Abstract
The establishment of a benign system for the nanoparticle (NPs) synthesis, is a key in nanotechnology for the environmental and health care industries. Therefore, enrichment of novel biological systems for the green synthesis is in significant demand, to lift up these compounds in the biomedical industries. The present work, reports the green synthesis of ZnO NPs, employing a novel thermophile, identified as Bacillus haynesii (GeneBank: MG822851) isolated from the leaf of date palm plant (Phoenix dactylifera), as an eco-friendly nanobiofactory. Physiochemical characterization of ZnO NPs (50 ± 5 nm in size), was achieved by Fourier transform infrared spectroscopy (FT-IR), X-ray diffraction (XRD), diffuse reflectance UV-Visible spectroscopy (DR UV-Vis spectroscopy), Thermogravimetry analysis (TGA), scanning electron microscopy (SEM) and transmissiom electron microscopy (TEM). The morphogenesis and antimicrobial activity of synthesized ZnO NPs, was studied by evaluating the minimum inhibitory/bactericidal concentration (MIC&MBC) against Escherchia coli (8 and 16 mg/mL) and Staphylococcus aureus (4 and 8 mg/mL), respectively. The present study encourages the use of B. haynesii for the green synthesis of ZnO NP. To the best of our knowledge, this is the first report on the study of thermophilic, B. haynesii for green synthesis of NPs in general and ZnO NPs in particular.
Introduction
Presently, nanotechnology is of immense interest and is broadly classified into different areas like physical, chemical and biogenic [Citation1]. Although all these methods have been utilized for the synthesis of NPs, the chemically synthesized NPs are being avoided in the food and pharma industries, because of the use of toxic chemicals during the process [Citation2,Citation3]. However, the preferred method for the synthesis of NPs is the green synthesis process, which has several advantages over the other processes [Citation4]. In addition to being eco-friendly, one of the important features of biologically derived NPs is its optical, photoelectrical and chemical properties, making them diverse in industrial applications [Citation5]. The green synthesized NPs have a vast variety of biomedical applications, e.g. they act as carriers for targeted drug delivery, gene therapy, cancer treatment, antibacterial agents, biosensors, magnetic resonance imaging (MRI), etc. [Citation6].
For the green synthesis of NPs, a number of microorganisms, isolated from different sources including plants, have been studied which includes bacteria, fungi, algae, etc., [Citation7–11]. Among them, bacteria are the favored one, due to easy handling and genetic manipulation, such as, Bacillus sp, like B. subtilis, B. indicus, B. cecembensis and B. amyloliquefaciens [Citation11–13]. Green synthesis of metal NPs has achieved much interest in numerous fields of research and development. Zinc oxide NPs (ZnO NPs) are one of the important particles to have received appreciable attention, due to their antimicrobial, high optical, catalytic, electrical properties and UV filtering characteristics [Citation9]. These properties have led them to be used more as biosensors, solar and nanoelectronics cells. Zinc NPs are successfully used as food additives and in the cosmetic sunscreens, due to its transparency and the ability to absorb UV rays [Citation14–17]. ZnO NPs are also being used as antimicrobial agent, targeted drug delivery system and probes for bioimaging [Citation18–20]. ZnO NPs are also being considered for the utilization in fabric for electrospun mats, which gives antimicrobial, durability and texture [Citation9]. Additionally, the use of ZnO NPs in agricultural purposes is studied for the use of pesticides, without affecting the soil fertility as compared to traditional antimicrobial agents [Citation21].
The present study is an attempt to isolate the thermophilic bacterium, inhabiting one of the most important plant of Saudi Arabia, i.e. a date palm tree, P. dactilerfera. The isolated thermophile was explored for the green synthesis of extracellular ZnONPs and further subjected to characterization and evaluation of antibacterial efficacy against human pathogens.
Experimental design
Isolation of endophytic bacteria
The leaves of Phoenix dactylifera were collected from the campus of Maternity and Children Hospital Campus, Dammam, Saudi Arabia (altitude 6 m/ft.), for the isolation of endophytic bacteria. Fresh and asymptomatic leaves were washed thoroughly under running tap water. Later surface sterilized in 75% ethanol for 3 min, 0.5% NaOCl (Sodium hypochlorite) for 3 min and again with 70% ethanol for 3 min. Subsequently, three washings were done with sterile distilled water. The efficiency of surface sterilization was assured by plating the water from the final washing of the sample. The surface sanitized leaves were air dried under sterile conditions. The dried leaves were crushed, in a sterile pestle motor and serially diluted and plated onto water agar medium amended with fluconazole 30 µg/mL to inhibit fungal growth. The plates were incubated at different temperature, ranging from 20 to 45 °C for the period of 3 to 6 days and were periodically checked for any growth. Visible colonies were picked and streaked on fresh nutrient agar (NA) [Citation22,Citation23].
Study of phenotypic characteristics of CDL3
The isolated bacterial strain labelled as CDL3 was grown on different growth media, like NA, Luria–Bertani agar (LB), trypticase soy agar (TSA), tryptone glucose yeast extract agar (TGY). Motility was examined using semi-solid agar following the previously described protocol [Citation24]. The range of temperature for growth was tested at 5, 10, 15, 25, 30, 35, 40, 45 and 55 °C and sodium chloride (NaCl) tolerance 0–12% (w/v) were tested on NA. The optimum pH range for growth was determined in pH adjusted TSB. Oxidase and catalase activities were also determined [Citation25,Citation26].
Cell morphology and structural details of the isolate were investigated by SEM and TEM. The TEM was regulated at accelerating voltage of 80 kV. A 24 h culture was used for the morphology and structural details of CDL3, while 48 h culture was used for spore assessment. For TEM, cell suspension and spore preparation were analyzed using chemical fixation [Citation27]. Briefly, the suspension of cell/spores was centrifuged to obtain specimen pellets. The pellets were then fixed in the primary fixative (a mixture of 4% glutaraldehyde and 2.5% paraformaldehyde buffered with 0.1 M PIPES). The supernatant was then removed by centrifuge and rinsed 2–3 times in PIPES buffer. The pellets were cut into 1–2 mm cubes. The cubes were further processed with a second fixative (buffered osmium tetra-oxide and incubated for 1 h at room temperature. The osmium tetroxide was removed and rinsed with distilled water. Later, samples were dehydrated by graded ethanol (30, 50, 70 and 90%) each for 10 min. The specimens were passed through absolute ethanol three times, each for 10 min. The fixed cells/spores were then exposed to propylene oxide and infiltered with propylene oxide. Cells/spores were embedded in pure resin mixture in beam capsule and cured in an oven at 60–70 °C for 48 h. Afterwards, ultrathin sections of each specimen were made by ultramicrotomy and few sections were transferred onto TEM grids. Finally, TEM grids holding cell/spore sections were stained by contrast enhancing agent uranyl acetate [Citation28,Citation29]. Stained grids were mounted into TEM and taken several micrographs of the spores/cells at different magnifications.
16S rRNA gene amplification, sequencing and analysis of CDL3
Total genomic DNA from CDL3 was extracted using Gentra Puregene Yeast/Bact. Kit (Qiagen, Hilden, Germany) as per the manufacturer’s instruction. The 16SrRNA gene (1490 bp) of the strain CDL3 was amplified using the primers (Forward 5′-AGAGTTTGATCCTGGCTCAG-3′ and Reverse 5′-TACGGCTACCTTGTTACGACTT’), (Applied Biosystems, Foster City, California, United States) as described earlier with minor modifications [Citation30]. The PCR reaction was carried out using TopTaq polymerase (Qiagen, Hilden, Germany) in Biometra T-Professional thermocycler (Biometra; Goettingen, Germany) with the annealing temperature at 61 °C for 35 cycles. The PCR amplicons were visualized using 1% agarose gel electrophoresis and purified using QIAquick PCR Purification Kit (Qiagen, Hilden, Germany). The purified products were sequenced with the same forward reverse primers using 3500 genetic analyzers (Applied Biosystems, Foster City, California, United States) through BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, California, United States). The 16S rRNA gene sequence of CDL3 was aligned by Basic Local Alignment Search Tool [Citation31] and submitted to the GeneBank (Accession: MG822851). The 16S rRNA gene sequence was analyzed using the EzBioCloud server [Citation32]. An evolutionary relationship of CDL3 was constructed using neighbour-joining phylogenetic tree, UPGMA method and Maximum Likelihood method with MEGA7 software package [Citation33].
Green synthesis of zinc NPs using CDL3
For the green synthesis of ZnO NPs, CDL3 cells were grown overnight at 55 °C with RPM of 120 and centrifuged. 100 mL of cell free supernatant of CDL3 and 100 mL of zinc sulphate solution (1 mM) were taken and the mixture was placed on a stirrer at room temperature for 24 h. The color change was observed and subsequently the sample was dried at 80 °C. This dried sample was annealed at 700 °C for 5 h [Citation34].
Characterization of biosynthesized ZnO NPs
X-ray diffraction pattern of ZnO NPs was analyzed using bench top Rigaku Multiplex system (Rigaku, Japan). The spectrum was recorded in 2-θ range between 10° and 70°. The functional groups were identified using FT-IR spectroscopy equipped with attenuated total reflectance (ATR) (Perkin Elmer, USA). The solid spectra of ZnO and Zinc sulphate were measured using diffuse reflectance UV-Vis spectroscopy (V-750, JASCO). Thermogrammetric analysis of as-synthesized ZnO was carried out using Simultaneous Thermal Analyzer (STA) 6000, Perkin Elmer. During analysis, sample (1.5 mg) was placed in the ceramic pan and heated between 25–900 °C at a heating rate of 10 °C/min under argon atmosphere. The elemental distributions of the sample were investigated using SEM-EDS. SEM was performed using (JSM-6610LV from JEOL). The prepared powder was dispersed onto doubled sided tape holder and examined under 20 kV. EDS were obtained using Aztec software from oxford company. The sample for TEM was prepared by dispersing a small amount of sample in ethanol, sonicating for 5–7 min and depositing onto TEM grids. The grids were examined by a TEM instrument (FEI, Morgagni 68, Czech Republic) at a working voltage of 80 kV. The structure of the NPs was confirmed by electron diffraction patterns. For SEM, the dispersion was placed onto metallic SEM stub, covered with adhesive carbon tape. The sample was dried overnight prior to examining by SEM (FEI, Inspect S50, Czech Republic). SEM was operated at an accelerating voltage of 20 kV and TEM at 80 kV.
Evaluation of antibacterial activity of biosynthesized ZnO NPs
Preparation of nanomaterial: Known amount of ZnO NPs was dissolved in sterile distilled water with continuous stirring to obtain the homogenized solution.
Inoculum preparation: Escherchia coli ATCC35218 and Staphylococcus aureus ATCC29213 strains were selected for the study. Test strains were grown overnight at 37 °C in Mueller Hinton Broth (MHB). After the incubation period, turbidity was adjusted to approximately 106 CFUs/mL.
MIC and MBC for NPs were evaluated by the Macrobroth-dilution method. Control experiments in the absence of NPs as the negative control, were also carried out. Briefly, 10 mL nutrient broth supplemented with 4, 8, 16 and 32 mg/mL ZnO NPs was prepared. The prepared solution was inoculated with 100 µL of overnight bacterial culture (106 CFU/mL) and further incubated for 24 h (shaking) at 35 ± 2 °C. The minimum concentration of the ZnO NPs that showed its inhibitory action in the form of no apparent growth (absence of turbidity), was taken as the MIC value of the compound. Subsequently, 10 µL of each set was streaked out on MHA plate and further incubated at 35 ± 2 °C for 24 h. The plates were observed by the naked eye for determining the lowest concentration (MBC) that blocked bacterial growth or with CFU less than three [Citation35].
Study of morphogenesis of treated cells by SEM
Escherchia coli and S. aureus cells treated with biosynthesized ZnO NPs, were examined for their morphogenesis. Briefly, the overnight treated cells were harvested and washed with PBS to remove the residual media. Later, the cells were fixed with 2.5% glutaraldehyde for 4 h and washed with PBS several times. Cells were fixed again with 1% osmium tetroxide and subsequently, washed with PBS. Fixed cells were dehydrated by a series of ethanol (30, 50, 70, 80, 95, and 100% for 10 min) at room temperature. The samples were dried by using a desiccator. Later, cells were mounted on SEM metallic stubs and coated with gold. The structural changes of the cells were observed by SEM (FEI, Inspect S50, Czech Republic) at an accelerating voltage of 20 kV [Citation35].
Results
Phenotypic characteristics of CDL3
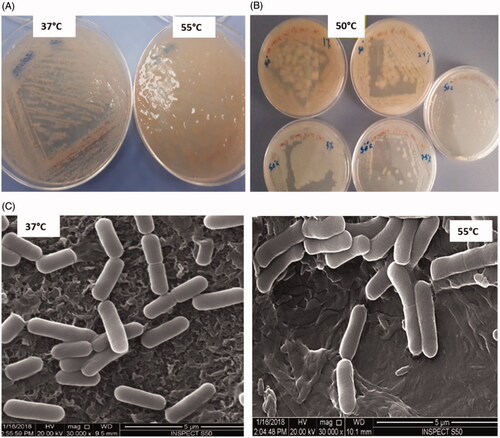
The selected bacterial strain (CDL3) isolated from the leaf of P. dactylifera, is a thermophilic, gram-positive, facultative anaerobe, motile and endospore-forming rod shaped. Colonies formed on NA, after overnight incubation at 55 °C, appear as creamy white, mucoid, translucent, raised and highly moistened, ranging 3–4 mm in diameter, whereas, colonies below 37 ± 2 °C, are presented with a dry texture (). CDL3 is found to grow on NA, LB, TSA, MHA agar, pH 5.5–10 with temperatures from 15 to 55 °C. CDL3 has a NaCl tolerance of 0–12% (w/v) at the temperature of 15 to 55 °C (). The cells were also found as oxidase-negative and catalase-positive. Analysis of CDL3 cells by SEM at 37 °C, appeared to be uniform in shape and size of 2.2 μm, approx., while the CDL3 cells at 55 °C, appeared enlarged (2.6 μm) (), suggesting the absorption of water content at elevated temperature.
Figure 1. Bacillus haynesii (CDL3) (A) NA plate showing cultural characteristics at different temperature, (B) NaCl tolerance of 0–10% (w/v) at the temperature of 50 °C (C) SEM images at different temperatures.
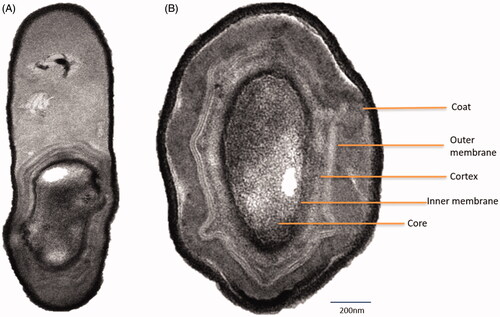
TEM analysis revealed that CDL3 rod is presented with paracentral and ellipsoidal endospores. Cell wall and cell membrane measuring with cellular inclusion were seen (). Coating revealed spores with a consistent characteristic appearance, having various layered structures. They possess a thin outer coat and a thick inner coat, with a core wall. The plasma membrane, surrounding a compact spore core is seen strongly dehydrated to immobilize the proteins. Around the core, the inner membrane can be seen which gives protection. After that, the germ cell wall could be spotted, which evolves into the cell wall after germination.
16S rRNA gene sequencing of CDL3
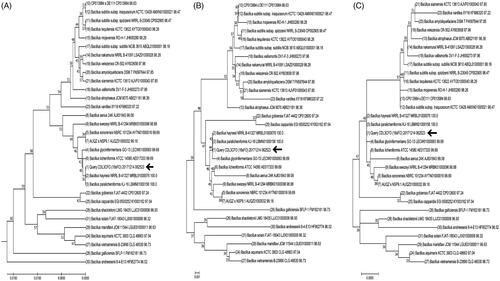
The sequence alignment of the 16S rRNA gene of CDL3 on the EzBioCloud server, revealed that strain CDL3 (https://www.ncbi.nlm.nih.gov/nuccore/MG822851.1) is similar to strain Bacillus haynesii NRRL B-41327 (MRBL01000076.1) with a similarity score of 100%. The phylogenetic trees constructed from partial 16S rRNA gene sequence of top 31 hits from the EzBioCloud server using the UPGMA method, neighbor-joining method and maximum-likelihood method (). The overall mean distance on the maximum-likelihood method is 0.018. Nucleotide composition of the partial 16S rRNA gene sequence of top 31 hits from the EzBioCloud server and the strain CDL3 revealed that the GC content of 54.8% in the 979 bp of the CDL3 strain. Further, the phenotypic and biochemical analysis of the CDL3 supported that the strain CDL3 is B. haynesii.
Green synthesis and characterization of ZnO NPs
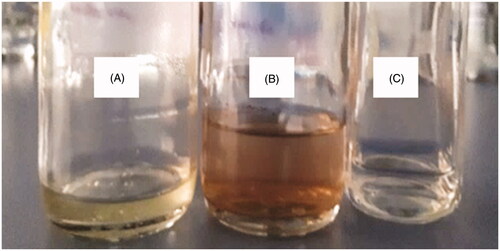
The green synthesis of zinc NPs by a thermophilic strain i.e. B. haynesii (CDL3) was confirmed and characterized by different techniques. The reaction mixture of zinc sulphate was analyzed initially along with the control by visual observation. The solution color changed from pale yellow to brown, while in the control experiments, no notable color changes were observed, indicating that the biotransformation of metal ions has occurred only in the presence of the CDL3 cell supernatant to Zinc NPs (. This color change is attributed to the excitation of surface plasmon resonance (SPR) and the results obtained are in accordance with Manokari et al. [Citation36].
Figure 4. Green synthesis of ZnO NPs using B. haynesii (CDL3). (A) cell free supernatant without Zinc Sulphate solution (control 1), (B) cell free supernatant with zinc sulphate solution showing color change due to synthesis of NPs, (C) zinc sulphate solution without cell free supernatant (control 2).
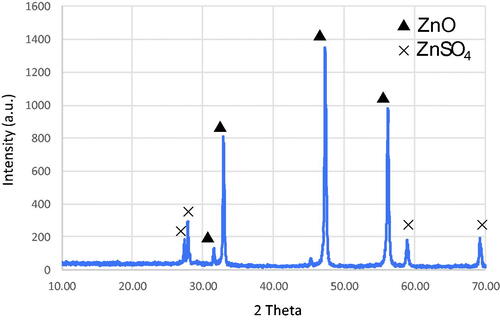
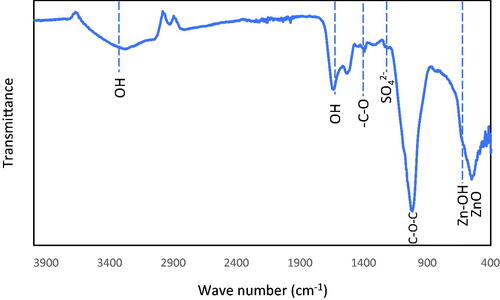
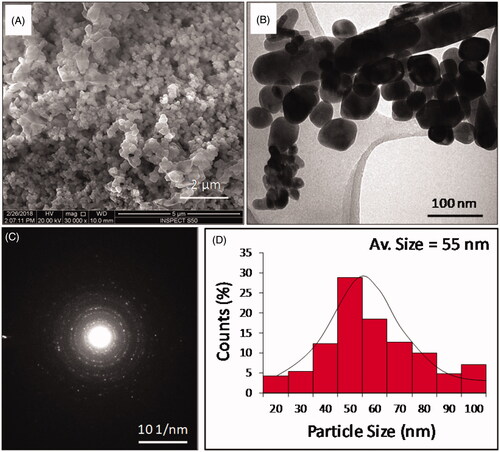
shows the X-ray diffraction pattern of ZnO NPs prepared using CDL3. The sample was scanned over a 2-θ range between 10° and 70°. The crystalline peaks corresponding to the ZnO indexed to hexagonal Zincite and ZnSO4 were clearly observed. The peaks at 2-θ value of 31.7°, 34.4°, 47.5°, 56.5° and 69.0° were ascribed to ZnO, while less intense peaks appeared at 28.4°, and 58.9° corresponding to ZnSO4. shows the FT-IR spectrum of ZnO NPs analyzed in the range 400–4000 cm−1. The peaks corresponding to ZnO and Zn-OH were observed between 400–600 cm−1, respectively. The peak corresponding to SO42− group was observed at 1200 cm−1. The presence of carbon–oxygen bond and an intense broad peak corresponding to –C–O–C stretching appears at 1387 and 1020 cm−1, respectively. The presence of an intense and broad peak between 2980–3600 cm−1 and a sharp peak at 1642 cm−1 indicates the presence of stretching and bending vibrations due to hydroxyl groups.
Diffuse reflectance spectroscopy was used to confirm the ZnO NPs. The characterization tool is a powerful technique to determine the coordination geometry of ZnO. The absorption maxima spectra of each metal oxide are unique, which can be used to identify the synthesized metal oxide species. In the present case, the spectra of synthesized NPs were analyzed between 200–700 nm. shows the spectra of commercially available bulk ZnO (Sigma Aldrich), ZnO prepared in this study using CDL3 and bulk Zinc sulphate. The bulk ZnO showed the presence of intense absorption peak at 370 nm (), whereas zinc oxide nanoparticles synthesized in this study showed similar absorption peak maxima but at a lower wavelength of 354 nm (). The observed absorption peak typical to the absorption band of one-dimensional array ZnO coincides well with the parent ZnO absorption band reported by Shen et al. [Citation37]. Such lower band gap of ZnO clearly shows the presence of nanosized ZnO compared to bulk ZnO. In the case of ZnSO4, a broad absorption occurs with peak maxima at about 320 nm (). The absorbance band differences among three samples clearly indicate the major phase of ZnO compared to ZnSO4.
Figure 7. Diffuse reflectance UV-visible spectra of (a) Bulk ZnO, (b) ZnO prepared using CDL3 and [Citation3] Bulk ZnSO4.
![Figure 7. Diffuse reflectance UV-visible spectra of (a) Bulk ZnO, (b) ZnO prepared using CDL3 and [Citation3] Bulk ZnSO4.](/cms/asset/aef20d40-b999-4a4c-a52b-6c62a0cb95eb/ianb_a_1620254_f0007_c.jpg)
In order to know the mechanistic aspect of ZnO formation, as-synthesized ZnO obtained from CDL3 was analyzed using TGA coupled with DTA. Zinc sulphate, also known as Zinc hydroxide sulphate, undergoes different decomposition phenomenon to transform into ZnO [Citation38]. In our case, raw sample ZnO showed dehydration similar to that of pure ZnSO4 between 80 and 140 °C (step 1). In the temperature ranging between 175 and 350 °C, the decomposition shows the formation of ZnO and Zinc oxy-sulphate (step 2). Though the formation of other phases in the form of Zinc hydroxy sulphate were also reported to occur. The transformation of Zn3O2(SO4) or Zn5O2(SO4)3 has been reported with the deposition temperature of 350 °C. However, the formation of different oxide forms is still controversial. Additional phase transition was reported from Zn3(OH)2(SO4)2 to Zn3O(SO4)2. Further decomposition at a temperature ranging between 600–800 °C corresponds to transformation of 2 molecules of Zn3O(SO4)2 to form ZnO, SO2 and O2 (step 3) (Figure S1).
The prepared ZnO NPs’ elemental distributions have been shown in Figure S2. The presence of Zinc and Oxygen were clearly seen along with elements like Na, Cl and C. The presence of Na, Cl and C, could be due to the presence of microbial byproducts. The absence of sulphate shows that fewer impurities were present. The major portion is ZnO and shows the effective transformation of zinc sulphate to ZnO with an annealing temperature of 700 °C.
The morphology of the synthesized product was investigated by SEM and TEM. shows the SEM image of a prepared ZnO NPs at a scale bar of 5 μm. The morphological analysis shows the presence of agglomerated crystals of ZnO NPs. TEM offers the morphological study of the nanomaterials with a high resolution. TEM analysis showed the presence of spherical shaped ZnO NPs, distributed between 20–100 nm (). The average size of the NPs was calculated as 50 ± 5 nm. A few rod-shaped ZnO NPs in the range between 100 and 300 nm were also observed. For size histogram, rod-shaped particles were excluded during the measurement. The selected area electron diffraction (SAED) pattern was displaying several uniform dotty rings, confirming the crystalline structure of the particles that is consistent with the XRD pattern shown in .
Evaluation of antibacterial activity of biosynthesized ZnO NPs
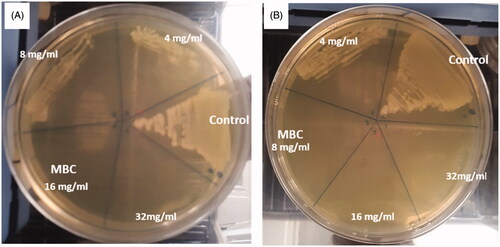
In the current study, the bactericidal activity of ZnO NPs against E. coli and S. aureus was determined by a standard broth dilution method. The MIC/MBC values were >8 and 16 mg/mL, respectively for E. coli and 4 and 8 mg/mL, respectively for S. aureus (. It was found that the biosynthesized NPs showed significant activity by inhibiting the growth of S. aureus at 4 mg/mL.
Study of morphogenesis of treated cells by SEM
By examining the SEM, the untreated S. aureus cells were seen as typically grape arranged cells, smooth and intact cell surface with no damage seen (). However, treated S. aureus cells were seen with changes in shape with distorted cell surface (). In S. aureus, the spherical-shaped cell was no longer intact and appeared as deformed, damaged and few in number. The change of cells could be attributed to damage caused by NPs attached to the cell surface. Particles of ZnO NPs were also found attached to the E. coli cells (), probably at positions that have more negatively charged functional groups. The treated cells depicted distorted cell structure, indicating the loss of membrane integrity of bacterial cells, however the untreated E. coli cells were found to appear normal and abundant in colony number ().
Discussion
The gram-positive, motile, rod-shaped, endospore-forming bacillus labelled as CDL3, has been isolated from an indigenous date palm plant of Saudi Arabia i.e. P. dactilerfera. The selected plant is already reported to harbor plethora of endophytic microbes including, Bacillus sps e.g B. safensis, B. sonorensis, B. subtilis, and B. cereus [Citation39–41]. However, to the best of our knowledge, there is no previous report on the thermophilic B. haynesii, not only from the date palm tree, even from any other existing plant sps across the world, so far. The phenotypic and biochemical analysis of the CDL3 indicated that, gram-positive isolate recovered from palm date leaf tissue, share the characteristics of previously reported Bacillus sps from the desert soil [Citation42]. This was further confirmed by phylogenetic analysis of the 16S rRNA gene analysis, which determined that it is sharing the similarity to B. haynesii NRRL B-41327 (MRBL01000076.1) with the similarity score of 100% [Citation41].
We also demonstrated that CDL3 is an extremophile for its ability to grow in the presence of 0–12% NaCl, making it halotolerant and up to the temperature of 55 °C, making it thermotolerant. Extremophiles are known to survive in the extreme environments to which they had adapted to grow; indeed it goes in favor of CDL3, which is isolated from a desert plant, an inhabitant of extreme hot climate and water scarcity condition [Citation43]. During the study of cultural characteristic, it is assumed that CDL3 has the ability of retaining water, when grown at 50 to 55 °C, which is evident from the moist colonies on agar plate (), hence, making it thrive in extreme conditions. The structural analysis of B. haynesii by electron microscopy is previously unknown. Analysis by TEM shows, the features of structural organization of the spore, which may correlate with its physical and biological characteristics including the ability to survive at extreme conditions [Citation44].
Previously, several studies have reported, B. cereus, B. subtilis, B. licheniformis, as an agent for the formation of ZnO NPs [Citation6,Citation45–47]. In the present study, first time a novel thermophilic B. haynesii is reported to play a reducing agent in the green synthesis of ZnO NPs. Diffuse reflectance UV-Visible spectroscopy is used for identification of coordination site of metal oxide species. The absorption coordinative spectra of each metal oxide are unique, which can be used to identify the synthesized metal oxide species. In the present case, the spectra of synthesized NPs were analyzed between 200–700 nm using DR UV-Vis spectroscopy. The NPs showed a broad absorption with peak maxima at about 350 nm, typical to the absorption band of one-dimensional array ZnO. The observed absorption peak coincide well with the parent ZnO absorption band reported by Shen et al. [Citation37].
The significant antibacterial activity of CDL3 mediated ZnO NPs, was observed against both gram-positive and gram-negative bacteria, with a better activity against gram-positive, S. aureus. This could be simply explained due to the difference in the cell wall composition and thickness, gram-positive having less thick cell wall, provides a more efficient means for antibacterial activity [Citation21]. Additionally, the surface of ZnO NPs generates hydrogen peroxide (H2O2) which is being considered as an effective way for the antibacterial activity [Citation48]. Our finding of synthesized ZnO NPs, possessing pronounced anti gram-positive bacteria goes in agreement with the previous studies conducted by Premanathan et al. [Citation49]. The surface charge attraction between ZnO NPs and the pathogenic bacteria results in the leakage of cytoplasmic content leading ultimately to cell death [Citation21].
Conclusion
To conclude, we report a novel thermophile; B. haynesii as a nontoxic, ecofriendly and richly obtainable source for the biogenic synthesis of ZnO NPs, in the range of 50 ± 5 nm in size. The NPs synthesized by the bacteria, are biologically active against the tested human pathogens. Therefore, our findings encourage to isolate and screen unexplored thermophiles, which could be considered for utilization in the green synthesis of NPs for various applications in medical and non-medical fields.
Acknowledgements
Dr. AbdulHakeem Aldossary, Dept of Biophysics, (IRMC) is highly acknowledged for the training in Electron microscopy. Mr. Faisal Hassan Saad and Ms. Janaica Logan are also acknowledged for assistance in the lab.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Al-Dhabi N, Valan Arasu M. Environmentally-friendly green approach for the production of zinc oxide nanoparticles and their anti-fungal, ovicidal, and larvicidal properties. Nanomaterials 2018;8:500.
- Taner M, Sayar N, Yulug IG, et al. Synthesis, characterization and antibacterial investigation of silver–copper nanoalloys. J Mater Chem. 2011;21:13150–13154.
- Wang C, Kim YJ, Singh P, et al. Green synthesis of silver nanoparticles by Bacillus methylotrophicus, and their antimicrobial activity. Artific Cells, Nanomed Biotechnol. 2016;44:1127–1132.
- Ojo SA, Lateef A, Azeez MA, et al. Biomedical and catalytic applications of gold and silver-gold alloy nanoparticles biosynthesized using cell-free extract of Bacillus safensis LAU 13: antifungal, dye degradation, anti-coagulant and thrombolytic activities. IEEE Transon Nanobiosci. 2016;15:433–442.
- Dhandapani P, Maruthamuthu S, Rajagopal G. Bio-mediated synthesis of TiO2 nanoparticles and its photocatalytic effect on aquatic biofilm. J Photochem Photobiol B: Biol. 2012;110:43–49.
- Ali AA, Asif MA, Mashrai AM, et al. Green synthesis of ZnO nanoparticles using Bacillus subtilis and their catalytic performance in the one-pot synthesis of steroidal thiophenes. Euro Chem Bull. 2014;3:939–945.
- Kalimuthu K, Babu RS, Venkataraman D, et al. Biosynthesis of silver nanocrystals by Bacillus licheniformis. Coll Surf B: Biointerf. 2008;65:150–153.
- Vigneshwaran N, Kathe AA, Varadarajan PV, et al. Silver − protein (core − shell) nanoparticle production using spent mushroom substrate. Langmuir 2007;23:7113–7117.
- Jayaseelan C, Rahuman AA, Kirthi AV, et al. Novel microbial route to synthesize ZnO nanoparticles using Aeromonas hydrophila and their activity against pathogenic bacteria and fungi. Spectrochimica Acta Part A: Mol Biomol Spectrosc. 2012;90:78–84.
- Castro L, Blázquez ML, Muñoz JA, et al. Biological synthesis of metallic nanoparticles using algae. IET Nanobiotechnol. 2013;7:109–116.
- Shahzad R, Latif Khan A, Ali L, et al. Characterization of new bioactive enzyme inhibitors from endophytic Bacillus amyloliquefaciens RWL-1. Molecules 2018;23:114.
- Southam G, Beveridge TJ. The in vitro formation of placer gold by bacteria. Geochim Cosmochim Acta. 1994;58:4527–4530.
- Shivaji S, Madhu S, Singh S. Extracellular synthesis of antibacterial silver nanoparticles using psychrophilic bacteria. Proc Biochem. 2011;46:1800–1807.
- Kumar V, Wariar P, Prasad V, et al. A novel approach for the synthesis of nanocrystalline zinc oxide powders by room temperature co-precipitation method. Mater Lett. 2011;65:2059–2061.
- Wang P, Zakeeruddin SM, Humphry-Baker R, et al. A binary ionic liquid electrolyte to achieve ≥7% power conversion efficiencies in dye-sensitized solar cells. Chem Mater. 2004;16:2694–2696.
- Nohynek GJ, Lademann J, Ribaud C, et al. Grey goo on the skin? Nanotechnology, cosmetic and sunscreen safety. Crit Rev Toxicol. 2007;37:251–277.
- Nohynek G, Dufour E, Roberts MS. Nanotechnology, cosmetics and the skin: is there a health risk? Skin Pharmacol Physiol. 2008;21:136–149.
- Padmavathy N, Vijayaraghavan R. Enhanced bioactivity of ZnO nanoparticles-an antimicrobial study. Sci Technol Adv Mater. 2008;9:035004.
- Dong S, Roman M. Fluorescently labeled cellulose nanocrystals for bioimaging applications. J Am Chem Soc. 2007;129:13810–13811.
- Hrkach J, Von Hoff D, Ali MM, et al. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci Translat Med. 2012;4:128ra39–128ra39.
- Sharma D, Rajput J, Kaith B, et al. Synthesis of ZnO nanoparticles and study of their antibacterial and antifungal properties. Thin Solid Films 2010;519:1224–1229.
- Rehman S, Shawl A, Kour A, et al. An endophytic Neurospora sp. from Nothapodytes foetida producing camptothecin. Appl Biochem Microbiol. 2008;44:203–209.
- Kumar A, Singh R, Yadav A, et al. Isolation and characterization of bacterial endophytes of Curcuma longa L. 3 Biotech. 2016;6:60.
- Lin J, Cheng J, Chen K, et al. The icmF3 locus is involved in multiple adaptation-and virulence-related characteristics in Pseudomonas aeruginosa PAO1. Front Cell Infect Microbiol. 2015;5:70.
- Chen C, Xin K, Liu H, et al. Pantoea alhagi, a novel endophytic bacterium with ability to improve growth and drought tolerance in wheat. Scientific Rep. 2017;7:41564.
- Breznak JA, Costilow RN. Physicochemical factors in growth. Methods for general and molecular microbiology, Third Edition. Washington (DC): American Society of Microbiology; 2007. p. 309–329.
- Kuo J. Electron microscopy. Methods and protocols. Third ed. New York (NY): Springer, Humana Press; 2014.
- Hayat MA. Prinicpals and techniques in electron microscopy. Biological applications. 4th Edition ed. Cambridge, UK: Cambridge University Press; 2000.
- Bozzola JJ, Russell LD. (1999). Electron microscopy: principles and techniques for biologists. Sudbury (MA): Jones and Bartlett.
- Dalisay DS, Williams DE, Wang XL, et al. Marine sediment-derived Streptomyces bacteria from British Columbia, Canada are a promising microbiota resource for the discovery of antimicrobial natural products. PLoS One. 2013;8:e77078.
- Altschul S, Gish W, Miller W, et al. Basic local alignment search tool. J Mol Biol. 1990;215:403–410.
- Yoon S-H, Ha S-M, Kwon S, et al. Introducing EzBioCloud: a taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int J Syst Evol Microbiol. 2017;67:1613–1617.
- Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–1874.
- Rajabairavi N, Raju CS, Karthikeyan C, et al. Recent Trends in Materials Science and Applications. Cham, Switzerland: Springer; 2017. Biosynthesis of novel zinc oxide nanoparticles (ZnO NPs) using endophytic bacteria Sphingobacterium thalpophilum; p. 245–254.
- Ansari MA, Baykal A, Asiri S, et al. Synthesis and characterization of antibacterial activity of spinel chromium-substituted copper ferrite nanoparticles for biomedical application. J Inorg Organomet Polym Mater. 2018;28:2316–2327.
- Manokari M, Ravindran CP, Shekhawat MS. Biosynthesis of zinc oxide nanoparticles using Melia azedarach L. extracts and their characterization. Int J Pharma Sci Res. 2016;1:31–36.
- Shen Z, Zhou H, Chen H, et al. Synthesis of nano-zinc oxide loaded on mesoporous silica by coordination effect and its photocatalytic degradation property of methyl orange. Nanomaterials 2018;8:317.
- Moezzi A, Cortie MB, McDonagh AM. Zinc hydroxide sulphate and its transformation to crystalline zinc oxide. Dalton Trans. 2013;42:14432–14437.
- Siala R, Chobba IB, Vallaeys T, et al. Analysis of the cultivable endophytic bacterial diversity in the date palm (Phoenix dactylifera L.) and evaluation of its antagonistic potential against pathogenic Fusarium species that cause date palm bayound disease. J Appl Env Microbiol. 2016;4:93–104.
- Hallmann J, Quadt-Hallmann A, Mahaffee W, et al. Bacterial endophytes in agricultural crops. Can J Microbiol. 1997;43:895–914.
- Mahmoud FM, Krimi Z, Maciá-Vicente JG, et al. Endophytic fungi associated with roots of date palm (Phoenix dactylifera) in coastal dunes. Revista Iberoamericana de Micologia. 2017;34:116–120.
- Dunlap CA, Schisler DA, Perry EB, et al. Bacillus swezeyi sp. nov. and Bacillus haynesii sp. nov., isolated from desert soil. Int J Syst Evol Microbiol. 2017;67:2720–2725.
- Echigo A, Hino M, Fukushima T, et al. Endospores of halophilic bacteria of the family Bacillaceae isolated from non-saline Japanese soil may be transported by Kosa event (Asian dust storm). Saline Systems 2005;1:8.
- Smirnova T, Zubasheva M, Shevlyagina N, et al. Electron microscopy of the surfaces of bacillus spores. Microbiology 2013;82:713–720.
- Hussein MZ, Azmin W, Mustafa M, et al. Bacillus cereus as a biotemplating agent for the synthesis of zinc oxide with raspberry-and plate-like structures. J Inorg Biochem. 2009;103:1145.
- Alkaim AF. Eco friendly synthesis, characterization and antibacterial activity of ZnO nano particles using bacillus subtilis against multi-drug resistant bacteria. J Glob Pharma Technol. 2017;9:207–213.
- Raliya R, Tarafdar JC. ZnO nanoparticle biosynthesis and its effect on phosphorous-mobilizing enzyme secretion and gum contents in Clusterbean (Cyamopsis tetragonoloba L.). Agric Res. 2013;2:48–57.
- Yamamoto O. Influence of particle size on the antibacterial activity of zinc oxide. Int J Inorg Mat. 2001;3:643–646.
- Premanathan M, Karthikeyan K, Jeyasubramanian K, et al. Selective toxicity of ZnO nanoparticles toward Gram-positive bacteria and cancer cells by apoptosis through lipid peroxidation. Nanomed: Nanotechnol, Biol Med. 2011;7:184–192.