Abstract
Osteoarthritis (OA) is a common joint disease for which a safe and reliable treatment has yet to be developed. Here, we demonstrated the potential benefit of treatment with paeonol, a derivative of Paeonia suffruticosa, in the treatment and prevention of OA. Chondrogenic cell line ATDC5 cells were cultured with IL-1β and the effects of paeonol were assessed through qRT-PCR, western blot analysis, MTT, ELISA, and NF-κB luciferase reporter gene assay. Our findings demonstrate a novel ability of paeonol to inhibit numerous factors of OA, including expressions of IL-6, TNF-α, NOX2, PTGS2, NUCB2/nesfatin-1, ICAM-1, VCAM-1, MMP-3/13, degradation of type II collagen, and NF-κB activation through the rescue of IκBα. Additionally, we assessed the effects of paeonol on cell viability to confirm its safety. These findings implicate a valuable potential role of paeonol in the treatment and prevention of OA.
Introduction
Although osteoarthritis (OA) is a common debilitating disease worldwide, the pathophysiology of OA remains poorly understood. Overexpression of cytokines such as IL-1β plays a key role in the pathogenesis of OA by upregulating expression of degradative enzymes and proinflammatory cytokines, among other things [Citation1]. One of the major hallmarks of OA is excessive loss of extracellular matrix (ECM) in the articular tissues, including type II collagen, which provides cartilage tissue with structural rigidity, thereby allowing joints to withstand heavy mechanical loading. It is degraded by matrix metalloproteinases (MMPs), which are upregulated in OA chondrocytes [Citation2,Citation3]. MMP-3 and MMP-13 are shown to induce degradation of type II collagen [Citation4]. Additionally, MMP-3 induces expression of various pro-MMPs, including pro-MMP-13 [Citation5]. MMP-13 is reported to specifically cleave type II collagen, making it a valuable target for therapies against cartilage degradation [Citation6].
Another major factor driving the development and progression of OA is oxidative stress. The NADPH oxidase isoform NOX2 has been shown to induce the generation of ROS upon incubation with IL-1β. While the role of NOX2 in OA has not yet been fully elucidated, a recent study using human chondrogenic cell line ATDC5 cells showed that NOX2 may be involved in the loss of articular ECM by the activation of hyaluronidase through intra- and extra-cellular acidification [Citation7]. Prostaglandin-endoperoxide synthase 2 (PTGS2) is an isoenzyme produced during inflammation that has been shown to regulate prostaglandin biosynthesis and pain response in OA [Citation8]. Inhibition of PTGS2 has been considered as a potential method for managing pain and inflammation in OA [Citation9]. The adipokine nucleobindin 2 (NUCB2/nesfatin-1) is a calcium-binding protein that regulates calcium homoeostasis and inflammation by inducing IL-6 and IL-8 when administered in combination with IL-1 in ATDC5 chondrocytes [Citation10]. As a recent discovery in chondrocytes, there exists very little information on the function of NUCB2/nesfatin-1 in OA.
Numerous investigations have revealed the participation of proinflammatory cytokines in the pathological development of OA by driving expression of MMPs and degradative enzymes [Citation11–14]. Two vascular adhesion molecules, ICAM1 and VCAM1, are important for promoting the inflammation in OA synovial tissue by regulating the recruitment of leukocytes through the vascular wall and into inflamed synovial tissue, where they interact with other cell types [Citation15]. Increased expression of ICAM1 and VCAM1 have been shown to be correlated with the development of knee OA [Citation16,Citation17]. Additionally, ICAM1 and VCAM1 have been associated with the senescence-associated secretory phenotype (SASP), which causes chondrocytes to lose their normal functionality and instead promote inflammation and expression of cytokines, chemokines, and MMPs, thus perpetuating the inflammatory response and driving degradation of ECM [Citation18,Citation19]. One of the most important factors in OA is the NF-κB signalling pathway. Activation of NF-κB governs the expression of numerous genes in joint destruction, inflammation and homoeostasis. NF-κB activation is triggered by cytokines and chemokines, activation of other signalling pathways, and byproducts of cartilage degradation. [Citation20,Citation21]. Phosphorylation of IκBα induced by IL-1β triggers translocation of the dimers of NF-κB, including p65 protein, to the nucleus where activation of NF-κB transcription occurs [Citation22,Citation23].
Paeonol is the active compound found in Paeonia lactiflora Pallas, Cynanchum paniculatum, and Paeonia suffruticosa Andr and was recently shown to have anti-inflammatory effects in a rheumatoid arthritis model using human fibroblast-like synoviocytes stimulated with IL-1β [Citation24]. Here, we examined the roles of treatment with paeonol on IL-1β-induced expression of cytokines, chemokines, adhesion molecules, ECM degradation and activation of IκBα/NF-κB in chondrogenic cell line ATDC5 cells. Our findings demonstrate that paeonol has a significant protective role against these factors of OA, thereby suggesting the potential value of paeonol in OA treatment.
Materials and methods
Cell cultures and treatments
ATDC5 cells were cultured in DMEM with 10% FBS and 1% P/S and allowed to differentiate into mature chondrocytes [Citation25]. Paeonol was obtained from the National Institute for Food and Drug Control (Beijing, China). Cells were stimulated with IL-1β (10 ng/mL) with or without paeonol (50, 100 μM) for 24 h.
Quantitative real time PCR
RNA from ATDC5 cells was extracted using Qiazol (Qiagen, USA). The isolated RNA (2 μg) was reverse-transcribed into cDNA. Expression of target genes was determined using real-time PCR with an SYBR Green PCR Master Mix. The 2−ΔΔCt approach was applied to calculate the level of detected genes, using GAPDH as an internal control [Citation26].
Western blot analysis
Proteins in ATDC5 cells were isolated from using RIPA buffer with protease inhibitors. Nuclear protein from ATDC5 cells was extracted using a Nuclear/Cytosol Fractionation Kit (Biovision Incorporated, USA). Total protein or nuclear protein (20 μg) was then separated on 8–12% Bis-Tris Precast Gels (Bio-Rad, USA) by electrophoresis. Proteins were then transferred onto nitrocellulose membranes. After blocking with 5% milk, membranes were probed with the following primary antibodies: type II collagen (1:2000, CAT# 14695–1-AP, Proteintech, Chicago, USA), p-IκBα (1:1000, Cat# sc-8404, Santa Cruz Biotechnology, Santa Cruz, USA), IκBα (1:1000, Cat#9242, Cell Signaling Technology, Danvers, USA), lamin B1 antibody (1:3000, CAT#sc-377000, Santa Cruz Biotechnology, Santa Cruz, USA), β-actin antibody (1:5000, CAT# sc-10731, Santa Cruz Biotechnology, Santa Cruz, USA) overnight at 4 °C. and appropriate secondary antibodies. Bands were then detected using enhanced chemiluminescence [Citation27].
Measurement of cell viability
Viability of ATDC5 cells was measured using 3–(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT). Briefly, MTT (5 mg/mL) was put into each well of cell culture. After incubation at 37 °C for 4 h, the product formazan was dissolved in DMSO and OD values recorded at 570 nm were used to index the viability percentage [Citation28].
ELISA assay
ATDC5 cells were cultured with IL-1β (10 ng/mL) with or without paeonol (50, 100 μM) for 24 h. Secretions of TNF-α, IL-6 and intracellular levels of ICAM-1, VCAM-1, MMP-3 and MMP-13 were examined using ELISA kits purchased from R&D Systems (Minneapolis, USA).
NF-κB luciferase reporter gene assay
The activity of NF-κB was examined using an NF-κB luciferase reporter gene assay. Briefly, NF-κB promoter-luciferase and β-galactosidase plasmids (Clontech, USA) were transfected into ATDC5 cells [Citation29]. 24 h later, stimulated with IL-1β (10 ng/mL) with or without paeonol (50, 100 μM) for 24 h. Cells were then lysed and centrifuged at 15,000 × g. Supernatants were then harvested and used to determine luciferase and β-galactosidase activity (Thermo Fisher Scientific, USA) on a luminometer. Luciferase activity of NF-κB in ATDC5 cells was normalized to β-galactosidase activity.
Statistical analysis
The results are demonstrated as means ± SEM. Comparisons between different groups were made using analysis of variance (ANOVA) method. P values < .05 was considered of statistical significance.
Results
Determination of cell viability
The molecular structure of paeonol is shown in . To measure the effects of paeonol on cell viability, human chondrocytic cell line ATDC5 cells were stimulated with 0, 0.1, 1, 10, 50, 100 and 500 μM paeonol for 48 h. As shown in , the dose of 100 µM has a slight effect on cell viability, but only the dose of 500 µM paeonol elicited a significant decline in cell viability, thereby indicating that lower concentrations of paeonol are safe. Therefore, we chose the highest concentrations considered to be safe, 50 and 100 µM, for the following experiments.
Figure 1. The effects of paeonol on cell viability in the chondrogenic cell line ATDC5 cells. (A) Molecular structure of paeonol, 2′-hydroxy-4′-methoxyacetophenone. (B) ATDC5 cells were stimulated with 0, 0.1, 1, 10, 50, 100 and 500 μM paeonol for 48 h. Viability of ATDC5 cells was measured by MTT assay. Note: Since 500 μM paeonol reduced cell viability, doses of 50 and 100 μM paeonol were used in the following experiments (*, P < .01 vs. vehicle control).
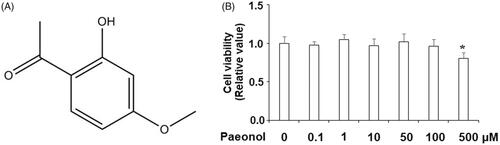
Figure 2. Paeonol reduces inflammatory mediators in chondrogenic cell line ATDC5 cells. ATDC5 cells were incubated with 10 ng/mL IL-1β with or without paeonol (50, 100 μM) for 24 h. (A) mRNA of NOX2 synthase; (B) mRNA of PTGS2; (C) mRNA of NUCB2/nesfatin-1 (*, #, $, P < .01 vs. previous control group).
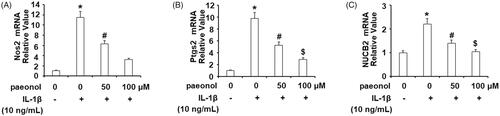
Paeonol reduces expression of NOX2, PTGS2 and NUCB/nesfatin-1
Next, we examined the effects of paeonol on factors of oxidative stress in ATDC5 chondrocytes. As shown in , NOX2 is barely expressed by ATDC5 cells at baseline, but IL-1β increased expression of NOX2 by roughly 11-fold. However, IL-1β-induced upregulation of NOX2 was prevented by paeonol (50, 100 µM), with 100 µM paeonol reducing NOX2 expression to approximately 3-fold. Similarly, treatment with IL-1β increased expression of PTGS2 by roughly 10-fold, which was reduced by paeonol (). Next, we assessed the effect of paeonol treatment on IL-1β-induced upregulation of NUCB2/nesfatin-1. As shown in , exposure to 10 ng/mL IL-1β nearly doubled mRNA expression of NUCB2/nesfatin-1, which was reduced by paeonol. Notably, the dose of 100 µM paeonol returned NUCB2/nesfatin-1 expression to almost baseline levels.
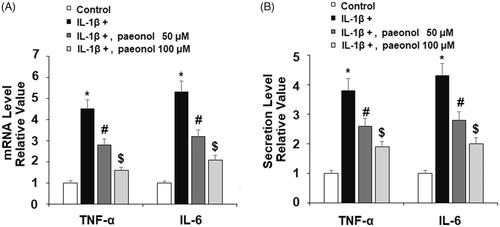
Paeonol inhibits expression of proinflammatory cytokines
Proinflammatory cytokines play a vital role in the pathogenesis of OA. Thus, we investigated the effects of paeonol on IL-1β-induced IL-6 and TNF-α in ATDC5 chondrocytes. Briefly, cells were exposed to insult from IL-1β for 48 h with or without 50 and 100 µM paeonol. As shown in , exposure to IL-1β increased TNF-α and IL-6 at the gene level by about 4.5- and 5.7-fold, respectively. However, paeonol reduced expression of these cytokines, with 100 µM paeonol decreasing TNF-α and IL-6 to only 1.6 and 2.1-fold, respectively. We further confirmed these effects of paeonol using ELISA analysis. As shown in , treatment with paeonol significantly reduced IL-1β-induced TNF-α and IL-6, reducing the expression of both cytokines to roughly 2-fold basal levels. These findings implicate a strong anti-inflammatory property in chondrocytes treated with paeonol, suggesting that paeonol may be of use in the treatment of OA.
Figure 3. Paeonol inhibits the expression and secretion of pro-inflammatory cytokines in chondrogenic cell line ATDC5 cells. ATDC5 cells were incubated with 10 ng/mL IL-1β with or without paeonol (50, 100 μM) for 24 h. (A) mRNA levels of TNF-α and IL-6; (B) Secretions of TNF-α and IL-6 (*, #, $, P < .01 vs. previous control group).
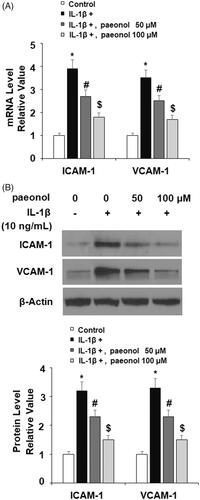
Paeonol attenuates expression of ICAM-1 and VCAM-1
Expressions of the adhesion molecules ICAM1 and VCAM1 have been shown to be elevated in OA and high levels of these molecules are associated with greater risk of knee or hip replacement [Citation17,Citation30]. To assess the effect of treatment with paeonol on IL-1β-induced expression of cellular adhesion molecules, ATDC5 chondrocytes were exposed to insult from IL-1β with or without 50 and 100 µM paeonol for 24 h. As shown in , IL-1β induced an about 3-fold increase in expression of both ICAM1 and VCAM1, which was attenuated by paeonol. The dose of 100 µM significantly lowered the mRNA levels of the two cytokines to less than 2-fold baseline. Concordantly, western blot analysis revealed that IL-1β led to a more than 3-fold increase in protein expression, which was lowered to roughly 1.5-fold baseline by the higher dose of paeonol.
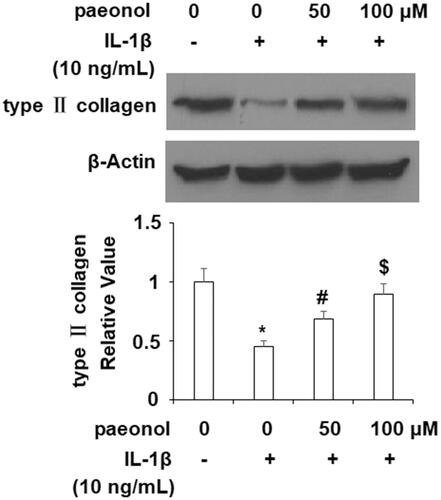
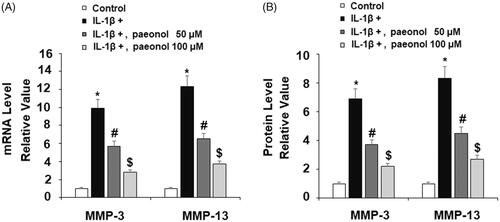
Paeonol reduces expression of MMP-3/13 and reduction of type II collagen
Expression of MMPs degrades type II collagen. To investigate the effect of paeonol on the expression of MMPs, ATDC5 cells were cultured with IL-1β (10 ng/mL) with or without paeonol (50, 100 µM) for 24 h. The results show that insult from IL-1β gave rise to approximately 10- and 12-fold increases in MMP-3 and MMP-13, respectively, at the gene level, which were reduced to 3- and 4-fold basal levels by paeonol (). Similarly, as revealed by the results of ELISA in , IL-1β increased MMP-3 and MMP-13 to 7- and 8-fold basal levels, which was reduced by paeonol to 2- and 3-fold basal levels (). Importantly, an insult from IL-1β obviously induced reduction of type II collagen, which was rescued to near basal levels by paeonol (). These findings suggest a new biological function of paeonol in preventing loss of type II collagen by suppressing MMP-3 and MMP-13.
Figure 5. Paeonol suppresses the expression of matrix metalloproteinases in chondrogenic cell line ATDC5 cells. ATDC5 cells were incubated with 10 ng/mL IL-1β with or without paeonol (50, 100 μM) for 24 h. (A) mRNA levels of MMP-3 and MMP-13; (B) Elisa results of MMP-3 and MMP-13 (*, #, $, P < .01 vs. previous control group).
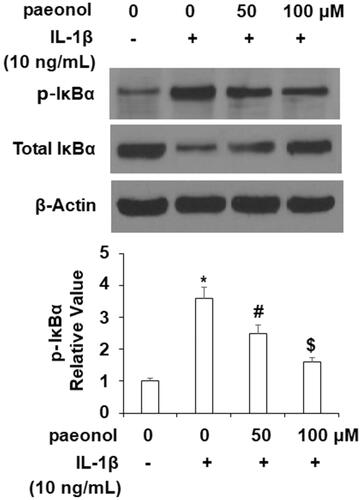
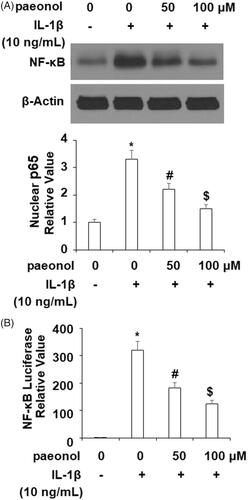
Inhibits phosphorylation of IκBα and activation of NF-κB
Activation of the NF-κB pathway is strongly correlated with the inflammatory response in OA. To elucidate the effects of paeonol on this pathway, we assessed the phosphorylation of IκBα and activity of NF-κB. As demonstrated in , an insult from IL-1β (10 ng/mL) increased phosphorylation of IκBα by more than 3.5-fold, which was decreased by paeonol to about 1.75-fold basal levels. Finally, we assessed the influence of paeonol on nuclear levels of p65 and subsequent luciferase activity of NF-κB. As shown in , there was a remarkable increase in nuclear p65 and luciferase activity of NF-κB by IL-1β, which was reduced by paeonol.
Discussion
OA is a disease characterized by excessive degradation of cartilage, chronic inflammation, infiltration of synovial tissue by immune cells, and eventual irreversible joint destruction. In the pathophysiology of OA, proinflammatory cytokines and cellular signalling pathways contribute greatly to disease progression and to sustaining a mild chronic inflammatory state [Citation31]. Therefore, inhibition of the release of proinflammatory cytokines and blockage of cellular signalling pathways is regarded as an attractive option for the management and treatment of OA. Paeonol is a commonly used compound in traditional Chinese medicine (TCM) and has been demonstrated to have antimicrobial, anticancer, antioxidant, analgesic, antipyretic and anti-inflammatory activities [Citation24,Citation32,Citation33]. However, little is known regarding the potential of this natural therapeutic agent until recently. Here, we used human chondrogenic cell line ATDC5 cells cultured with IL-1β to explore the potential effects of paeonol treatment in OA. We confirmed the safety of treatment with paeonol by performing a cell viability assay, which showed that doses under 500 µM had little to no effect on the cell viability of ATDC5 cells (). Additionally, our findings demonstrate that paeonol possesses anti-oxidative stress and anti-inflammatory effects by inhibiting expression of NOX2, PTGS2 and NUCB2/nesfatin-1 in ATDC5 chondrocytes, which have been suggested as a potential treatment targets for OA [Citation34–36]. This beneficial effect suggests a novel role of paeonol against the expression of chemokines and adipokines by OA chondrocytes.
Expression of cytokines, especially IL-1β, IL-6 and TNF-α, are often considered to be the main factors driving the pathogenesis of OA. Hence, blockade or inhibition of these is considered a valuable approach for the management of OA. In the present study, we used insult from IL-1β to simulate the condition of OA in ATDC5 chondrocytes as previously described [Citation37]. Our findings demonstrate a remarkable ability of paeonol to inhibit TNF-α and IL-6 induced by IL-1β. Another factor driving the pathology of OA is the recruitment of monocytes to the synovium by ICAM-1 and VCAM-1, resulting in chronic low-grade inflammation [Citation38]. Our findings show that paeonol significantly reduced ICAM-1 and VCAM-1 in ATDC5 cells. Thus, treatment with paeonol may be a safe therapeutic option for reducing the expression of these molecules in a myriad of diseases. To our knowledge, this study is the first to demonstrate the beneficial role of paeonol in inhibiting ICAM-1 and VCAM-1 in an OA chondrogenic cell model.
Perhaps the most prominent feature of OA, inhibition of impairment of the articular cartilage matrix is a primary treatment target. Here, we demonstrated the ability of paeonol to suppress MMP-3 and MMP-13 and further confirmed the ability of paeonol to prevent reduction of type II collagen, a primary component of articular cartilage, in chondrogenic ATDC5 cells. These findings agree with the results of a recent study demonstrating the ability of paeonol to inhibit expression of MMPs and loss of type II collagen in rabbit chondrocytes. Also in accordance with the findings of the same study, we reveal that paeonol treatment inhibited activation of NF-κB [Citation39]. Activation of NF-κB triggered by IκBα phosphorylation and subsequent nuclear translocation of p65 governs the expression of a massive array of inflammatory processes. Thus, blockade of NF-κB signalling is often cited as a valuable therapeutic target. Importantly, we found that treatment with paeonol could inhibit activation of NF-κB by significantly inhibiting IκBα and nuclear translocation of p65 protein induced by IL-1β.
In summary, the results of this study are among the first to demonstrate the potential of paeonol as an effective therapeutic agent against the pathogenesis of OA by virtue of its ability to downregulate expression of proinflammatory cytokines, chemokines, cellular adhesion molecules, degradative enzymes and to inhibit the IκBα/NF-κB signalling pathway.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- van Dalen SC, Schelbergen R, Sloëtjes A, et al. Locally administered adipose derived mesenchymal stem cells reinforce their anti-inflammatory effect through IL-1β mediated attraction of neutrophils into knee joints with experimental osteoarthritis. Osteoarthr Cartil. 2015;23:A379–A380.
- Huang CY, Lai KY, Hung LF, et al. Advanced glycation end products cause collagen II reduction by activating Janus kinase/signal transducer and activator of transcription 3 pathway in porcine chondrocytes. Rheumatology 2011;50:1379–1389.
- Kapoor M, Martel-Pelletier J, Lajeunesse D, et al. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33.
- Gudmann NS, Karsdal MA. Biochemistry of collagens, laminins and elastin. Waltham, MA: Academic Press; 2016. Chapter 2, Type II collagen; p. 13–20.
- Ma JD, Zhou JJ, Zheng DH, et al. Serum matrix metalloproteinase-3 as a noninvasive biomarker of histological synovitis for diagnosis of rheumatoid arthritis. Mediat Inflamm. 2014;2014:1.
- Chen YJ, Tsai KS, Chan DC, et al. Honokiol, a low molecular weight natural product, prevents inflammatory response and cartilage matrix degradation in human osteoarthritis chondrocytes. J Orthop Res. 2014;32:573–580.
- Funato S, Yasuhara R, Yoshimura K, et al. Extracellular matrix loss in chondrocytes after exposure to interleukin-1β in NADPH oxidase-dependent manner. Cell Tissue Res. 2017;368:135–144.
- Dearth CL, Slivka PF, Stewart SA, et al. Inhibition of COX1/2 alters the host response and reduces ECM scaffold mediated constructive tissue remodeling in a rodent model of skeletal muscle injury. Acta Biomaterialia. 2016;31:50–60.
- Arendt-Nielsen L, Egsgaard LL, Petersen KK. Evidence for a central mode of action for etoricoxib (COX-2 inhibitor) in patients with painful knee osteoarthritis. Pain 2016;157:1634–1644.
- Scotece M, Conde J, Abella V, et al. NUCB2/nesfatin‐1: a new adipokine expressed in human and murine chondrocytes with pro‐inflammatory properties, an in vitro study. J Orthop Res. 2014;32:653–660.
- Ji Q, Xu X, Zhang Q, et al. The IL-1β/AP-1/miR-30a/ADAMTS-5 axis regulates cartilage matrix degradation in human osteoarthritis. J Mol Med. 2016;94:771–785.
- Wang D, Qiao J, Zhao X, et al. Thymoquinone inhibits IL-1β-induced inflammation in human osteoarthritis chondrocytes by suppressing NF-κB and MAPKs signaling pathway. Inflammation 2015;38:2235–2241.
- Nummenmaa E, Hämäläinen M, Pemmari A, et al. TRPA1 as a factor and drug target in osteoarthritis: TRPA1 (transient receptor potential ankyrin 1) mediates interleukin-6 expression in chondrocytes. Osteoarthr Cartil. 2018;26:S124.
- Hu G, Zhao X, Wang C, et al. MicroRNA-145 attenuates TNF-α-driven cartilage matrix degradation in osteoarthritis via direct suppression of MKK4. Cell Death Dis. 2017;8:e3140.
- Schett G, Kiechl S, Bonora E, et al. Vascular cell adhesion molecule 1 as a predictor of severe osteoarthritis of the hip and knee joints. Arthritis Rheum. 2009;60:2381–2389.
- Hoeven TA, Kavousi M, Ikram MA, et al. Markers of atherosclerosis in relation to presence and progression of knee osteoarthritis: a population-based cohort study. Rheumatology 2015;54:1692–1698.
- Karatay S, Kiziltunc A, Yildirim K, et al. Effects of different hyaluronic acid products on synovial fluid levels of intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in knee osteoarthritis. Ann Clin Lab Sci. 2004;34:330–335.
- Kandhaya-Pillai R, Miro-Mur F, Alijotas-Reig J, et al. TNFα-senescence initiates a STAT-dependent positive feedback loop, leading to a sustained interferon signature, DNA damage, and cytokine secretion. Aging 2017;9:2411.
- Jeon OH, David N, Campisi J, et al. Senescent cells and osteoarthritis: a painful connection. The J Clin Investigat. 2018;128:1229–1237.
- Roman-Blas JA, Jimenez SA. NF-κB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthr Cartil. 2006;14:839–848.
- Olivotto E, Otero M, Marcu KB, et al. Pathophysiology of osteoarthritis: canonical NF-κB/IKKβ-dependent and kinase-independent effects of IKKα in cartilage degradation and chondrocyte differentiation. RMD Open. 2015;1:e000061.
- Mukherjee SP, Quintas PO, McNulty R, et al. Structural characterization of the ternary complex that mediates termination of NF-κB signaling by IκBα. Proc Nat Acad Sci. 2016;113:6212–6217.
- Rigoglou S, Papavassiliou AG. The NF-κB signalling pathway in osteoarthritis. Int J Biochem Cell Biol. 2013;45:2580–2584.
- Zhai KF, Duan H, Luo L, et al. Protective effects of paeonol on inflammatory response in IL-1β-induced human fibroblast-like synoviocytes and rheumatoid arthritis progression via modulating NF-κB pathway. Inflammopharmacology 2017;25:523–532.
- Gómez R, Lago F, Gómez‐Reino JJ., et al. Expression and modulation of ghrelin O‐acyltransferase in cultured chondrocytes. Arthritis Rheum. 2009;60:1704–1709.
- Vural AC, Odabas S, Korkusuz P, et al. Cranial bone regeneration via BMP-2 encoding mesenchymal stem cells. Artif Cells Nanomed Biotechnol. 2017;45:544–550.
- Gao F, Zhang S. Salicin inhibits AGE-induced degradation of type II collagen and aggrecan in human SW1353 chondrocytes: therapeutic potential in osteoarthritis. Artif Cells Nanomed Biotechnol. 2019;47:1043–1049.
- Ahangarpour A, Oroojan AA, Khorsandi L, et al. Antioxidant effect of myricitrin on hyperglycemia-induced oxidative stress in C2C12 cell. Cell Stress Chaperones. 2018; 23:773–781.
- Ma S, Bai Z, Wu H, et al. The DPP-4 inhibitor saxagliptin ameliorates ox-LDL-induced endothelial dysfunction by regulating AP-1 and NF-κB. Eur J Pharmacol. 2019;851:186–193.
- Benito MJ, Veale DJ, FitzGerald O, et al. Synovial tissue inflammation in early and late osteoarthritis. Annals of the Rheumatic Diseases. 2005;64:1263–1267.
- Robinson WH, Lepus CM, Wang Q, et al. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12:580.
- Yang SY, Du LD, Lu Y. Natural small molecule drugs from plants. Singapore: Springer; 2018. Paeonol; p. 439–444.
- Shakibaei M, John T, Schulze-Tanzil G, et al. Suppression of NF-κB activation by curcumin leads to inhibition of expression of cyclo-oxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: implications for the treatment of osteoarthritis. Biochem Pharmacol. 2007;73:1434–1445.
- Ehrich EW, Schnitzer TJ, McIlwain H, Rofecoxib Osteoarthritis Pilot Study Group, et al. Effect of specific COX-2 inhibition in osteoarthritis of the knee: a 6 week double blind, placebo controlled pilot study of rofecoxib. J Rheumatol. 1999;26:2438–2447.
- Cremers NAJ, Koenders MI, van de Loo FAJ, et al. The role of NOX2-derived reactive oxygen species in collagenase-induced osteoarthritis. Osteoarthr Cartil. 2018;30:1e11.
- Scotece M, Conde J, Lopez V, et al. FRI0029 Nucb2/nesfatin-1: a new adipokine expressed in human and murine chondrocytes with pro-inflammatory properties. Ann Rheum Dis. 2013;72:A377.
- Ji B, Guo W, Ma H, et al. Isoliquiritigenin suppresses IL-1β induced apoptosis and inflammation in chondrocyte-like ATDC5 cells by inhibiting NF-κB and exerts chondroprotective effects on a mouse model of anterior cruciate ligament transection. Int J Mol Med. 2017;40:1709–1718.
- Cremers N, Geven E, Blom A, et al. S100A8/A9 increases the mobilisation of LY6C high monocytes to the synovium during experimental osteoarthritis. Arthr Res Ther. 2017;19:217.
- Lou Y, Wang C, Tang Q, et al. Paeonol inhibits IL-1β-induced inflammation via PI3K/Akt/NF-κB pathways: in vivo and vitro studies. Inflammation 2017;40:1698–1706.