Abstract
Impairment of type II collagen caused by MMPs in response to overproduction of IL-1β is an important step in the pathological progression of osteoarthritis (OA). Lunasin, a well-known peptide present in the soybean, has displayed a positive impact on numerous physiological functions. Little information in the effects of lunasin on cartilage degradation has been sought in clinical research before. Here, we report that lunasin suppressed the increase in MMP-3 and MMP-13 caused by IL-1β. In addition, we found that lunasin could prevent the decrease in TIMP-1 and TIMP-2 expressions caused by IL-1β. Notably, lunasin suppressed reduction of type II collagen, the basis for articular cartilage. Lunasin also attenuated activation of the JAK2/STAT1/IRF-1 pathway. These effects of lunasin suggest that it might become a promising therapeutic agent for chondro-protective therapy.
Introduction
Osteoarthritis (OA) is commonly seen in the elderly, and it affects millions of the population worldwide. Multiple biochemical and mechanical factors contribute to the initiation of OA [Citation1]. Mild inflammation associated with overproduction of IL-1β plays a critical role in the pathological progression of OA. IL-1β results in the induction of matrix metalloproteinases (MMPs) and nitric oxide (NO) in chondrocytes. MMPs have been recognized as crucial factors in OA [Citation2]. Among them, MMP-3 and MMP-13 act as the two most important collagenases involved in the degradation of the cartilage matrix in OA [Citation3]. MMP-3 can cleave multiple extracellular matrices including proteoglycans [Citation4]. MMP-13 is responsible for the digestion of type II collagen [Citation5,Citation6]. The physiological activities of MMPs are antagonized by tissue inhibitors of metalloproteinases (TIMPs), such as TIMP-1 and TIMP-2 [Citation7]. Inhibiting the activity of MMPs and degradation of ECM has become a promising strategy to blunt OA progression.
Lunasin, a well-known natural peptide, was found in the soybean [Citation7]. It has displayed positive impacts on numerous physiological functions in living beings. Increasing evidence shows that lunasin has protective properties that are effective in the treatment of various diseases, including neurodegenerative diseases, heart disease and bone disorders [Citation8]. Patents taking lunasin have demonstrated that this peptide exhibits a diverse range of biological activities, which include anti-inflammatory, anti-diabetes and anti-cancer properties [Citation9]. Due to these beneficial roles, numerous nutritional supplements containing lunasin are offered. It has been identified that daily dietary supplementation with 125 mg lunasin exerts protective effects in terms of heart health and cholesterol management. Additionally, a kind of lunasin extract from Mexico has been successfully applied for developing soy beverages and functional foods [Citation10]. The anti-inflammatory function of lunasin has been studied in recent investigations. Significantly, lunasin could inhibit the secretion of IL-1β [Citation11]. However, the pharmacological role of lunasin in OA has not been known. Here, we report that lunasin treatment prevented IL-1β-mediated loss of type II collagen by inhibiting MMP-3 and MMP-13.
Materials and methods
Cell isolation and treatment
Normal knee joint cartilage was obtained from transplant donors (n = 21) who were undergoing joint cartilage head replacement surgeries. The cartilage was cut and the samples were digested with 0.2% collagenase at 37 °C for 4 h. Isolated chondrocytes were grown in DMEM with 10% FBS and 0.1% antibiotics. Lunasin was provided by KaiJie Bio (Chengdu, China). Analytical HPLC and ESI-MS was used for quality control of the synthesized lunasin by the manufacturer. The purity of lunasin was higher than 95% (Impurities: undesired fragments of peptides generated in the process of chemical synthesis with different molecular weights). Cells were cultured with IL-1β (10 ng/ml) with or without lunasin (50, 100 μM).
Cell proliferation measurement
After the indicated treatments, MTT reduction assay was performed to examine cell proliferation [Citation12]. Briefly, cells were grown in 96-well culture plates. After stimulation, we put 1 mg/mL MTT to the culture medium for 3 h in darkness. One-hundred microlitre of dimethyl sulphoxide was used to dissolve the resultant insoluble products. OD at 490 nm was detected using a microtitre plate reader (model 680, Bio-Rad, UK).
Real-time PCR
Real-time PCR was performed as previously reported in other studies [Citation13]. Qiazol was used to isolate total RNA from chondrocytes. RNA concentration was assessed by measuring the A260/A280 ratio. Reverse transcription PCR was performed to synthesize cDNA with 1 μg total RNA. Experiment was carried out using cDNA template, primers and the SYBR green master mix system (12.5 µl). Assay precision has been verified at the dynamic range over which a reaction is linear [Citation14]. Quantification was achieved by normalizing the target gene to GAPDH using the 2–ΔΔCT method and compared with the control.
Western blot analysis
Chondrocytes were lysed. Protein concentration was assessed using a BCA assay. 20 μg samples were subjected to 8% SDS-PAGE and transferred to PVDF membranes. After blocking, blots were incubated with antibodies against IRF-1, p-JAK2, type II collagen, JAK2, MMP-3, p-STAT1, MMP-13, STAT1 and β-actin overnight in a cold room. Membranes were then probed with the secondary HRP-conjugated antibodies. The blots were developed using ECL [Citation15].
Determination of MMP concentrations
The secretion of active MMP-3 and MMP-13 was assessed using a commercially available ELISA kit (AnaSpec, USA). The fluorescence of 5-FAM was read and monitored (excitation: 490 nm; emission: 520 nm).
Statistical analysis
Experimental data are presented as means ± SD. Data were assessed by ANOVA. Differences were thought as significant when p was <.05.
Results
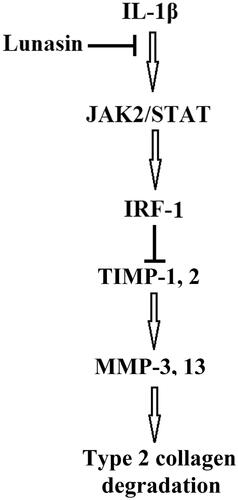
To evaluate the effects of lunasin on proliferation of chondrocytes, a MTT assay was performed. Our results indicate that the administration of lunasin at final concentrations of 50 and 100 μM did not affect cell proliferation of chondrocytes. However, mean cell proliferation was reduced by 18% (p < .01) after treatment with lunasin using the final concentration of 500 μM (). Notably, administration of lunasin at final concentrations of 50 and 100 μM did not have any impact on basal levels of MMP-3 and MMP-13 (Figure S1). Therefore, we administered lunasin at concentrations of 50 and 100 μM. First, we examined whether lunasin affects the inhibitory role of IL-1β on chondrocytes proliferation. We demonstrate that IL-1β treatment (48 h) remarkably reduced cell proliferation by 45% (p < .01), which was prevented by lunasin (). We then profiled the levels of MMP-3 and MMP-13 after IL-1β stimulation in the absence or presence of lunasin. Results shown in indicate that following treatment with IL-1β, mRNA levels of MMP-3 and MMP-13 had increased 4.5 and 5.1 times, respectively. This was prevented by lunasin. Consistently, ELISA assay revealed that the secretion of these enzymes was elevated after IL-1β treatment, which was suppressed by lunasin (). The biological activities of MMPs are mainly inhibited by TIMP-1 and TIMP-2. Therefore, we determined the roles of lunasin on TIMP-1 and TIMP-2. Results in show that IL-1β remarkably decreased TIMP-1 and TIMP-2 at the mRNA level by 69% and 65%, respectively, which was obviously prevented by lunasin. Consistently, the presence of lunasin restored the protein expression of these two TIMPs ().
Figure 1. Lunasin ameliorated IL-1β-mediated reduction of cell proliferation. (A) Chondrocytes were incubated with lunasin (50, 100 and 500 μM) for 48 h. Cell proliferation was assessed by MTT. (B) Chondrocytes were cultured with IL-1β with or without lunasin (50, 100 μM). Chondrocytes proliferation was examined with MTT (*, #, p < .01).
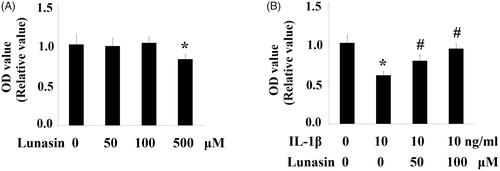
Figure 2. Lunasin ameliorated IL-1β-mediated induction of MMPs. Chondrocytes were stimulated with IL-1β or lunasin (50, 100 μM). (A) mRNA level of MMP-3, MMP-13. (B) Protein level of MMP-3, MMP-13 (*, #, p < .01).
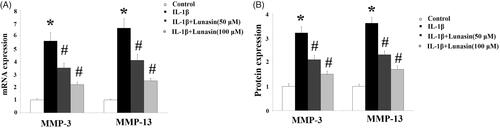
Figure 3. Lunasin ameliorated IL-1β-mediated decrease in TIMP-1 and TIMP-2. Chondrocytes were stimulated with IL-1β or lunasin (50, 100 μM). (A) mRNA level of TIMP-1, TIMP-2. (B) Protein level of TIMP-1, TIMP-2 (*, #, p < .01).
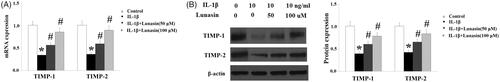
The level of type II collagen was reduced by 59% (p < .01) after IL-1β treatment. As expected, lunasin suppressed IL-1β-mediated loss of type II collagen (. MMP-3 and MMP-13 are transcriptionally governed by IRF-1. Hence, we investigated whether lunasin affects the levels of IRF-1. Notably, we found that treatment with lunasin abolished the upregulation of IRF-1 (. The JAK2/STAT1 pathway is important for the activation of IRF-1. Significantly, it was shown that IL-1β treatment markedly elevated the phosphorylation of JAK2 by 2.5 times (p < .05) as well as STAT1 at Ser727 by 2.1 times (p < .05), which was reduced by lunasin (). These results imply that lunasin might suppress the expression of MMPs by inhibiting activation of the JAK2/STAT1/IRF-1 pathway. To further confirm the participation of this pathway, cells were incubated with IL-1β and lunasin (100 μM) with or without the specific JAK2 activator coumermycin A1 (50 nM) or the specific JAK2 inhibitor AG490 (10 nM). Results display that the presence of coumermycin A1 negated the effects of lunasin on MMPs expression (. In contrast, AG490 enhanced the inhibitory effects of lunasin on MMPs expression. This suggests the participation of the JAK2/STAT1/IRF-1 pathway in these processes.
Figure 4. Lunasin mitigated IL-1β caused reduction of type II collagen. Chondrocytes were stimulated with IL-1β or lunasin (50, 100 μM). Col II, type II collagen. (A) Representative bands of type II collagen. (B) Quantitative analysis (*, #, p < .01).
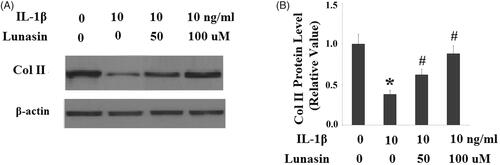
Figure 5. Lunasin abolished IL-1β-induced upregulation of IRF-1. Chondrocytes were stimulated with IL-1β or lunasin (50, 100 μM). (A) mRNA level of IRF-1. (B) Protein level of IRF-1 (*, #, p < .01).
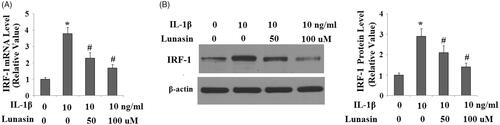
Figure 6. Lunasin suppressed IL-1β- induced activation of JAK2 and STAT1. Chondrocytes were stimulated with IL-1β or lunasin (50, 100 μM). (A) Representative bands of phosphorylated JAK2 and STAT1 at Ser 727, (B) Quantitative analysis (*, #, p < .01).
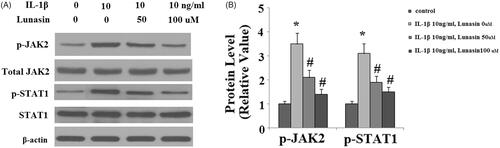
Figure 7. The JAK2/STAT1/IRF-1 pathway participates in the inhibitory effects of lunasin on MMPs. Chondrocytes were cultured with IL-1β and lunasin (100 μM) with or without the specific JAK2 activator coumermycin A1 (50 nM) or the specific JAK2 inhibitor AG490 (10 nM) for 24 h. (A) mRNA levels of MMPs. (B) Protein levels of MMPs (*, #, $, p < .01).

Discussion
Here, we provide evidence to identify a new pharmacological function of lunasin in abrogating ECM degradation in chondrocytes. First, we found that lunasin inhibited MMP-3 and MMP-13. Second, it was shown that lunasin reversed the decrease in TIMP-1 and TIMP-2. Third, lunasin abrogated IL-1β-induced activation of IRF-1 by regulating the JAK2/STAT1 pathway. A graphical explanation of the molecular mechanism is presented in .
MMPs are essential enzymes that play key roles in regulating the balance of the extracellular matrix metabolism. Under normal physiological conditions, they assist in the creation and maintenance of interstitial spaces. Under pathological conditions, they cause excessive degradation of extracellular matrix proteins by facilitating multiple inflammatory processes [Citation16]. Among them, MMP-3 is capable of cleaving proteoglycans, collagens, gelatins and aggrecan link proteins. MMP-13 is another important enzyme capable of efficiently degrading type II collagen and aggrecans in cartilage. Selective MMP-13 inhibitors have been reported to block degradation of type II collagen [Citation17,Citation18]. An equilibrium of MMPs and TIMPs is necessary for maintaining the integrity of articular cartilage [Citation19–22]. It should be noted that treatment with lunasin alone at the concentrations of 50 and 100 μM did not affect the basal levels of MMPs under normal conditions (Figure S1). However, when cells were exposed to IL-1β, lunasin significantly prevented the induction of MMP-3 and MMP-13 (). These results suggest that lunasin displays a strong protective effect against inflammatory stimuli by activating anti-inflammatory signalling in cells. In the current study, we report that lunasin treatment increased expression of TIMP-1 and TIMP-2, while decreasing expression levels of MMP-3 and MMP-13. Indeed, our subsequent study demonstrates that lunasin abrogated degradation of its substrate type II collagen. Lunasin could suppress the generation of lipopolysaccharide (LPS)-induced inflammation markers by suppressing the central inflammation regulator NF-κB [Citation23]. Furthermore, lunasin reduces the secretion of TNF-α and IL-6 and the generation of ROS [Citation11]. Interestingly, lunasin displays its anti-inflammatory property by suppressing IL-6, TNF-α and MCP-1 not only in RAW264.7 macrophages but also in 3T3-L1 adipocytes [Citation24]. Lunasin was shown to suppress cell proliferation and induce apoptosis of rheumatoid arthritis (RA) synovial fibroblasts [Citation25]. Lunasin-enriched preparation prevented inflammasomes activation by decreasing IL-1β, IL-18 and ROS in macrophages [Citation26]. Notably, lunasin could inhibit the migration of human MCF-7 cells [Citation27]. RGD-peptide lunasin reduced pro-inflammatory factors through regulating αVβ3 integrin in human and murine macrophages [Citation28–32]. Integrins such as β1 and αvβ3 subunits play a role in mechanotransduction and in the ability of chondrocytes to sense the local microenvironment [Citation33]. These results suggest that suppression of integrin signalling by lunasin might be involved in the effects of lunasin against IL-1β-caused reduction of type II collagen.
Although a number of novel bioactivities of lunasin have been reported over the past decade, there are still certain limitations and challenges. Firstly, existing findings including our results regarding the pharmacological capacities of lunasin are still not mature. A number of these findings have only been reported by individual scientists. Secondly, most of the current findings on the protective effects of lunasin are based on in vitro and animal models. Evidence from human experiments is limited. Thus, future clinical trials are necessary to study the benefits and side effects of this peptide.
In conclusion, our results provide evidence of the chondroprotective effects and abilities of lunasin. These results suggest that lunasin might become a novel pharmacological agent for chondroprotective therapy.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Kang B, Ryu J, Lee CJ, et al. Luteolin inhibits the activity, secretion and gene expression of MMP-3 in cultured articular chondrocytes and production of MMP-3 in the rat knee. Biomol Ther. 2014;22:239–245.
- Haseeb A, Haqqi TM. Immunopathogenesis of osteoarthritis. Clin Immunol. 2013;146:185–196.
- Muto T, Kokubu T, Mifune Y, et al. Temporary inductions of matrix metalloprotease-3 (MMP-3) expression and cell apoptosis are associated with tendon degeneration or rupture after corticosteroid injection. J Orthop Res. 2014;32:1297–1304.
- Zeng JZ, Ma LF, Meng H, et al. Effect of Ginkgo biloba extract on matrix metalloproteinase-3 expression in a rat model of chondrocyte injury. Genet Mol Res. 2015;14:18280–18286.
- Mitchell PG, Magna HA, Reeves LM, et al. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest. 1996;97:761–768.
- Roughley PJ. The structure and function of cartilage proteoglycans. ECM. 2006;12:92–101.
- Serra A, Gallart-Palau X, See-Toh RS, et al. Commercial processed soy-based food product contains glycated and glycoxidated lunasin proteoforms. Sci Rep. 2016;6:26106.
- Galvez AF, de Lumen BO. A soybean cDNA encoding a chromatin-binding peptide inhibits mitosis of mammalian cells. Nat Biotechnol. 1999;17:495–500.
- Chatterjee C, Gleddie S, Xiao CW. Soybean bioactive peptides and their functional properties. Nutrients. 2018;10:1211.
- Fernández-Tomé S, Hernández-Ledesma B. Current state of art after twenty years of the discovery of bioactive peptide lunasin. Food Res Int. 2019;116:71–78.
- Herna ́ndez-Ledesma B, Hsieh CC, de Lumen BO. Antioxidant and anti-inflammatory properties of cancer preventive peptide lunasin in RAW264.7 macrophages. Biochem Biophys Res Commun. 2009;390:803–808.
- Hasandoost L, Akbarzadeh A, Attar H, et al. In vitro effect of imatinib mesylate loaded on polybutylcyanoacrylate nanoparticles on leukemia cell line K562. Artif Cells Nanomed Biotechnol. 2017;45:665–669.
- Dehghanizade S, Arasteh J, Mirzaie A. Green synthesis of silver nanoparticles using Anthemis atropatana extract: characterization and in vitro biological activities. Artif Cells Nanomed Biotechnol. 2018;46:160–168.
- Wittwer CT, Herrmann MG, Moss AA, et al. Continuous fluorescence monitoring of rapid cycle DNA amplification. Biotechniques 1997;22:130–138.
- Sheng B, Wang X, Su B, et al. Impaired mitochondrial biogenesis contributes to mitochondrial dysfunction in Alzheimer's disease. J Neurochem. 2012;120:419–429.
- Troeberg L, Nagase H. Proteases involved in cartilage matrix degradation in osteoarthritis. Biochim Biophys Acta. 2012;1824:133–145.
- Burrage PS, Mix KS, Brinckerh CE. Off matrix metalloproteinases: role in arthritis. Front Biosci. 2006;11:529–543.
- Piecha D, Weik J, Kheil H, et al. Novel selective MMP-13 inhibitors reduce collagen degradation in bovine articular and human osteoarthritis cartilage explants. Inflamm Res. 2010;59:379–389.
- Davidson RK, Waters JG, Kevorkian L, et al. Expression profiling of metalloproteinases and their inhibitors in synovium and cartilage. Arthritis Res Ther. 2006;8:R124.
- Burrage PS, Brinckerhoff CE. Molecular targets in osteoarthritis: metalloproteinases and their inhibitors. Cdt. 2007;8:293–303.
- Dean DD, Martel-Pelletier J, Pelletier JP, et al. Evidence for metalloproteinase and metalloproteinase inhibitor imbalance in human osteoarthritic cartilage. J Clin Invest. 1989;84:678–685.
- Zhang FJ, Yu WB, Luo W, et al. Effect of osteopontin on TIMP-1 and TIMP-2 mRNA in chondrocytes of human knee osteoarthritis in vitro. Exp Ther Med. 2014;8:391–394.
- Cam A, de Mejia EG. RGD-peptide lunasin inhibits Akt-mediated NF-κB activation in human macrophages through interaction with the αVβ3 integrin. Mol Nutr Food Res. 2012;56:1569–1581.
- Hsieh CC, Chou MJ, Wang CH. Lunasin attenuates obesity-related inflammation in RAW264.7 cells and 3T3-L1 adipocytes by inhibiting inflammatory cytokine production. PLoS One. 2017;12:e0171969.
- Jia S, Zhang S, Yuan H, et al. Lunasin inhibits cell proliferation via apoptosis and reduces the production of proinflammatory cytokines in cultured rheumatoid arthritis synovial fibroblasts. Biomed Res Int. 2015;2015:1.
- Price SJ, Pangloli P, Dia VP. Pepsin-pancreatin hydrolysis reduced the ability of lunasin-enriched material to inhibit activation of the inflammasomes in THP-1 human macrophages. Food Funct. 2017;8:4449–4458.
- Jiang Q, Pan Y, Cheng Y, et al. Lunasin suppresses the migration and invasion of breast cancer cells by inhibiting matrix metalloproteinase-2/-9 via the FAK/Akt/ERK and NF-kappaB signaling pathways. Oncol Rep. 2016;36:253–262.
- Shidal C, Inaba JI, Yaddanapudi K, et al. The soy-derived peptide Lunasin inhibits invasive potential of melanoma initiating cells. Oncotarget. 2017;8:25525–25541.
- Inaba J, McConnell EJ, Davis KR. Lunasin sensitivity in non-small cell lung cancer cells is linked to suppression of integrin signaling and changes in histone acetylation. IJMS. 2014;15:23705–23724.
- Cam A, Sivaguru M, de Mejia EG. Endocytic mechanism of internalization of dietary peptide lunasin into macrophages in inflammatory condition associated with cardiovascular disease. PLoS One. 2013;8:e72115.
- Dia VP, de Mejia EG. Lunasin induces apoptosis and modifies the expression of genes associated with extracellular matrix and cell adhesion in human metastatic colon cancer cells. Mol Nutr Food Res. 2011;55:623–634.
- Cam A, de Mejia EG. RGD-peptide lunasin inhibits PI3-kinase/Akt-mediated NF-κ B activation in human and murine macrophages through interaction with alpha v beta 3 integrins. FASEB J. 2012;56:1569–1581.
- Chai DH, Arner EC, Griggs DW, et al. Alphav and beta1 integrins regulate dynamic compression-induced proteoglycan synthesis in 3D gel culture by distinct complementary pathways. Osteoarthritis Cartilage. 2010;18:249–256.