Abstract
Bionanotechnology has pivotal role in the development of a novel therapy, applications of gold nanoparticles (AuNPs) in the treatment of cancer. In this study, we found that therapeutics, pharmaceutics and diagnostic effectiveness of photosynthesized Catharanthus roseus (CR) AuNPs induces mitochondrial-mediated apoptotic signalling pathways via reactive oxygen species (ROS) induced cytotoxicity in cervical cancer cell line (HeLa) by in vitro model. The present examinations were for the most part centred around the gold chloride and photosynthesis AuNPs from the fluid leaf concentrate of CR and their harmful impacts on HeLa cell lines. The synthesized AuNPs were characterized using numerous biophysical analyses such as UV-vis, DLS, EDX, HR-TEM, SAED, FTIR and AFM. The synthesized AuNPs in the particle size range of 25–35 nm was confirmed by HR-TEM. The element gold and the crystalline nature of AuNPs were finalized using EDX, respectively. Anticancer potential of CR-AuNPs was studied using HeLa cells and the cytotoxic mechanism has been evaluated using MTT, mitochondrial-mediated apoptotic pathway through AO/EtBr staining assay, pro-apoptotic (Bax), anti-apoptotic (Bcl-2 and Bid) protein expression western blotting analysis and caspases activity using ELISA analysis. In in vitro study, the IC50 of HeLa cells was found to be 5 µg/ml confirmed using MTT assay. The present data revealed that drug delivery vehicles developed on CR-AuNPs nanocomplexes might include extensive purpose in human cancer diagnosis and treatment.
Introduction
Advanced cervical carcinoma, one of the most relative young women worldwide detected in over 70% of cases and most aggressive gynaecological diseases [Citation1]. Therefore, new advanced chemotherapies applications are very urgently needed this cancer. It remains a present most vital reason for malignant growth related passing for women in creating nations. The continual infection through extreme risk of human papillomavirus is major significant risk factor. Moreover, HPV positive cervical cancer cells have an inborn resistance to chemotherapy-induced programmed cell death and inhibition of cell proliferation [Citation2].
Nanoparticles (NPs) are lesser than 100 nm in dimension and the particles targeted drug therapies aim to shuttle drugs. In addition, the NPs target particular contaminated site so as to transport efficient concentrations of cancer therapeutic because they preferentially localize to cancer cell proliferation and easily enter tissue and molecular level [Citation3–5]. More than the earlier period few years, natural syntheses of NPs in advance growing energy due to their gorgeous physicochemical chattels and superior biocompatibility. Mostly, plant arbitrated synthesis of NPs has emerged as imperative branches of recent bio nanotechnology [Citation6]. To date, more than two hundred plants were monitored for that potent to making various metal NPs including gold [Citation7]. Unquestionably, the unique contacts between the nanoscale materials and cell lines can encourage the improvement of innovative and potent to more effectual action move towards diminishing the compound concentration and getting the better response of carcinoma cells [Citation8].
AuNPs are utilizing in imaging and analytical purposes. Since the improvement of ann innovative advance in the green synthesis of metal NPs is enormous significance, the requirement, an easy and secure system for the production of AuNPs [Citation9]. While cancer continues individual of the world's predominantly overwhelming diseases and in progress cancer remedy contains surgical involvement, chemotherapeutic drugs and radiation, frequently also toxic to normal cells and cause remedy through cancer patient. Novel therapeutic protocol must contain lower side effects for human cancer patients [Citation10]. AuNPs are widely scrutinized for genetic purpose and pharmaceutical applications fitting to their exclusive optical components and electrochemical stability [Citation11].
In drug curable tumour growth, apoptosis is a foremost mechanism linked with the induction of cancer remission [Citation12]. Thus, a drug that triggers apoptosis might accomplish significant efficacy in cancer treatment. Furthermore, timely observations of apoptosis know how to successfully help early identification of correlated diseases and simultaneous evaluation of the value of drugs. Consequently, developing sophisticated protocol for apoptosis examination has concerned more and more notice [Citation13].
Apoptosis evaluation is not only a hot topic for biologists and important takes for toxicology. Apoptotic section progress through different stages of programmed cell death. Carcinoma cells underwent cell death were explored as a targeted novel treatment for anticancer therapeutics were recently thought [Citation14]. Promptly accessible is an extensive history of anticancer medications anticipated from normally gotten therapeutic important plants can be produced increasingly solid cure against malignant growth.
CR, a medicinal plant, is famous to make secondary metabolites, which are derived compounds terpenoid indole alkaloids and broadly used the world-over in usual herbal medicine [Citation15]. Vincristine and vinblastine, the two major anticancer vinca alkaloids in chemotherapy regimens, are formed in this plant annually worldwide for treating different cancers [Citation15]. CR is helpful in traditional medicine not only for the treatment of several cancers but skin diseases, menorrhagia, diabetes, hypertension and cancer [Citation16]. Vinblastine to cure some kind of cancer and Hodgkin’s disease to treat leukaemia via oxidation process [Citation17].
Therefore, in the present study, biologically AuNPs was synthesized using the CR leaf extract and might have anticancer, therapeutic and preventive medicinal properties. On the basis, efforts are made to identify the therapeutic and preventive medicinal value of CR functionalized AuNPs. Then, the ROS mediated apoptotic effects of capped AuNPs with medicinal CR leaf extract were studied against human cervical cancer (HeLa) cells.
Methods
Chemicals
Antibodies against for B-cell lymphoma 2 (Bcl-2), Bcl-2-associated X protein (Bax), Bid, caspase-3 & -9 and HRP conjugated β-actin were procured from Santa Cruz Biotechnology. The animal cell culture Dulbecco’s modified eagle’s medium (DMEM), fetal bovine serum (FBS), trypsin-EDTA, antibiotics (streptomycin and penicillin), phosphate buffer saline (PBS), dimethyl sulfoxide (DMSO), 3–(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT), 2’,7’-dichlorofluorescein diacetate (DCFH-DA), acridine orange (AO), Ethiduim bromide (EtBr) and 4’,6-diamidino-2-phenylindole (DAPI) staining were obtained from the Sigma-Aldrich (USA). Chloroauric acid/gold chlorides (HAuCl4) were obtained for the Himedia (USA).
Collection of plant material
Preparation of plant extract and AuNPs synthesis
The collected leaf of CR was shaded dried for 15 days under the dark room, then converted into powder. Powdered leaf (1 gm) were subsequently mixed by 100 ml of distilled water in an Erlenmeyer flask then boiled at 50 °C for 20 min. After, suspension was sieved using Whatman paper no. 1 and set aside 4 °C for additional trial. AuNPs were described by following the method of Ghosh et al. [Citation18]. 1 ml of the leaf aqueous extract of CR with added the 9.0 ml of 1.0 mM of HAuCl4 independently. The reaction was executed in a static stipulation & dark room for 24 h and confirmed the colour of the property of synthesizing in AuNPs.
Characterization of CR-AuNPs
Spectroscopic analysis
The photosynthesizing bionanoparticles, CR leaf extract, HAuCl4 solutions were recorded as a function of absorbance by Ultraviolet-visible (UV-Vis) spectroscopy in the wavelength of using 300–700 nm.
Dynamic light scattering (DLS) analysis
The hydrodynamic size, zeta potential and polydispersity index of synthesized CR-AuNPs were evaluated through dynamic light scattering (DLS) measurements machine at 25 °C. All through this analysis, CR-AuNPs diluted samples was sighted in a folded capillary cell holder with platinum electrodes and loaded into the sample holder of the analyzer.
Energy dispersive X-ray (EDX) analysis
The obtained NPs were characterized using to EDX investigation were accompanied in directly on top of instrument connect by way of thermo EDX to prove the being thereof synthesized AuNPs various elemental structure of the illustration in CR leaf extract.
Microscopic analysis
High resolution transmission electron microscopy (HR-TEM) and selected area electron diffraction (SAED)
The different size, shape and morphological of synthesized AuNPs to the CR leaf extract was determined using HR-TEM. SAED pattern was obtained using the above instrument to measure the crystalline nature. The electron beam diffracted has produces, the crystalline symmetry, unit cell parameter and other microstructure characters like texture that were learned. It is based on the Bragg’s law, that the diffracted spots are recognized and proved the structure of the particles.
Fourier Transform Infrared Spectroscopy (FTIR) and Atomic Force Microscopy (AFM)
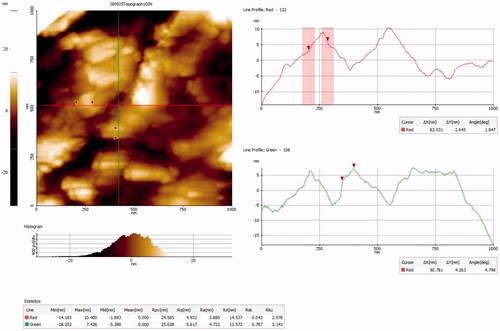
FTIR analysis was carried out to find functional groups and other surface chemical residues connected to the surface of CR-AuNPs, then the complete decline of the Au3+ ions by CR leaf extract; further samples were registered in an array of 500–4000 cm−1 at 4 cm−1 resolution. The height of the newly synthesized AuNPs was carried out using AFM. The AFM samples of CR-AuNPs were diluted with milli Q water and shifted on glass slide for ventilated by applying vacuum at 25 °C for 24 h. Diluted samples were then examined using AFM.
Cell culture maintenance
Human cervical cancer cell (HeLa) and 3T3 cell lines were obtained from Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai, China). HeLa cells were maintained in DMEM supplemented with FBS and antibiotics. Cells were maintained at 37 °C in a 5% CO2 atmosphere.
MTT assay
HeLa cell proliferation suppressed was obtained absorbance by viable cells and computed using the ELISA reader. In brief, cells were seeded into 96-well microtiter plates separately (5 × 103) and incubated at 37 °C for 24 h until growth to 90% confluence. HeLa cell lines with various concentrations of CR leaf extract and freshly synthesized AuNPs (1–15 µg/ml). After the incubation, the cells were washed with PBS, added a concentration of 5 mg/ml and 10 µl of MTT dye in each well and allowed in the incubate dark for 4 h. Then, 100 µl of DMSO added that decrystallize the purple colour formazone, and absorbance was read at 570 nm in microplate using ELISA reader.
Measurement of ROS
The measurement of intracellular ROS levels was based on the oxidative conversion of DCFH-D into highly fluorescent dicholorofluorescsin (DCF) [Citation19]. Briefly, CR-AuNP 5 and 10 µg/ml concentration with HeLa cells were loaded in 6 well plates (2 × 106 cells/well) with 10 µl of DCFH-DA for 30 min at 37 °C in the dark. Fluorescent was determined at 485/530 nm using spectrofluorometer.
AO/EtBr dual staining
The HeLa cells were plated at 1 × 106 cells/well and incubated for 24 h. After confluence, the HeLa cells were allowed with CR-AuNPs (5 and 10 µg/ml) for 24 h. From this nuclear analysis study, the monolayer of HeLa cells was stained with 1 mM acridine orange and ethidium bromide for 5 min. After incubation in the stained cells, morphological changes in the apoptotic nuclei, DNA damage and condensed chromatin were observed under fluorescence microscopy.
Caspases activity assay
The protease activity of caspase-3 and -9 in HeLa cells was carried out according to the orders of the manufacturer for the colourimetric assay kit (Promega, Madison, WI, USA). About 2 × 106 cells were treated with CR-AuNPs (5 and 10 μg/ml for 24 h), and the cells were rinsed three times using PBS for protein concentrations estimation and were quantified using the Bradford assay. Samples of total protein containing 200 μg were assayed for caspase-3 and -9 activities with DEVD-pNA as a caspase-3 specific substrate. Finally, the reaction samples were read at 405 nm in a microplate reader.
Western blotting
HeLa cells were seeded (1 × 105 cells/well) with CR-AuNPs in DMEM containing tissue culture plates and incubated for 24 h. Further cells lysed by the addition of cell lysis buffer and centrifuged at 1.3 × 104 rpm for 10 min. However, the 50 μg of two-part amounts of protein samples were separated using SDS-PAGE. These were afterwards transferred with a nitrocellulose transfer PVDF membrane. However, blocking the PVDF membrane with fresh 5% non-fat milk in TBST at 35 °C for 2 h, after incubation, the membrane was probed with primary antibodies Bcl-2, Bax and Bid and β-actin next to incubation with an appropriate secondary antibody conjugated to HRP. The immunoreactive bands were detected using enhanced chemiluminescence kit. The density of the immunoreactive bands was performed using IISP flatbed scanner and quantities with Total lab 1.11 software.
Statistical analysis
Statistical analysis was carried out using one-way analysis of variance (ANOVA), followed by DMRT using SPSS version 17.0 for windows. All values were represented as means ± SD. Values are represented as mean ± SD, and p < .05 were measured statistically significant.
Results and discussion
Analysis of CR-AuNPs
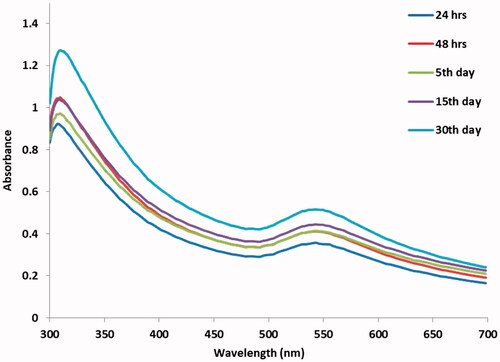
In the present findings, the green synthesis using a plant CR leaf extract as a novel reduces stable AuNPs showed the colour alteration of the test sample from light yellow to ruby red colour at 5 days. The newly synthesized CR-AuNPs surface Plasmon resonance band is centred at 540 nm different time period (). The similar results were in good agreement of ruby red colour for CR-AuNPs synthesis and also with a maximum absorbance at 540 nm [Citation20].
Analysis of DLS and EDX 0f CR-AuNPs
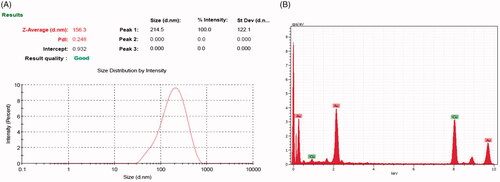
Particle size (10 nm and 55 nm) distribution scrutinization was carried out using DLS measurements. Synthesized CR-AuNPs average diameter was discovered to be 214.5 d.nm at 100% intensity while standard deviation 122.1 (). The synthesized CR-AuNPs particle size value obtaining in DLS technique is higher in comparison with HR-TEM microscopic techniques because of hydrodynamic radius probed with DLS. Tomoaia et al. [Citation21] stated that, during DLS dimensions studies, the DLS was assessed to be of greater size due to organic coating on synthesized AuNPs while in HR-TEM, only the particle size. The DLS pattern conforms some distribution CR-AuNPs at lower and higher range of element size.
The EDX spectra of the photosynthesized CR-AuNPs have been observed to determine the elemental composition of CR-AuNPs. The presence of O and C peaks were concluding that the CR-AuNPs were capped by photoconstituents by an oxygen atom. EDX spectrum unambiguously disclosed strong energy peaks for the signal assigned for CR-AuNPs are in the range of 2–4.5 keV of signals which synchronize with the preliminary detections made on Alfalfa spp and Artemisia nilagirica and also reported that the purity of the synthesized AuNPs by EDX [Citation22,Citation23]. The EDX of Mesembryanthemum edule – AuNPs is the evidence of gold atoms for a strong signal at 12.30 keV [Citation24].
Analysis of HR-TEM and SAED of CR-AuNPs
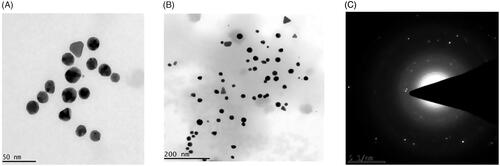
The particle size, distribution, shape and morphology of CR-AuNPs were categorized using HR-TEM. CR-AuNPs were on the whole sphere-shaped with an average particle size of 50 and 200 nm (). The SAED spectra proved so as to CR-AuNPs synthesized are crystalline. shows the SAED examination of the synthesized AuNPs. The SAED spots illustrate face centred cubic of different crystallographic planes. Philip et al. ([Citation25] accounted that the AuNPs synthesized from Murraya koenigii leaf were bringing homogeneous and that was spherical ∼10 nm and ∼20 nm, respectively. The high-resolution TEM descriptions of AuNPs synthesized in the current effort denoted that NPs were good crystalline form. These similar findings were correlated with our present findings.
Analysis of FTIR and AFM of CR-AuNPs
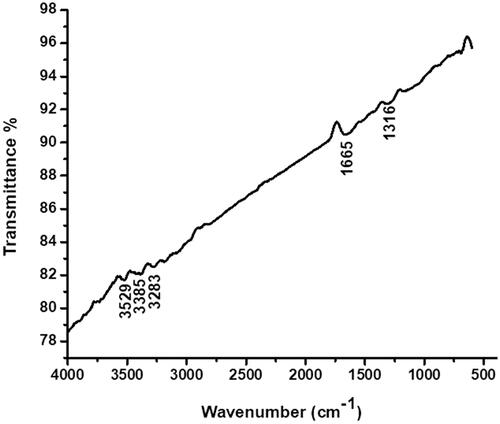
Attendance of biomaterials in CR leaf extract answerable for bioreduction and stabilization of CR-AuNPs was probed using FTIR analysis (). Furthermore, the three dimensional (3D) structures of synthesized CR-AuNPs was analyzed using AFM ().
Anti-proliferative activity of CR-AuNPs
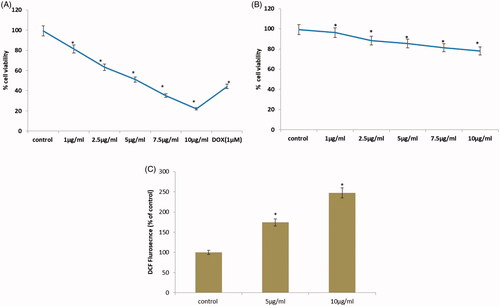
As shown in , the effect of cell proliferation on cervical cancer cells was determined by CR-AuNPs. We first investigated the potentiality of CR-AuNPs on cell viability using MTT evaluate, a convenient technique for calculating drug sensitivity. Proliferation of cancer HeLa cell line was significantly inhibited by (1–15 µg/ml) 24 h treatment with different concentration of CR-AuNPs. From these doses, the IC50 values of CR-AuNPs were (5 µg/ml) for HeLa cells and therefore used in further experiments. However, doses higher than 5 µg/ml of CR-AuNPs consequence in a decline in the cell number.
Figure 6. (A) Effect of CR-AuNPs on morphological characteristics and cell viability of HeLa cell lines at 24 h using MTT assay. Doxorubicin (Dox) is used as positive control. (B) cell viability of 3T3cell lines at 24 h using MTT assay. (C) CR-AuNPs induces ROS production in HeLa cells using DCFH-DA staining assay. HeLa cells were treated with CR-AuNPs at various concentrations (5 and 10 µg/ml) for 24 h. ROS measurement was carried out using spectrofluorimetry. Results in a different dose enhance in ROS production as shown through increased % DCF staining ratio. Values were accessible as mean + SD of three experiments in every group ANOVA followed by DMRT. Asterisks indicate statically different from control: *p < .05.
Measurement of intracellular ROS level in CR-AuNPs
Intracellular ROS changes are one of the major biochemical changes in cancer cells after the treatments with anticancer agents is in ROS generation, which is frequently enhanced considered as a cancer-promoting factor [Citation26]. Thus, to determine the generation of ROS level, HeLa cells were allowed for 24 h with various concentration (5 and 10 µg/ml) of CR-AuNPs, intended using DCFH-DA through spectrofluorimetry. Upon treating HeLa cells for 30 min with CR-AuNPs, ROS production was analyzed by dose-dependent manner (). The fluorescent intensity values of HeLa control cells were 100%, whereas the values change to 146 and 198, respectively, when cells allowed with different dosages of CR-AuNPs bring higher ROS production were higher in HeLa cells probably lead apoptotic cell death through the mitochondrial-mediated pathway. The effect of CR-AuNPs evidenced in A431 cells concentration-dependent increase of ROS. Similar results were found in our study.
Induction of apoptosis in HeLa cells dual stained with Ao/EtBr
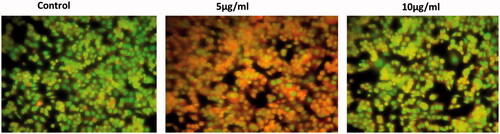
Apoptosis is a step dependent mechanism and is a genetically controlled cell death process. To detect and quantification of apoptosis and necrosis were comparatively studied by Ao/EtBr staining for fluorescent microscopy. This procedure interlinks with the various uptake of fluorescence DNA binding dye of Ao/EtBr. We evaluated cell death by assessing Ao/EtBr staining after 24 h of treatment with various concentration of CR-AuNPs (5 and 10 µg/ml) (). In HeLa cells, CR-AuNPs induced apoptosis of Ao/EtBr positive cells at a concentration of 5 µg/ml after 24 h, this % up-regulation and most of the cells were dual positive to Ao/EtBr staining illustrated morphological changes when compared to control HeLa cells. The control cells have shown homogeneous bright green nuclei and cytoplasm for AO stained positive cells and in EtBr stain uptake cells shows small number of positive cells, whereas the cells treated with CR-AuNPs explored characteristics modification apoptosis, i.e., cell shrinkage, nuclear condensation, fragmentation and formation of apoptosis bodies in Ao/EtBr staining. From this, our present study concluded that CR-AuNPs can stimulate cell death in HeLa cells via ROS mediated mitochondrial pathways leads apoptotic process, which is suggestive of their preventive and therapeutic drug efficacy [Citation27]. The benefits of the CR-AuNPs as potentially positive drug implicating cell death and inhibit progression.
Figure 7. Effect of CR-AuNPs induces apoptotic incidence. HeLa cells treated with control and CR-AuNPs at different concentrations (5 and 10 µg/ml) at 24 h, stained with AO/EtBr dye (40×). White arrow show green fluorescence; Orange arrow show apoptotic bodies; Blue arrow show apoptotic cells; Yellow arrow show necrotic cells.
Effects of CR-AuNPs of caspase-3 and -9 in HeLa cell line
Aberrant activation of cell proliferation signalling pathways was topic places in various types of carcinogenesis. Suppression of apoptosis induction may play a vital role in the carcinoma progression [Citation28]. Caspases mediated apoptotic pathways are targeted therapeutically mechanism inducing cervical cancer. Gupta et al. [Citation29] demonstrated that induced caspase-mediated apoptotic pathways in cancer cells may be characterized changes through cell shrinkage, nucleic chromatin, DNA fragmentation and several biochemical reactivities and also the commencement of death receptor pathways, mitochondrial pathways and/or caspase cascades and so on.
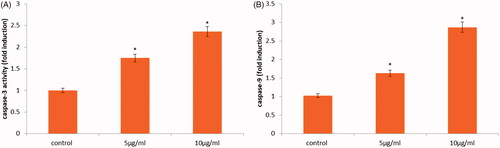
To corroborate it, caspases were the most important molecule monitor as shown in . To find out whether caspase establishment is involved in the CR-AuNPs induces cell death, the performance of the indicator caspase-9 and effectors caspase-3 was deliberated using ELISA assay. revealed that administration of 10 µg/ml CR-AuNPs significantly augmented the induction of caspase-3 and -9 in a various concentration manner, which recommended that dual induction of death receptor and mitochondria-mediated apoptotic pathways are concerned in the CR-AuNPs induced apoptosis. Ashe and Berry [Citation30] demonstrated that caspases are very critical for the execution of programmed cell death, which are synthesized as procaspases, after that proteolytically processed to their active forms. Caspases interacted with caspase-9 is the genetically of the apoptotic property. It is induction in the apoptotic cell both via intrinsic (mitochondrial) and extrinsic (death receptor) pathways and also death receptor pathway primarily activates procaspase-9 [Citation28]. In our finding, activation of caspase-9 initiator caspases such as caspase-3 (effectors caspases) might be observed in cancer HeLa cells treatment with CR-AuNPs. The results may suggest that CR-AuNPs activated apoptosis in HeLa cells via apoptotic mediated extrinsic and intrinsic pathway.
Effects of CR-AuNPs on Bcl-2, Bax and Bid in HeLa cells
After administration of cancer cells with CR-AuNPs for 24 h, the protein level of pro-apoptotic Bax, Bid and anti-apoptotic Bcl-2 protein was evaluated in control and treated through CR-AuNPs using western blotting. Increased expression of Bax and Bid proteins and down-regulation of Bcl-2 proteins was reported by CR-AuNPs in treated and control HeLa cells. Our results demonstrated that pro-apoptotic protein expression enhanced after treatment of CR-AuNPs this was accompanied by a concurrent diminish in anti-apoptotic proteins. Inactive precursor of Bid localized in the cytosolic region is a pro-apoptotic member from Bcl-2 family [Citation31] although caspase-8 to generate Bid. Bid translocation to the mitochondria stimulates cyt-C release, which leads to multifaceted with Apaf 1 and caspase-9. In addition, bid interacts by the way of both anti-apoptotic family member Bcl-2 and pro-apoptotic protein Bax. Our information provides a suggestion that CR-AuNPs treated HeLa cells increased Bid construction length with increased Bax and decreased Bcl-2 in cancer HeLa cells (). Taken together, the data can be further clarified that CR-AuNPs activated apoptosis in HeLa cells through mitochondrial pathways ().
Figure 9. CR-AuNPs induce apoptosis pathway on HeLa cells. (A) Protein expression of Bcl-2, Bax & Bid. (B–D) The representative graph shows the relative protein expression of fold changes in immunoblots. Values are expressed as mean ± SD for three experiments. *p < .05 compared to the control.
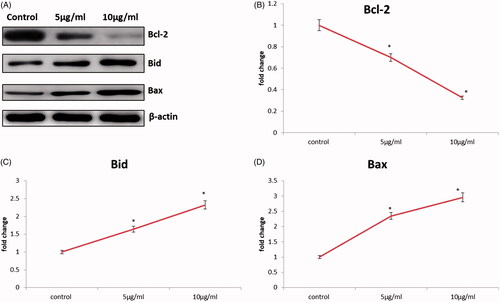
Figure 10. (A) Schematic representation of cellular modulation induced by CR-AuNPs in human cervical cancer (HeLa) cells. CR-AuNPs (5 and 10 µg/ml) induced in HeLa cells through ROS level in low and high increased in dose-dependent manner. Depending on ROS level generated by CR-AuNPs autophagy induction might be related with megakaryocytic disintegration and/or fragmentation in HeLa cells. Although high doses of CR-AnUPs (10 µg/ml) induced autophagy apoptosis, low dose (5 µg/ml) did not lead to any proliferation inhibitory or cytotoxic effects in HeLa cells. (B) The fundamental signalling pathway of CR-AuNPs induced apoptosis in cervical cancer HeLa cells.
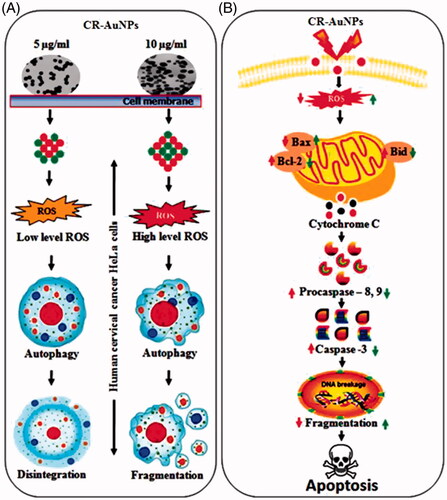
Caspase-3 is considered to be the central regulation of apoptosis while caspase-8 and caspase-9 act as indicators of the death receptor mediates cell apoptosis and mitochondrial apoptosis pathways. CR-AuNPs induce caspase reliant cell death in HeLa cells. Caspases are involved in cell apoptosis, some as mediators and some as effectors. Activation of caspase cascade can lead to cell apoptosis (). Pattarachotanant et al. [Citation16] demonstrated that stems of CR have potent to dose-dependent inhibit the keratin 17 expression phosphorylation of STAT-3 via JAK/STAT pathway in psoriasis and cancer. Kuriakose et al. [Citation32] reported that eaty pella spp or P14 CR based anti-proliferative on human squamous cell line – A431. Furthermore, they showed that fungal vincristine has been accomplished of apoptosis formation through ROS synthesis, and the regulation of the intrinsic pathway contributes to the loss of MMP. Similar results were found in our present study. CR medicinal plants produce important compounds such as vinca alkaloids, vinblastine and vincristine [Citation33]. These compounds treat some types of cancers such as breast cancer, testicular cancer, leukaemia and Hodgkin’s disease including cervical cancer cell lines.
In summary, biologically synthesized CR-AuNPs explored cytotoxicity, and apoptosis was increased in human cervical cancer (HeLa) cells. Exposure to CR-AuNPs for 24 h induced cleavage of caspase-3. These findings suggest that CR-AuNPs contributes to apoptotic cell death in human cervical cancer (HeLa) cells (). Therefore, this study offers further evidence on cytotoxicity as well as the anticancer efficacy increased ROS through induction of caspase-mediated apoptosis which warrants further cancer therapeutics and preventive potential in cervical cancer treatment.
Acknowledgements
I gratefully acknowledge the department of Biology, College of Science, Majmaah University, Kingdom of Saudi Arabia for providing the resources and platform to complete this research.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Catarino R, Petignat P, Dongui G, et al. Cervical cancer screening in developing countries at a crossroad: emerging technologies and policy choices. WJCO. 2015;6:281–290.
- Golemis EA, Scheet P, Beck TN, et al. Molecular mechanisms of the preventable causes of cancer in the United States. Genes Dev. 2018;32:868–902.
- Monge-Fuentes V, Muehlmann LA, de Azevedo RB. Perspectives on the application of nanotechnology in photodynamic therapy for the treatment of melanoma. Nano Rev. 2014;5:eCollection 2014.
- Vinardell MP, Mitjans M. Antitumor activities of metal oxide nanoparticles. Nanomaterials (Basel). 2015;5:1004–1021.
- Prabakar K, Sivalingam P, Rabeek SIM, et al. Evaluation of antibacterial efficacy of phyto fabricated silver nanoparticles using Mukia scabrella (Musumusukkai) against drug resistance nosocomial gram negative bacterial pathogens. Colloids Surf B Biointerfaces. 2013;104:282–288.
- Huang J, Lin L, Sun D, et al. Bio-inspired synthesis of metal nanomaterials and applications. Chem Soc Rev. 2015;44:6330–6374.
- Molnar Z, Bódai V, Szakacs G, et al. Green synthesis of gold nanoparticles by thermophilic filamentous fungi. Sci Rep. 2018;8:3943.
- Tang L, Yang X, Yin Q, et al. Investigating the optimal size of anticancer nanomedicine. Proc Natl Acad Sci USA. 2014;111:15344–15349.
- Baharara J, Ramezani T, Divsalar A, et al. Induction of apoptosis by green synthesized gold nanoparticles through activation of caspase-3 and 9 in human cervical cancer cells. Avicenna J Med Biotechnol. 2016;8:75–83.
- Arruebo M, Vilaboa N, Sáez-Gutierrez B, et al. Assessment of the evolution of cancer treatment therapies. Cancers (Basel). 2011;3:3279–3330.
- Dykman LA, Khlebtsov NG. Gold nanoparticles in biology and medicine: recent advances and prospects. Acta Naturae. 2011;3:34–55.
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100:57–70.
- Sellers and Fisher. Apoptosis and cancer drug targeting. J Clin Invest. 1999;104:1655–1661.
- Liu B, Bhatt D, Oltvai ZN, et al. Significance of p53 dynamics in regulating apoptosis in response to ionizing radiation, and polypharmacological strategies. Sci Rep. 2014;4:6245.
- Koul M, Lakra NS, Chandra R, et al. Catharanthus roseus and prospects of its endophytes: a new avenue for production of bioactive metabolites. Int J Pharm Sci Res. 2013;4:2705–2716.
- Pattarachotanant N, Rakkhitawatthana V, Tencomnao T. Effect of Gloriosa superba and Catharanthus roseus extracts on IFN-γ-induced keratin 17 expression in HaCaT human keratinocytes. Evid Based Complement Alternat Med. 2014;2014:1.
- Moudi M, Go R, Seok Yien CY, et al. Vinca alkaloids. Int J Prev Med. 2013;4:1231–1235.
- Ghosh Chaudhuri R, Paria S. Core/shell nanoparticles: classes, properties, synthesis mechanisms, characterization, and applications. Chem Rev. 2012;112:2373–2433.
- Suresh Mickymaray Hafer K, Iwamoto KS, Schiestl RH. Refinement of the dichlorofluorescein assay for flow cytometric measurement of reactive oxygen species in irradiated and bystander cell populations. Radiat Res. 2008 Apr;169:460–8. doi:10.1667/RR1212.1.
- Shankar SS, Rai A, Ahmad A, et al. Rapid synthesis of Au, Ag, and bimetallic Au core-Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J Colloid Interface Sci. 2004;275:496–502.
- Tomoaia G, Horovitz O, Mocanu A, et al. Effects of doxorubicin mediated by gold nanoparticles and reservatrol in two human cervical cancer cell lines. Colloid Surf B. 2015;135:726–734.
- Gardea-Torresdey E, Gomez JR, Peralta-Videa JG, et al. Jose-Yacaman Alfalfa sprouts: a natural source for the synthesis of silver nanoparticles. Langmuir. 2003;19:1357–1361.
- Sundararajan B, Ranjitha Kumari BD. Novel synthesis of gold nanoparticles using Artemisia vulgaris L. leaf extract and their efficacy of larvicidal activity against dengue fever vector Aedes aegypti L. J Trace Elem Med Biol. 2017;43:187–196.
- Ahn S, Singh P, Jang M, et al. Gold nanoflowers synthesized using Acanthopanacis cortex extract inhibit inflammatory mediators in LPS-induced RAW264.7 macrophages via NF-κB and AP-1 pathways. Colloids Surf B Biointerfaces. 2018;162:398–404.
- Philip D, Unni C, Aromal SA, et al. Murraya Koenigii leaf-assisted rapid green synthesis of silver and gold nanoparticles. Spectrochim Acta A Mol Biomol Spectrosc. 2011;78:899–904.
- Kumari S, Badana AK, G MM, et al. Reactive oxygen species: a key constituent in cancer survival. Biomark Insights. 2018;13:1177271918755391
- Thatte U, Bagadey S, Dahanukar S. Modulation of programmed cell death by medicinal plants. Cell Mol Biol (Noisy-le-Grand). 2000;46:199–214.
- Kaufmann SH, Gores GJ. Apoptosis in cancer: cause and cure. Bioessays. 2000;22:1007–1017.
- Gupta S, Afaq F, Mukhtar H. Selective growth-inhibitory, cell-cycle deregulatory and apoptotic response of apigenin in normal versus human prostate carcinoma cells. Biochem Biophys Res Commun. 2001;287:914–920.
- Ashe PC, Berry MD. Apoptotic signaling cascades. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:199–214.
- Wei H, Leeds P, Chen RW, et al. Neuronal apoptosis induced by pharmacological concentrations of 3-hydroxykynurenine: characterization and protection by dantrolene and Bcl-2 overexpression. J Neurochem. 2000;75:81–90.
- Kuriakose T, Man SM, Subbarao Malireddi RK, et al. ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. Sci Immunol. 2016;1:aag2045.
- Gajalakshmi S, Devi R. Pharmacological activities of Catharanthus roseus: a perspective review. Int J Pharma Biosci. 2013;4:431–439.