?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Synthesis of silver and silver based nanoparticles using microorganisms has received profound interest because of obtaining nanoparticles with unique physicochemical and biological properties. In the current study, for the first time, synthesis of silver chloride nanoparticles (AgClNPs) using cell-free supernatant of Escherichia coli culture is reported. Prepared AgClNPs were characterized by EDS, XRD and FESE. Data revealed the synthesized nanoparticles, mostly, have a spherical shape with an average size of 13 nm. Additionally, MTT assay elucidated a dose-dependent cytotoxicity of AgClNPs against MCF-7 cells (IC50 = 44 µg/mL). Quantitative real-time reverse transcription-PCR and colourimetric assays were employed to investigate the mechanism of cell toxicity in several cell death pathways. The results revealed the ability of AgClNPs to upregulate Bax/Bcl-2 ratio and p53 at mRNA level. Moreover, other apoptotic factors such as caspase-3, 8 and 9 were also upregulated at both mRNA and proteome levels. Finally, apoptosis induction was confirmed by Annexin-V/PI detection assay. Based on the obtained data, biosynthesized AgClNPs using E. coli cell-free supernatant exhibit a cytotoxic effect on human breast cancer cells through up-regulation of apoptotic factors, which suggest them as anti-tumour agents for further investigations.
Introduction
Nanotechnology, which involves the production and use of nanomaterials, is growing rapidly and has a wide range of applications in various fields [Citation1–4]. Advances in nanotechnology and deep understanding of cellular processes have helped the researchers to design and synthesize nanoparticles for both therapeutic and diagnostic purposes [Citation5].
Different methods have been developed for synthesizing metal nanoparticles including mechanical, chemical, plant and microbial synthesis. The latest has significant advantages over other methods, such as environmental compatibility, synthesis at ambient temperature and pressure, and precise control of size and shape [Citation6–8]. Therefore, high-throughput screening of microorganisms is an important step for finding new potential sources. Nanoparticle synthesis occurred at different locations in bacteria; (i) intracellular, and (ii) extracellular. Intracellular synthesis of nanoparticles, however, requires labor-intensive purifications steps like ultrasonication or treatment with appropriate detergents for bacterial lysis. While the extracellular biosynthesis is cheap and has a simple downstream process and could be applied for large-scale production of nanoparticles. Therefore, many studies have reported the extracellular synthesis of metal nanoparticles using bacterial cultures [Citation9–11]. It must be noted that different preparation methods cause different structural properties in the synthesized nanoparticles which consequently leads to different functional characteristics [Citation12]. For example, various investigations have shown that the antiproliferative activity of silver nanoparticles on tumour cells, is completely dependent on the method of synthesis [Citation13].
Silver nanoparticles are one of the most attractive metal nanoparticles which have antimicrobial [Citation14–16], adjuvanticity [Citation17], and anti-tumour properties [Citation18–20]. Moreover, silver-based nanoparticles have attracted attention, because they show enhanced physicochemical and biological characteristics based on the type of additional elements used in the nanoparticle preparations. AgClNPs have a variety of photochemical and biomedical applications such as fabricating antiseptic catheters, bone cement, and fabrics due to the antibacterial activity. Despite such advantages, preparation of controlled sized AgClNPs is limited to methods such as micro-emulsion technique, ultrasound irradiation, matrix-based technique or mixing silver nitrate (AgNO3) with hydrochloride acid in the presence of poly(vinyl pyrrolidone) [Citation21–23]. According to the authors’ knowledge, the biosynthesis of AgClNPs has not been reported so far using bacterial cultures. Therefore, in the current study, AgClNPs were prepared by a facile method just using two precursors: silver nitrate and potassium chloride. this reaction occurred in an aqueous environment containing extracellular culture of a k12 strain of Escherichia coli. The fabricated nanoparticles were characterized using different spectroscopic and microscopic techniques such as EDS, XRD and FESEM. Additionally, the mechanism of synthesized AgClNPs in apoptosis induction against human breast cancer cells was investigated at both transcriptional and translational levels.
Materials and methods
Cell-free supernatant preparation and nanoparticle synthesis
For the preparation of cell-free supernatant (CFS), E. coli K12 strain was inoculated into a 250 mL Erlenmeyer flask containing 100 mL sterile Czapek–Dox broth and the flask was then incubated overnight with 150 rpm shaking at 37 °C. After incubation, the culture was centrifuged at 2000 g for 30 min and the supernatant was filtered through a 0.22 µm syringe filter. For nanoparticle synthesis, the KCl solution was added to CFS at a final concentration of 4 mM. Then, AgNO3 solution was added stoichiometrically equivalent molar ratio with KCl to the mixture under stirring (100 rpm) at room temperature for a period of 24 h. Visual observations were conducted periodically to check the nanoparticle formation by a colour change (white to medium purple). When the reaction was completed, the solution was centrifuged at 14,000 g for 20 min and the sediment was washed with distilled water three times.
Nanoparticle characterizations
The existence of silver and chloride was studied using energy-dispersed spectroscopy (EDS), which automatically identifies the elements responsible for the peaks in the energy distribution. The particle size and surface morphology were investigated by X-ray diffraction (XRD) (X’ Pert Pro, Panalytical, UK) and field emission scanning electron microscopy (FESEM) (Philips, Amsterdam, Netherlands, XL30).
In vitro studies
Cell lines and culture conditions
MCF-7 and HEK293 cell lines was purchased from National Cell Bank of Iran (NCBI, Pasteur Institute of Iran). The cells were grown in DMEM medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 1.0 mM sodium pyruvate, 2 mM L-glutamine and 1X Pen-Strep (All purchased from Gibco, Paisley, Scotland) at 37 °C in humidified atmosphere containing 5% CO2.
Cell viability assay
Cell viability was determined by 3-(4,5-dimethylthiazol2yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Briefly, the cells were seeded into 96-well plates (SPL Life Sciences, South Korea) with a density of 1 × 104 cells per well and allowed the cells to attach during 24 h. Then, different concentrations of AgClNPs (0, 3.9, 7.8, 15.6, 31.2, 62.5, 125, 250 and 500 µg/mL) were added to the wells in eight replicates, and the plates were left intact for 24 h. Afterward, MTT was added (0.05 mg/well) and the plates were incubated for 4 h at 37 °C. Finally, the purple formazan crystals were dissolved by addition of 100 µL dimethyl sulfoxide (DMSO) (Merck, Germany) and absorbance was measured at a wavelength of 570 nm using a microplate reader (Bio-Rad, Hercules, CA).
Quantitative real-time reverse transcription-PCR (qRT-PCR)
A SYBR Green real-time quantitative PCR was employed to quantify the expression levels of Bax, Bcl-2, p53, caspase-3, caspase-8, and caspase-9 genes after the treatment of MCF-7 cells. Briefly, cancerous cells were seeded into 6-well plates (5 × 105 cells/well) and incubated for 24 h. The cells were then exposed to AgClNPs with a final concentration of 44 μg/mL and incubated for an additional 24 h. Total mRNA was then extracted from the cells and converted to complementary DNA using RNA-isolation kit (Qiagen, RNeasy Plus Mini Kit 50, Valencia, CA) and PrimeScriptTM first strand cDNA Synthesis Kit (Takara, Tokyo, Japan), respectively, according to the manufacturer’s protocol. The primers used for real-time PCR are shown in . The expression of the target genes was studied using ABI 7300 real-time PCR system (Applied Biosystems, Foster City, CA) and GAPDH was used as internal control. Each amplification reaction was performed in a 20 µL reaction mixture containing 10 µL Power SYBR Green PCR Master Mix (2X), 1 µL of each primer (2 µM), 2 µL cDNA (100 ng), and 6 µL double-distilled water. After denaturation at 95 °C for 10 min, 40 cycles were followed by 95 °C for 15 s, and 60 °C for 1 min. Amplification step was followed by a melting step at 95 °C for 20 s, 60 °C for 60 s and 95 °C for 20 s. The level of gene expressions was determined using ΔΔCT method.
Table 1. Primer sequences used in qRT-PCR.
Caspase-3, -8 and -9 activity assays
A colorimetric method was employed for caspase activity measurements using caspase 3, 8 and 9 assay kits (Abnova). The cells were treated with AgClNPs with a final concentration of 44 μg/mL and incubated at 37 °C for 24 h in 5% CO2. Trypsinized cells were then centrifuged at 600 g for 5 min, and the pellets were lysed by adding 50 µL of chilled cell lysis buffer and incubation on ice for 10 min. The lysate was centrifuged at 10,000 g for 1 min using a microcentrifuge and the supernatant was transferred to a fresh tube. For each assay, 200–300 µg of protein was added to 50 µL cell lysis buffer and used as a sample. Fifty microlitres of 2X reaction buffer containing 10 mM DTT were added to each sample. Then, pNA conjugated specific substrates (in a final concentration of 200 µM) were individually added into each tube and incubated at 37 °C for 2 h. Finally, absorbance was determined using a MultiSkan plate reader (Bio-Rad, Hercules, CA) at a wavelength of 400 nm. Related caspase activities were calculated using EquationEquation (1)(1)
(1) . All protein concentrations were measured using a Bradford assay [Reference is needed].
(1)
(1)
Annexin V/propidium iodide apoptosis assay
To evaluate the mechanism of cell toxicity, a fluorescein isothiocyanate (FITC)-annexin V and propidium iodide (PI) apoptosis detection kit (Hoffman-La Roch Ltd, Basel, Switzerland) was used. MCF-7 cells (1 × 105 cells/well) were treated with AgClNPs (a final concentration of 44 and 150 μg/mL as a mid and high dose) in a 24-well plate and un-treated MCF-7 cells were the negative control.
Statistical analysis
One-way analysis of variance (ANOVA) followed by a Tukey post-hoc test was used for MTT data analysis. Student’s t-test was used for qRT-PCR and caspase activity assay analysis. All statistical analyses were performed using SPSS 15.0 package (SPSS Inc., Chicago, USA). Data were presented as mean ± SD and P values <.05 were considered to be statistically significant.
Results
Characterization of AgClNPs
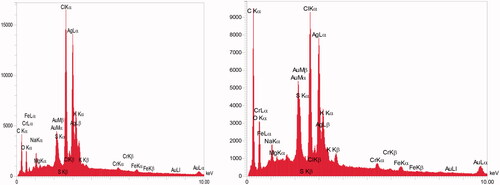
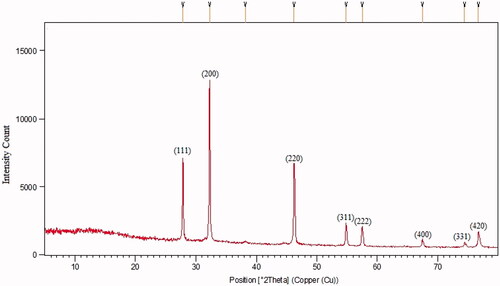
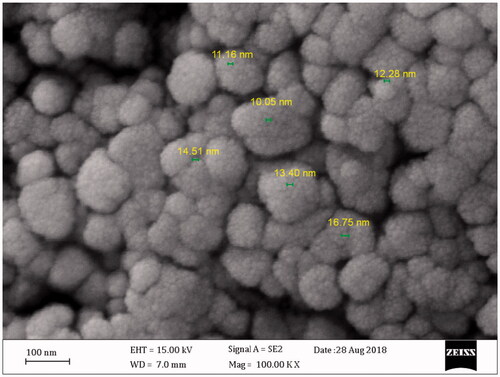
After addition of AgNO3 into the flask containing KCl and CFS, a change in the solution from colourless to a purple colour occurred within 60 min (). The colour change was assumed a significant criterion for the reduction of silver and chloride ions to silver chloride nanoparticles. The existence of silver and chloride, as the major constituents, was confirmed by EDS. The spectrum showed the related peaks, which confirmed the presence of silver and chloride in the nanoparticles (). Other signals in the spectrum such as carbon, oxygen and sulfur indicate the presence of compounds, which may be related to the adsorbed biomolecules on the nanoparticles. The morphological characteristics of the synthesized AgClNPs were also observed under FESEM (). The result showed that AgClNPs were agglomerated. However, they have a spherical shape with an average size of 13 nm in diameter. The XRD spectrum is compared with the standard spectrum by Joint Committee on Powder Diffraction Standards (JCPDS, file no. 85–1355) and confirmed the identity of synthesized nanoparticles ().
Figure 1. Colour change during nanoparticle synthesis. (From left to right: initial time, AgClNPs and AgNPs).

Figure 2. FESEM image of the synthesized AgClNPs. As seen, the nanoparticles have a spherical shape with a size around 13 nm in diameter.
MTT assay
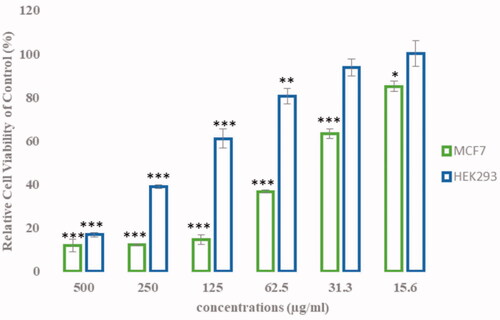
The effect of different concentrations of AgClNPs on the viability of the MCF-7 and HEK cells is shown in . AgClNPs showed a cytotoxic effect on breast cancer cells in a dose-dependent manner. The IC50 was calculated as 44 and 190.5 µg/mL for MCF-7 and HEK cells, respectively, after treatment with the nanoparticles.
Apoptosis-related gene expression profile
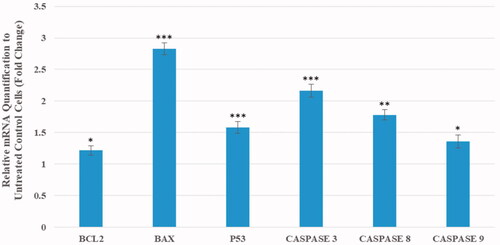
The changes in the expression level of apoptosis-related genes in the cancerous cells after the treatment with the nanoparticles were shown in . Our findings exhibit the mRNA level of Bax gene was significantly upregulated by a 2.8-fold (p < .001). While the expression of anti-apoptotic Bcl-2 gene was increased by a 1.2-fold compared to the control (p < .05). Also, the expression of p53 gene was significantly upregulated by a 1.58-fold in the treated cells (p < .01). Furthermore, the expression of caspase-3, -8, and -9 genes were significantly upregulated by a 2.16, 1.78 and 1.36-fold, respectively, compared to the untreated cells (p < .001, p < .01, and p < .05, respectively).
Cell death assessments at the translational level
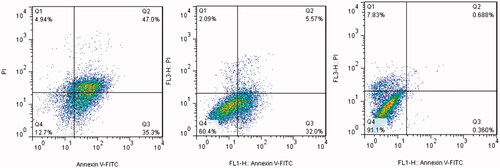
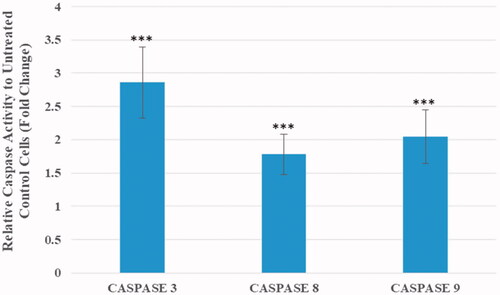
Caspase activity measurements and annexin V/PI flow cytometry were employed to identify the mechanism of cell death in the treated cells. As shown in , the activity of caspases-3, 8 and 9 were increased by 2.86, 1.78 and 2.05 times respectively in MCF7 cells treated with AgClNPs in compared to untreated cells which confirms the qRT-PCR results.
Figure 7. Caspase activity measurements in MCF-7 cells after 24 h of incubation with AgClNP at a medium dose (44 μg/mL). Data are normalized to the untreated cells and reported as mean ± SD. Asterisks (*) indicate a significant difference with the control group (***p < .001).
Dot plots of Annexin V/PI staining are shown in . Exposure of MCF-7 cells to mid-dose of AgClNPs (44 µg/mL) exhibited a 32% early stage apoptosis and a 5.5% late stage apoptosis. Moreover, MCF-7 cells treated with AgClNPs at a concentration of 150 µg/mL (a high dose) exhibited a 35% early stage apoptosis and a 47% late stage apoptosis.
Discussion
Several lines of evidence suggest the potential application of silver nanoparticles in cancer therapy. Recently, many studies report the ability of AgNPs to suppress tumour cell growth both in vivo and in vitro [Citation18–20]. However, there is a lack of literature regarding the use of AgClNPs as anticancer agents against cancer cells. In the present study, we have shown, for the first time, the anticancer activity of AgClNPs against human breast cancer cells and determined the mechanism of action. Moreover, an eco-friendly biofabrication of silver chloride nanoparticles using cell-free supernatant of E.coli culture was reported.
Although the principle of AgClNPs synthesis is similar to AgNPs, identification of AgClNPs is more difficult than AgNPs during the synthesis process. Usually, a colour change in the reaction mixture is an indicator for nanoparticle formation. For example, a colour change from colourless to brown indicates the formation of silver nanoparticles. But it is not simple for AgClNPs synthesis because AgClNPs are highly sensitive to light. In this study, during the synthesis of AgCl nanoparticles, a colour change from colourless to medium purple was observed in the reaction. In contrast to our observation, there was a report that light causes colour change to gray in the AgCl containing solutions [Citation24]. To check our observation, a simple experiment was conducted to determine the effect of sunlight on AgClNPs. AgCl was prepared, then re-suspended in distilled water, and finally exposed to the light. It was found that the white colour of AgCl changed to purple (data not shown). Based on this experiment, we found that the formation of purple colour during the synthesis of nanoparticles could be due to the formation of AgCl. Although it is suggested that the release of silver ions from silver chloride nanoparticles causes change in the colour, but the reason is not completely clear because the release of silver ions cannot be exactly related to purple colour. Analytical tests like EDS elucidated chloride and silver are the main elements of synthesized nanoparticles by observation of strong absorption peaks at 2.6 and 3 keV, respectively. However, other trace elements were also determined probably due to the impurities derived from the cell-free supernatant used in the nanoparticle synthesis. Moreover, the XRD spectrum was compared with the standard (JCPDS, file no. 85–1355) and confirmed the identity of synthesized nanoparticles. Paulkumar et al. used Bacillus subtilis biomass as to synthesize AgClNPs. The colour change reported during the synthesis was a brownish colour but the X-ray diffraction pattern was the same as our study [Citation25].
In vitro cell viability assay clearly indicated AgClNPs were severely toxic to human breast cancer cells even more than AgNPs (data not shown). Interestingly, the synthesized AgClNPs had lower cytotoxicity effect on HEK non-tumour derived cell line. An increase in the percentage of apoptotic cells, measured by flow cytometry, plainly clarified an induction of programmed cell death in the cancer cells after treatment with AgClNPs. More investigations at transcriptional and translational levels revealed the molecular mechanism of apoptosis induction. Activation of caspases 3, 8 and 9, as well as an increase in Bax/Bcl-2 ratio and p53 expression, suggest that AgClNPs induce apoptosis through the mitochondrial pathway. Several studies have reported AgNPs-mediated apoptosis is independent of p53 [Citation14,Citation15]. But, as shown in our study, AgClNPs increase the expression level of p53 in the cancer cells by a 1.5-fold. But it remains to be determined whether AgClNPs directly induce DNA damage in the S-phase, which causes a cell arrest, or whether AgClNPs interfere with the replication, causing a collapse in the replication forks which detected as DNA damage. These data suggest that the anticancer activity of AgClNPs occurs through multiple pathways. Nonetheless, it seems AgClNPs act mainly through p53 gene overexpression. In essence, activation of p53 causes an increase in Bax/Bcl-2 ratio and activates intracellular caspases which promote DNA damage and ultimately apoptosis induction.
Conclusion
We demonstrated the anticancer activity of AgClNPs, as well as the mechanism of action on human breast cancer cells. Moreover, the formation of silver chloride nanoparticles by the cell-free supernatant of E.coli is interesting because of precise control on the size and shape of nanoparticles, simple purification steps, and lower cost production. Our study showed that synthesized AgClNPs decrease cell viability of human breast cancer cells in a dose-dependent manner. Data obtained from qRT-PCR, flow cytometry, and caspase activity assays revealed the cytotoxicity occurred through apoptosis induction rather than necrosis. Taken together, our study elucidates that AgClNPs could be considered as an anticancer candidate in human breast cancer for further investigations.
Acknowledgements
Authors deeply thank Javid Biotechnology Institute for their technical support.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Bhattacharyya S, Kudgus RA, Bhattacharya R, et al. Inorganic nanoparticles in cancer therapy. Pharm Res. 2011;28:237–259.
- Boisselier E, Astruc D. Gold nanoparticles in nanomedicine: preparation, imaging, diagnostics, therapies and toxicit. Chem Soc Rev. 2009;38:1759–1782.
- Asgary V, Shoari A, Afshar Moayad M, et al. Evaluation of G2 citric acid-based dendrimer as an adjuvant in veterinary rabies vaccine. Viral Immunol. 2018;31:47–54.
- Sadat Shandiz SA, Shafiee Ardestani M, Shahbazzadeh D, et al. Novel imatinib-loaded silver nanoparticles for enhanced apoptosis of human breast cancer MCF-7 cells. Artif Cells Nanomed Biotechnol. 2017;45:1–10.
- Baskar G, Chandhuru J, Sheraz Fahad K, et al. Anticancer activity of fungal L-asparaginase conjugated with zinc oxide nanoparticles. J Mater Sci Mater Med. 2015;26:5380.
- Kim Y, Lee BG, Roh Y. Microbial synthesis of silver nanoparticles. J Nanosci Nanotechnol. 2013;13:3897–3900.
- Narayanan KB1, Sakthivel N. Biological synthesis of metal nanoparticles by microbes. Adv Colloid Interface Sci. 2010;156:1–13.
- Pereira L, Mehboob F, Stams AJ, et al. Metallic nanoparticles: microbial synthesis and unique properties for biotechnological applications, bioavailability and biotransformation. Crit Rev Biotechnol. 2015;35:114–128.
- Shivaji S, Madhu S, Singh S. Extracellular synthesis of antibacterial silver nanoparticles using psychrophilic bacteria. Proc Biochem. 2011;46:1800–1807.
- Das VL, Thomas R, Varghese RT, et al. Extracellular synthesis of silver nanoparticles by the Bacillus strain CS 11 isolated from industrialized area. 3 Biotech. 2014;4:121–126.
- Deljou A, Goudarzi S. Green extracellular synthesis of the silver nanoparticles using thermophilic Bacillus sp. AZ1 and its Antimicrobial Activity against Several Human pathogenetic bacteria. Iran J Biotechnol. 2016;14:25–32.
- Zhang X, Liu Z, Shen W, et al. Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. IJMS. 2016;17:1534.
- Zhang XF, Liu ZG, Shen W, et al. Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int J Mol Sci. 2016;17:1534.
- Tang S, Zheng J. Antibacterial activity of silver nanoparticles: structural effects. Adv Healthcare Mater. 2018;7:1701503.
- Qing Y, Cheng L, Li R, et al. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int J Nanomed. 2018;13:3311–3327.
- Ahluwalia V, Kumar J, Sisodia R, et al. 2014;Green syntPotential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologieshesis of silver nanoparticles by Trichoderma harzianum and their bio-efficacy evaluation against Staphylococcus aureus and Klebsiella pneumonia Vivek. Ind Crop Prod. 55:202–206.
- Asgary V, Shoari A, Baghbani-Arani F, et al. Green synthesis and evaluation of silver nanoparticles as adjuvant in rabies veterinary vaccine. IJN. 2016;11:3597–3605.
- Hanan NA, Chiu HI, Ramachandran MR, et al. Cytotoxicity of plant-mediated synthesis of metallic nanoparticles: a systematic review. Int J Mol Sci. 2018;19:1725.
- De Matteis V, Cascione M, Toma CC, et al. Silver nanoparticles: synthetic routes, in vitro toxicity and theranostic applications for cancer disease. Nanomaterials (Basel) 2018;8:319.
- Sriram MI, Kanth SB, Kalishwaralal K, et al. Antitumour activity of silver nanoparticles in Dalton’s lymphoma ascites tumour model. Int J Nanomedicine. 2010;5:753–762.
- Abbasi AR, Morsali A. Synthesis and characterization of AgCl nanoparticles under various solvents by ultrasound method. J Inorg Organomet Polym Mater. 2013;23:286–292.
- Kim S, Chung H, Kwon JH, et al. Facile synthesis of silver chloride nanocubes and their derivatives. Bull Korean Chem. 2010;31:2918–2922.
- Reddy V, Currao A, Calzaferri G. Zeolite A and zeolite L monolayers modified with AgCl as photocatalyst for water oxidation to O2. J Mater Chem. 2007;17:3603–3609.
- Siddiqi KS, Rahman R, Tajuddin. Formation of Ag/AgCl nanoparticles. J Nanomed Res. 2017;5:00108.
- Paulkumar K, Rajeshkumar S, Gnanajobitha G, et al. Biosynthesis of silver chloride nanoparticles using Bacillus subtilis MTCC 3053 and assessment of its antifungal activity. ISRN Nanomaterials 2013;2013:1.