormity and malignant tumours has got widespread attention since the beginning of the 20th century [Citation15]. The function of centrosome ensuring the symmetry and dual polarity of cell division is a necessary condition for the right separation of chromosomes [Citation16]. It has been verified that centrosome dysfunction can lead to the abnormal chromosome separation in malignant tumours [Citation17]. Centrosomes number in almost all human solid tumours is abnormal. AURKA is a kind of functional kinase of the centrosome, and as an oncogene [Citation18], the amplification and over-expression of AURKA gene have been detected in variety of human tumours [Citation19]. AURKA polymorphisms have been proved by many studies to be the genetic factors of disrupting the centrosome functions [Citation20]. Results of previous studies are different and this may be due to different races, tumours and sample sizes of the studies [Citation21,Citation22].
Our study researched the association of AURKA gene T91A and G169A polymorphisms with oral cancer risk. We found that 91AA genotype was frequently observed in cases than in controls, indicating a significant association with the onset of oral cancer. Compared with 91T allele carriers, 91A allele carriers had 1.700 times increased risk of oral cancer. Meanwhile, 169AA obviously different between case and control groups, but the difference was not related to the occurrence of oral cancer. 169A allele of AURKA G169A polymorphism significantly associated with 1.978 times increased risk of oral cancer.
The occurrence of oral cancer is recognized as a complex process with the joint action of multiple steps and factors. Various genetic and environmental factors take part in the occurrence of oral cancer [Citation23, Citation24]. Epidemiological investigations show that, among these risk factors, there is a significant correlation between smoking and the occurrence of oral cancer [Citation25]. But not all of the smokers suffer from cancer, there might exist interaction between smoking status and genes. It was believed that when people smoke, plenty of free radicals will enter the body to attack the normal cells. Genes can be changed including chain scission and base modification. Thus oncogene mutations occur, dramatically accelerating cell differentiation, and finally canceration occurs. We also explored the interaction between smoking and AURKA gene polymorphisms (T91A and G169A), so as to certify the pathogenesis of oral cancer. We discovered high expression of 91TA and 91AA genotypes in smokers when compared with non-smokers. Smokers had high risk of oral cancer respectively in all of the genotypes of G169A polymorphism. Interactions of T91A and G169A polymorphisms with smoking promoted the occurrence of oral cancer. The conclusion might be helpful for the selection of individuals with high risk of oral cancer. Genetic polymorphisms also have the ability to act as tumour biomarkers. For instance, Gonzalez-Hormazabal et al. reported that IL-8 rs4073 polymorphism showed close association with overall survival of gastric cancer patients, which might be used for prognosis evaluation among the cases [Citation26]. Beysel et al. suggested that VDR gene Fokl T allele and TT genotype predicted aggressive disease progression, which might be an indicator for poor prognosis of patients with papillary thyroid cancer [Citation27]. Sierra-Martinez and colleagues proved that the presence of G/G genotype of Xbal increased the risk of postmenopausal breast cancer, which might be a potential predictive factor for breast cancer in Mexican women [Citation28]. All the evidence demonstrated the great possibility of genetic polymorphisms to be employed as cancer biomarkers. In our study, we proved that AURKA T91A and G169A combined with smoking significantly increased the risk of oral cancer. However, whether AURKA T91A and G169A polymorphisms could be acted as the diagnostic biomarkers of oral cancer remained poor unclear. Due to the complex of oral cancer aetiology and relatively small sample size in our study, the application of AURKA polymorphisms alone for early detection of oral cancer might cause diagnostic errors. Therefore, further investigations are still required to address the above issues.
In conclusion, the interactions of AURKA polymorphisms and smoking play an important role in the occurrence of oral cancer.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Papageorge MB. Etiology of oral cancer in the young patient: is tongue cancer becoming the other cancer in women? Oral Maxillofacial Surg Clin North Am. 2007;19:163–171.
- Jayaprakash V, Sullivan M, Merzianu M, et al. Autofluorescence-guided surveillance for oral cancer. Cancer Prev Res (Phila). 2009;2:966–974.
- Llewellyn CD, Johnson NW, Warnakulasuriya S. Factors associated with delay in presentation among younger patients with oral cancer. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:707–713.
- Irimie AI, Ciocan C, Gulei D, et al. Current insights into oral cancer epigenetics. Int J Mol Sci. 2018;19:E670.
- Choi S, Myers JN. Molecular pathogenesis of oral squamous cell carcinoma: implications for therapy. J Dent Res. 2008;87:14–32.
- Marron M, Boffetta P, Zhang ZF, et al. Cessation of alcohol drinking, tobacco smoking and the reversal of head and neck cancer risk. Int J Epidemiol. 2010;39:182–196.
- Tsai CW, Tsai MH, Tsou YA, et al. The joint effect of smoking and hOGG1 genotype on oral cancer in Taiwan. Anticancer Res. 2012;32:3799–3803.
- Jing G, Lv K, Jiao X. The p53 codon 72 polymorphism and the risk of oral cancer in a Chinese Han population. Genet Test Mol Biomarkers. 2012;16:1149–1152.
- Chu YH, Tzeng SL, Lin CW, et al. Impacts of microRNA gene polymorphisms on the susceptibility of environmental factors leading to carcinogenesis in oral cancer. PLoS One. 2012;7:e39777.
- Shackney SE, Shankey TV. Common patterns of genetic evolution in human solid tumors. Cytometry. 1997;29:1–27.
- Glover DM, Leibowitz MH, McLean DA, et al. Mutations in aurora prevent centrosome separation leading to the formation of monopolar spindles. Cell. 1995;81:95–105.
- Zhou H, Kuang J, Zhong L, et al. Tumour amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy and transformation. Nat Genet. 1998;20:189–193.
- Tang W, Qiu H, Jiang H, et al. Aurora-A V57I (rs1047972) polymorphism and cancer susceptibility: a meta-analysis involving 27,269 subjects. PLoS One. 2014;9:e90328.
- Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45:309–316.
- Duensing S. Analysis of centrosomes in human cancer. Methods Cell Biol. 2015;129:51–60.
- Kramer A, Neben K, Ho AD. Centrosome replication, genomic instability and cancer. Leukemia. 2002;16:767–775.
- Pannu V, Mittal K, Cantuaria G, et al. Rampant centrosome amplification underlies more aggressive disease course of triple negative breast cancers. Oncotarget. 2015;6:10487–10497.
- Leontovich AA, Salisbury JL, Veroux M, et al. Inhibition of Cdk2 activity decreases Aurora-A kinase centrosomal localization and prevents centrosome amplification in breast cancer cells. Oncol Rep. 2013;29:1785–1788.
- Zhang J, Li B, Yang Q, et al. Prognostic value of Aurora kinase A (AURKA) expression among solid tumor patients: a systematic review and meta-analysis. Jpn J Clin Oncol. 2015;45:629–636.
- Karthigeyan D, Prasad SB, Shandilya J, et al. Biology of Aurora A kinase: implications in cancer manifestation and therapy. Med Res Rev. 2011;31:757–793.
- Tang W, Qiu H, Ding H, et al. Association between the STK15 F31I polymorphism and cancer susceptibility: a meta-analysis involving 43,626 subjects. PLoS One. 2013;8:e82790.
- Qin J, He XF, Wei W, et al. Association between the STK15 polymorphisms and risk of cancer: a meta-analysis. Mol Genet Genomics. 2015;290:97–114.
- Rosenquist K. Risk factors in oral and oropharyngeal squamous cell carcinoma: a population-based case-control study in southern Sweden. Swedish Dent J Suppl. 2005;179:1–66.
- Kadashetti V, Chaudhary M, Patil S, et al. Analysis of various risk factors affecting potentially malignant disorders and oral cancer patients of Central India. J Can Res Ther. 2015;11:280–286.
- Lu X, Hua Z, Du G, et al. Scavenging of free radicals in gas-phase mainstream cigarette smoke by immobilized catalase at filter level. Free Radic Res. 2008;42:244–252.
- Gonzalez-Hormazabal P, Romero S, Musleh M, et al. IL-8-251T > A (rs4073) polymorphism is associated with prognosis in gastric cancer patients. Anticancer Res. 2018;38:5703–5708.
- Beysel S, Eyerci N, Pinarli FA, et al. VDR gene FokI polymorphism as a poor prognostic factor for papillary thyroid cancer. Tumour Biol. 2018;40:1010428318811766.
- Sierra-Martinez M, Hernandez-Cadena L, Garcia-Sanchez JR, et al. Predictive polymorphisms for breast cancer in postmenopausal Mexican women. J Cancer Res Ther. 2018;14:640–646.
