?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective
Our study aims to investigate the effect of arsenic trioxide (As2O3) on proliferation and apoptosis of Tca8113 tongue squamous carcinoma cells.
Methods
Cell proliferation and the expression of Id-1 mRNA in Tca8113 cells after treatment with different concentrations of As2O3 were detected by MTT and qRT-PCR, respectively. The expression of Id-1, cell proliferation and apoptosis in Id-1 silencing Tca8113 cells were detected by qRT-PCR, Western blot, MTT and flow cytometry, respectively. The pcDNA 3.1-Id-1 overexpression vector was transfected into Tca8113 cells combination with 3 μmol/L As2O3. The detection of cell proliferation, apoptosis and Caspase-3, Bax and Bcl-2 protein expression in transfected Tca8113 cells were performed by MTT, flow cytometry and Western blot assay, respectively.
Results
As2O3 of different concentration could inhibit the proliferation of Tca8113 cells and IC50 value was 3.004 ± 0.2379 μmol/L. The expression of Id-1 mRNA was down-regulated in Tca8113 cells treated with 3 μmol/L As2O3 for 48 h. The results of qRT-PCR, Western blot, MTT and flow cytometry indicated that the expression level of Id-1 and cell proliferation ability were decreased while the apoptosis rate was increased in Tca8113 cells after transfection of Id-1 siRNA. Overexpression of Id-1 could attenuate the inhibition or promotion of As2O3 on proliferation, apoptosis and Caspase-3, Bax and Bcl-2 protein expression in Tca8113 cells.
Conclusion
As2O3 could regulate the proliferation and apoptosis of Tca8113 cells by inhibiting the expression of Id-1.
Introduction
The overall incidence of malignant tumors is on the rise, seriously endangering the health of human. There were 4.29 million new cancer cases and 2.81 million deaths in China only in 2015. Among them, the number of oral cancer cases and deaths were 48,100 and 22,100, respectively [Citation1]. Tongue squamous cell carcinoma accounts for 43.4% of oral cancer. It is the most common oral cancer, with high lymph node metastasis rate and strong invasiveness [Citation2]. The incidence of tongue squamous cell carcinoma is related to age, sex and race. Smoking, alcohol abuse and poor oral hygiene can promote the occurrence of tongue squamous cell carcinoma. According to the survey, the incidence of tongue squamous cell carcinoma tends to be younger [Citation3,Citation4]. Surgical treatment, supplemented by radiotherapy, chemotherapy and other multidisciplinary treatment models are frequently used for the treatment of tongue squamous cell carcinoma. However, the tongue is special and slight carelessness seriously affects the patient’s speaking, swallowing ability and other functions and postoperative recurrence and metastasis limit the therapeutic effect. The 5-year survival rate of young patients with tongue squamous cell carcinoma is only 42% [Citation5]. Arsenic trioxide (As2O3) is one of the main components of traditional Chinese medicine arsenic, which has been widely used in the clinical treatment of acute promyelocytic leukemia [Citation6]. With continuous research, As2O3 has a certain effect on the treatment of a variety of solid tumors [Citation7]. In this paper, Tca8113 cells were used as the research object to observe the effect of As2O3 on Id-1 expression, cell proliferation and apoptosis.
Materials and methods
Materials
Tca8113 cell line was purchased from ATCC; fetal bovine serum (FBS) and RPMI1640 medium were purchased from Gibco-BRL, Gaithersburg, MD, USA; MTT was purchased from Sigma, St. Louis, MO, USA; Annexin V-FITC apoptosis detection kit was purchased from Coulter, USA; Lipofectamine 2000 transfection reagent was purchased from Invitrogen, Carlsbad, CA, USA; TaKaRa reverse transcription kit and TaKaRa real-time fluorescence quantitative kit were purchased from TaKaRa (Dalian, China) Co., Ltd.; Trizol reagent, PVDF membrane, RIPA lysate, ECL luminescent liquid were purchased from Beijing Solarbio Company (Solarbio, Beijing, China) ; Control siRNA and Id-1 siRNA were designed and synthesized by Shandong Vigene Biosciences Co., Ltd. (Biosciences, Shandong , China); pcDNA3.1-Id-1 overexpression vector was constructed by (Golden Rex Group Ltd, Zhongshan, China); Rabbit anti-human Bax polyclonal antibody was purchased from Shanghai XuanLing Biotechnology Co., Ltd. (XuanLing Biotechnology, Shanghai, China); Rabbit anti-human Bcl-2 polyclonal antibody, rabbit anti-human Caspase-3 polyclonal antibody, rabbit anti-human Id-1 polyclonal antibody, rabbit anti-human β-actin polyclonal antibody were purchased from Wuhan AmyJet Scientific Co., Ltd. (AmyJet Scientific, Wuhan, China); Horseradish peroxidase (HRP)-labeled secondary antibody was purchased from Wuhan Bohai Bioengineering Co., Ltd. (Bohai Bioengineering, Wuhan, China); MCO-18AC CO2 incubator was purchased from SANYO, Osaka, Japan; NanoDrop micronucleic acid analyzer was purchased from Shanghai Chuangmeng Biological Technology Co., Ltd. (Chuangmeng Biological Technology, Shanghai, China), Flow Cytometry was purchased from BD Company, San Jose, CA, USA, and Enzyme Label was purchased from Thermo Fisher Scientific, Rockford, IL, USA.
Cell culture and grouping
Tca8113 cells were cultured in RPMI 1640 medium containing 10% FBS at 37 °C, 5% CO2 incubator. The cells in the logarithmic growth phase were collected, seeded in a 24-well plate at 1 × 10 5 cells/well. After incubating for 24 h, fresh medium containing As2O3 with different concentration (0.25, 0.50, 1.00, 2.00, 4.00, 8.00, 16.00, 32.00 μmol/L) was added. The cells were cultured for another 48 h and labeled as 0.25 μmol/L group, 0.50 μmol/L group, 1.00 μmol/L group, 2.00 μmol/L group, 4.00 μmol/L group, 8.00 μmol/L group, and 16.00 μmol/L group and 32.00 μmol/L group. When Tca8113 cells were grown to a confluency of about 45%, the cells were transfected with siRNA and Id-1 siRNA and continuously cultured for 24 h according to the instructions of LipofectamineTM2000 transfection reagent. The cells were divided into si-con group and si-Id-1 group, respectively. After transfecting the cells with pcDNA 3.1 empty vector and pcDNA 3.1-Id-1 overexpression vector for 24 h, the culture medium was replaced by fresh medium containing 3 μmol/L As2O3 for 48 h, The cells were divided into As2O3+Ctrl group and As2O3+Id-1 group.
MTT assay was used to detect cell proliferation
Cells were cultured for 48 h with the control group, 0.25 μmol/L group, 0.50 μmol/L group, 1.00 μmol/L group, 2.00 μmol/L group, 4.00 μmol/L group, 8.00 μmol/L group, 16.00 μmol/L group, and 32.00 μmol/Group, respectively. When the cells were cultured for 24 h, 48 h, and 72 h, 20 μL MTT (5 mg/mL) was added and incubation was continued for 4 h. The superfluous medium was discarded and 150 μL of dimethyl sulfoxide (DMSO) was added to react for 10 min. The absorbance at 490 nm was measured by a microplate reader. Cell proliferation rate (%) = (experiment group OD value/control group OD value) × 100%.
qRT-PCR
Cells from the control group, As2O3 group, si-con group and si-Id-1 group were collected, fully lysed, and total RNA was extracted by Trizol method to detect RNA purity and concentration. The RNA was reverse transcribed into cDNA using the TaKaRa reverse transcription kit, and the reaction system was prepared according to the TaKaRa fluorescence quantification kit. The β-actin was used as an internal reference and was placed on an ABI-7300 real-time PCR instrument for amplification. The samples were repeated 3 times, and the experimental results were analyzed using the F = 2−ΔΔCt method. Id-1 primers: upstream: 5′-CTCCAACTGAAGGTCCCTGATGTAG-3′, downstream: 5′-ACGACATGAACGGCTGTTACTC AC-3′; β-actin primer: upstream: 5′-AGTGTGACGTGGACATCCGCAAAG-3′, downstream: 5′-ATCCACATCTGCTGGAAGGTGGAC-3′.
Western blot
Cells in control group, As2O3 group, si-con group, si-Id-1 group, As2O3+Ctrl group and As2O3+Id-1 group were ultrasonically pulverized, then RIPA lysate containing protease inhibitor was added and the cells were lysed on ice for 5 min, The supernatant was collected by centrifugation at 12,000 g for 15 min at 4 °C. The protein samples were electrophoresed on SDS-PAGE, transferred to PVDF membrane, and blocked with 5% skim milk powder for 2 h. Rabbit anti-human Bax polyclonal antibody (1:5000), rabbit anti-human Bcl-2 polyclonal antibody (1:1000), rabbit anti-human Caspase-3 polyclonal antibody (1:500), rabbit anti-human Id-1 polyclonal antibody (1:1000) and rabbit anti-human beta-actin polyclonal antibody (1:1000) were added respectively and incubated overnight at 4 °C. After rinsing with TBST for 3 times, HRP labeled secondary antibody was added for 2 h at room temperature. The samples were rinsed with TBST for 3 times, 10 min each time, ECL kit was developed by light, and each sample was repeated for three times.
Flow cytometry for apoptosis
The cells of the control group, As2O3 group, si-con group, si-Id-1 group, As2O3+Ctrl group and As2O3+Id-1 group were digested by trypsinase, washed with PBS for 3 times and collected. 5 μL Annexin V-FITC and 10 μL PI were added to each group according to the instructions of Annexin V-FITC/PI apoptosis detection kit, gently mixed, and incubated at room temperature for 15 min in the dark, and apoptosis was detected by flow cytometry.
Statistical analysis
The experimental data were expressed as ± s, and analyzed by SPSS 22.0 (SPSS, Inc., Chicago, IL,USA). The data were compared between the two groups by t-test. The data of multiple groups were compared by one-way analysis of variance. p < .05 was considered statistically significant.
Results
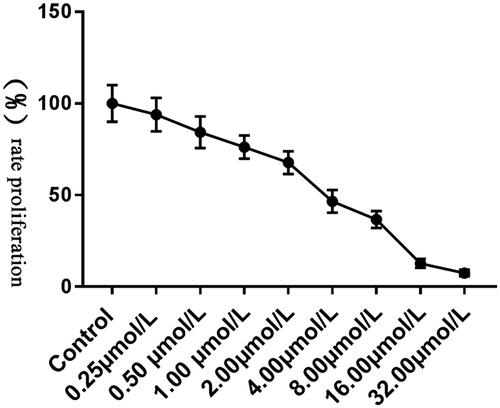
As2O3 inhibits Tca8113 cell proliferation
Tca8113 cells were treated with As2O3 of different concentration and cell proliferation was detected. The results () showed that As2O3 could inhibit Tca8113 cell proliferation. IC50 = 3.004 ± 0.2379 μmol/L and 3 μmol/L was used for subsequent experiments.
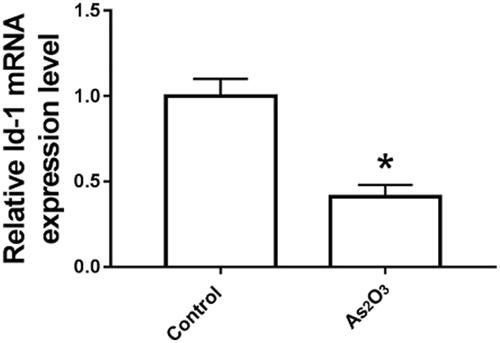
As2O3 inhibits Id-1 expression in Tca8113 cells
Tca8113 cells were treated with 3 mol/l As2O3 for 48 h, and Id-1 expression was detected by qRT-PCR. As shown in , the expression level of Id-1 mRNA in group As2O3 was significantly lower than that in the control group (p < .05).
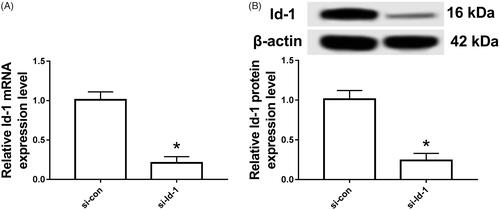
Detection of Id-1 gene silencing efficiency in Tca8113 cells
As displayed in , the expression of Id-1 mRNA and protein in Tca8113 cells transfected with Id-1 siRNA was significantly lower than that in si-con group (p < .05). The difference was statistically significant.
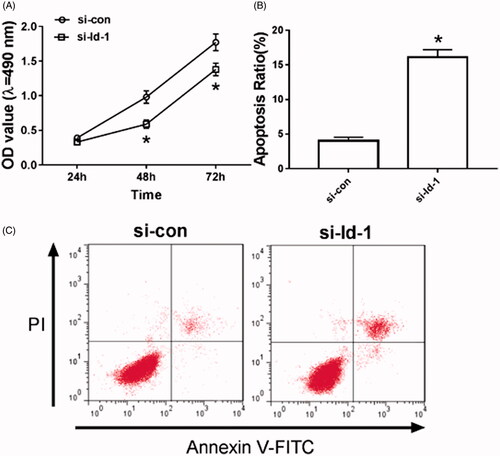
Silencing Id-1 inhibits proliferation and induces apoptosis in Tca8113 cells
As can be seen in , after Id-1 siRNA transfection, the cell proliferation ability was significantly decreased (p < .05), and the number of apoptotic cells was significantly increased in Tca8113 cells, (p < .05), and the difference was statistically significant.
Figure 4. Silencing Id-1 inhibits proliferation and induces apoptosis in Tca8113 cells. A: Silencing Id-1 inhibits the proliferation of Tca8113 cells; B: Statistics of apoptotic rate of Tca8113 cells after silencing Id-1; C: Detection of apoptotic rate of Tca8113 cells compared with si-con group. *p < .05.
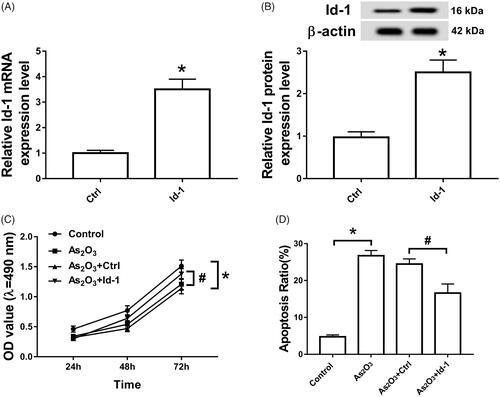
Overexpression of Id-1 attenuates the effect of As2O3 on proliferation and apoptosis of Tca8113 cells
As shown in , the intervention of 3 μmol/L As2O3 in Tca8113 cells inhibited cell proliferation and induced apoptosis (p < .05). However, the cell proliferation ability was restored (p < .05) and the apoptotic rate was reduced (p < .05) in Tca8113 cells transfected with Id-1 siRNA.
Figure 5. Overexpression of Id-1 partially restored the inhibition of As2O3 on proliferation and the promotion of apoptosis in Tca8113 cells. Note: A:qRT-PCR analyzed the expression of ld-1 mRNA of Tca8113 cells. B: Western blot determined the expression of ld-1 protein of Tca8113 cells. C: MTT assay determined the proliferation of Tca8113 cells. D:Cell apoptotic rate was detected using a flow cytometry of Tca8113 cells. *p < .05.
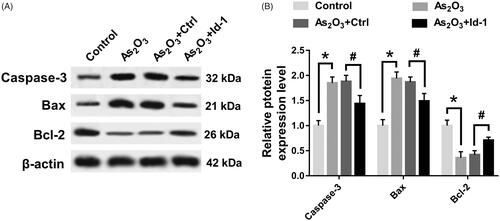
Id-1 ectopic expression partially restored the effect of As2O3 on the expression of apoptosis protein in Tca8113 cells
As can be seen in , As2O3 could inhibit the expression of Caspase-3 and Bax protein in Tca8113 cells (p < .05) and up-regulate the expression of Bcl-2 protein (p < .05). The expression of Caspase-3, Bax and Bcl-2 in Tca8113 cells were restored to a certain extent after Id-1 siRNA transfection.
Discussion
As2O3 is powdery, tasteless, soluble in acid, alkali, water, ethanol, etc. Once entering in the human body, it will show extremely strong toxicity and cause blood vessel and visceral damage, and seriously will lead to circulatory failure or even death. As2O3 was firstly used as a drug in the clinical treatment of tuberculosis and it has been confirmed by continuous research that As2O3 has an inhibitory effect on the growth and metastasis of various tumor cells. Clinical evidence indicates that [Citation8] the use of As2O3 in the treatment of multiple myeloma patients is of significant efficacy. It is reported that As2O3 could inhibit the growth and development of glioma cells [Citation9]. After treatment with As2O3, the survival rate of xenotransplantation model mice has been improved. In addition, As2O3 could regulate the biological processes such as apoptosis and proliferation of tumor cells. For example, Xia et al. [Citation10] found that As2O3 could induce apoptosis of breast cancer cells and inhibit cell growth by down-regulating the expression of Bcl-2 and nuclear factor-kappa B (NF-κB). Gao et al. [Citation11] found that As2O3 could regulate the cell cycle and stagnate the pancreatic cancer cell cycle in the G2/M phase, thereby effectively inhibiting cell proliferation and promoting apoptosis by regulating p53 expression; Moreover, Nakamura et al. [Citation12] analyzed the role of As2O3 in the pathogenesis of osteosarcoma and found that As2O3 could induce apoptosis by causing DNA damage and up-regulating the activity of caspase-3. However, there is a limited study of As2O3 on the pathogenesis of tongue squamous cell carcinoma. In this study, As2O3 was able to effectively inhibit the proliferation of Tca8113 cells by using As2O3 with different concentration to interfere with Tca8113 cells and detect cell proliferation. The IC50 was 3.004 ± 0.2379 μmol/L.
The transcription factor that regulates the activity of basic helix-loop-helix in the 11 groups of an inhibitor of DNA binding and/or differentiation (Id) was first cloned in 1990 [Citation13]. Mammals contain four Id factors, namely Id1, Id2, Id3, Id4, which are involved in the regulation of cell growth, differentiation, maturation, apoptosis, etc., and are abnormally expressed in tumors such as melanoma and pancreatic cancer [Citation14,Citation15]. A number of studies [Citation16,Citation17] have shown that the abnormal expression of Id-1 can be detected in prostate, ovarian and cervical cancer tissues, and its expression level is related to the tumor stage, disease grade, lymph node metastasis and survival rate of patients. Li et al. [Citation18] found that ectopic expression of Id-1 could regulate the cell cycle, promote cell proliferation and metastasis, while down-regulation of Id-1 expression had the opposite effect. Lai et al. [Citation19] used RNAi technology to knock down Id-1 expression in colorectal cancer cells. It was found that the cell proliferation ability decreased, the number of migration and invasion cells decreased significantly, and the expression of matrix metalloproteinase-2 decreased. It suggested that Id-1 could regulate cell proliferation and metastasis by regulating downstream target genes. Caspase-3, Bcl-2 and Bax proteins are key proteins in apoptosis, and BH1 and BH2 domains in Bcl-2 and Bax proteins can bind to form dimers, and Bcl-2/Bax homodimers are the main switch of apoptosis, when the expression of Bax in cells increases, the Bcl-2/Bax homologous dimer dissociates, promotes the release of cytochrome C, and then activates Caspase-3, initiates the caspase cascade reaction, promotes cell apoptosis. Evidence [Citation20] shows that Id-1 can inhibit Bax expression and up-regulate Bcl-2 expression in breast cancer cells, up-regulate Bcl-2 expression and reduce cell apoptosis. In this study, 3 μmol/L As2O3 treatment could inhibit the expression of Id-1, Caspase-3 and Bax in Tca8113 cells and promote the expression of Bcl-2. After silencing Id-1 by RNAi technology, the cell proliferation ability decreased and the number of apoptotic cells increased. Overexpression of Id-1 could attenuate the inhibition of As2O3 on the proliferation and apoptosis of Tca8113 cells to some extent.
In summary, As2O3 can effectively inhibit the proliferation of Tca8113 cells and induce apoptosis, accomplished by regulating the expression of apoptosis-related proteins and down-regulating Id-1 expression. The study is expected to provide an experimental basis for the clinical application of As2O3 in the treatment of tongue squamous cell carcinoma.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. Ca Cancer J Clin. 2016;66:115–132.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;60:277–300.
- Wade J, Smith H, Hankins M, et al. Conducting oral examinations for cancer in general practice: what are the barriers. Fam Pract. 2010;27:77–84.
- Patel SC, Carpenter WR, Tyree S, et al. Increasing incidence of oral tongue squamous cell carcinoma in young white women, age 18 to 44 years. JCO. 2011;29:1488–1494.
- Jeon JH, Min GK, Park JY, et al. Analysis of the outcome of young age tongue squamous cell carcinoma. Maxillofac Plast Reconstr Surg. 2017;39:41–48.
- Lo-Coco F, Avvisati G, Vignetti M, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369:111–121.
- Murgo AJ. Clinical trials of arsenic trioxide in hematologic and solid tumors: overview of the national cancer institute cooperative research and development studies. Oncologist. 2001;6:22–28.
- Hussein MA, Saleh M, Ravandi F, et al. Phase 2 study of arsenic trioxide in patients with relapsed or refractory multiple myeloma. Br J Haematol. 2004;125:470–476.
- Beauchamp EM, Ringer L, Bulut G, et al. Arsenic trioxide inhibits human cancer cell growth and tumor development in mice by blocking Hedgehog/GLI pathway. J Clin Invest. 2011;121:148–160.
- Xia J, Li Y, Yang Q, et al. Arsenic trioxide inhibits cell growth and induces apoptosis through inactivation of notch signaling pathway in breast cancer. IJMS. 2012;13:9627–9641.
- Gao JK, Wang LX, Long B, et al. Arsenic trioxide inhibits cell growth and invasion via down- regulation of skp2 in pancreatic cancer cells. Asian Pac J Cancer Prev. 2015;16:3805–3810.
- Nakamura S, Nagano S, Nagao H, et al. Arsenic trioxide prevents osteosarcoma growth by inhibition of gli transcription via DNA damage accumulation. PLoS One. 2013;8:e69466.
- Peng Y, Kang Q, Luo Q, et al. Inhibitor of DNA binding/differentiation helix-loop-helix proteins mediate bone morphogenetic protein-induced osteoblast differentiation of mesenchymal stem cells. J Biol Chem. 2004;279:32941–32949.
- Tajima K, Terai S, Takami T, et al. Importance of inhibitor of DNA binding/differentiation 2 in hepatic stellate cell differentiation and proliferation. Hepatol Res. 2007;37:647–655.
- Peretz Y, Wu H, Patel S, et al. Inhibitor of DNA binding 4 (ID4) is highly expressed in human melanoma tissues and may function to restrict normal differentiation of melanoma cells. Plos One. 2015;10:e0116839.
- Yu X, Xu X, Han B, et al. Inhibitor of DNA binding-1 overexpression in prostate cancer: relevance to tumor differentiation. Pathol Oncol Res. 2009;15:91–96.
- Kubelac MP, Fetica B, Vlad IC, et al. The role of inhibitor of DNA-binding 1 (ID-1) protein and angiogenesis in serous ovarian cancer. Anticancer Res. 2014;34:413–416.
- Li W, Zhang CH, Hong YL, et al. Inhibitor of DNA-binding-1/inhibitor of differentiation-1 (ID-1) is implicated in various aspects of gastric cancer cell biology. Mol Biol Rep. 2012;39:3009–3015.
- Lai X, Liao J, Lin W, et al. Inhibitor of DNA-binding protein 1 knockdown arrests the growth of colorectal cancer cells and suppresses hepatic metastasis in vivo. Oncol Rep. 2014;32:79–88.
- Kim H, Chung H, Kim HJ, et al. Id-1 regulates Bcl-2 and Bax expression through p53 and NF-kappaB in MCF-7 breast cancer cells. Breast Cancer Res Treat. 2008;112:287–296.