Abstract
Neonatal hypoxia–ischemia is a troublesome disease. Angelica polysaccharide (AP) is proved to have antioxidant effects. Our study was performed to confirm the effects of AP in hypoxia-exposed neural stem cells (NSCs). NSCs were pre-treated with AP and then stimulated with hypoxia. Viability of NSCs was examined by Cell Counting Kit-8 assay. Hypoxia-introduced apoptosis was observed by flow cytometry. Essential regulators of mTOR and Notch signalling pathways were examined by Western blot. mRNA expression was accessed using qRT-PCR. Bcl2/adenovirus EIB 19kD-interacting protein 3 (BNIP3) was altered by transfection. We noticed that NSCs were sensitive to hypoxia-induced apoptosis and showed decreased viability. Moreover, Beclin and light chain 3-II was upregulated while p62 was downregulated. However, AP reversed all these results. Similarly, hypoxia decreased the phosphorylation of mTOR and p70S6K and Notch1 expression while AP increased the phosphorylation of mTOR and p70S6K as well as the expression of Notch1. BNIP3 was upregulated by hypoxia while downregulated by AP. Further experiments demonstrated that overexpression of BNIP3 broken all the effects induced by AP shown in cell viability, apoptosis, autophagy and signalling pathways. Collectively, AP alleviated hypoxia-introduced NSCs damages by maintaining cell viability, blocking apoptosis and autophagy via downregulation of BNIP3 with the activation of mTOR and Notch signalling pathways.
Introduction
Neonatal hypoxia–ischemia is widely a causative factor of troublesome paediatric encephalopathy and referred to perinatal asphyxiation [Citation1]. In detail, hypoxia and ischemic induced cell injury contributes to the ref="#c4">4]. For example, resveratrol alleviates brain damages caused by hypoxia–ischemia in the neonatal rat [Citation5]. Among all these herb medicine, angelica polysaccharide (AP) brings amount of impact on treating injuries induced by different stimulus as AP exhibits multiple biological functions [Citation6,Citation7]. For instance, AP plays important roles in anti-apoptotic and anti-inflammatory effects in LPS-evoked excessive inflammation and apoptosis process in PC12 cells [Citation6]. Importantly, AP reveals potential functions in protecting rat cardiomyocytes H9c2 cells from hypoxia-stimulated inflammatory injury via regulating miR-22 [Citation8]. What makes AP the candidate in our study is dependent on its neuroprotective effect: protecting PC12 neuronal cells not only from H2O2-induced cytotoxicity but also inhibiting cell apoptosis to some extent [Citation9]. Therefore, we inferred that AP might have potential protective effects in neural stem cells (NSCs) against hypoxia-caused lesions.
Here, we established cell model in vitro using hypoxia to stimulate NSCs. Coincidentally, Bcl2/adenovirus EIB 19kD-interacting protein 3 (BNIP3) has been reported not only to exert key functions in neural precursor cells (NPCs) but also show potential roles in responding to hypoxia-induced injury [Citation10]. In addition, as the previous literature reported that AP normally achieves its functions through regulating some other factors, such as miR-223 [Citation7], miR-675 [Citation11] and activating transcription factor 6 (ATF6) pathway [Citation12]. Herein, is there possible regulating relationship between AP and BNIP3? This novel idea draws a blueprint about AP and BNIP3. Therefore, our study investigated the possible relationship between AP and BNIP3 in regulating hypoxia-induced NSCs injury. Moreover, the possible mechanism was further investigated. Our study might offer molecular information which might be helpful for the treatment and prevention of neonatal hypoxia–ischemia injury.
Material and methods
Cell obtain and treatment
All animals were used in accordance with the regulations of the ethics committee of the International Association for the Study of Pain and all protocols were approved by the Institutional Animal Care and Use Committees. NSCs were obtained from the hippocampus of E14 rats (Shandong University Laboratory Animal Center, Shandong, China). Briefly, chloral hydrate (2 mL/kg body weight) was applied to anesthetize rats, and the hippocampus was dissected and digested using 0.125% trypsin (Pierce, Appleton, WI, USA) to obtain the single-cell suspensions. Next, the cells were diluted into a density of 1 × 104 cells per mL in Dulbecco’s modified Eagle medium/nutrient mixture F-12 medium (DMEM/F12) (v/v, 1:1) (Gibco, Gaithersburg, MD, USA). The cell suspensions were plated into flasks at a density of 1 × 104 cells per mL with DMEM/F12, and the culture was maintained in a humidified incubator containing 95% air and 5% CO2 at 37 °C. Five days later, the neurospheres were divided into single-cells, and the obtained single-cell suspension was inoculated into 96-well plate around 1–2 cells per well. To obtain the enough neurospheres, the cells were passaged until three times. To generate hypoxic stress, NSCs were seeded in 96-well plates and cultured in DMEM/F12 medium containing 10% foetal bovine serum (Gibco). The cells were exposed to 1% O2, 94% N2 and 5% CO2 in a hypoxic chamber (STEMCELL, Vancouver, BC, Canada) for 8 h. AP with a purity of 95% was obtained from Ci Yuan Biotechnology Co., Ltd. Shanxi (Xian, China). AP was diluted into the concentration of 8 mg/mL for storage, and then diluted into various concentrations (10, 30, 50, 70 and 100 μM). NSCs were treated with AP at the indicated concentrations for 24 h before stimulated under a hypoxia environment.
Cell viability assay
Cell viability assay was performed using Cell Counting Kit-8 (CCK-8) (Yeasen, Shanghai, China) according to the supplier’s protocol. In brief, 5,000 NSCs were seeded in 96-well plate and treated with AP at different concentrations for 24 h. Continually, the cells were exposed to O2 for 8 h. Afterward, 10 μL of CCK-8 solution was added into plates. The absorbance at 450 nm was measured using a Microplate Reader (Bio-Rad, Hercules, CA, USA) after 1 h.
Cell apoptosis examination
Apoptosis assay was carried out using a flow cytometry based on staining with propidium iodide (PI) and fluorescein isothiocynate (FITC)-conjugated Annexin V (Yeasen, Shanghai, China). In brief, cells suspension was seeded in 96 well-plates in a density of 1 × 105 cells/well. After treatment with AP and exposure to hypoxia, the cells were washed two times in phosphate-buffered saline (Sigma-Aldrich, MO, USA). After centrifugation, the cells were suspended in binding buffer. Then, 5 μL Annexin V-FITC and 5 μL PI were added into the culture and mixed gently. The culture was next put in the dark and incubated for 15 min. The apoptotic cells rate was measured with flow cytometer (Beckman Coulter, USA).
Transfection
To identify the function of BNIP3, we established BNIP3-overexpressed NSCs. In short, cells (2 × 105) were seeded into 96-well plates and cultured until the confluence reached 70%–80%. Next, pc-BNIP3 and its corresponding negative control (NC) pcDNA3.1 (GenePharma Co., Shanghai, China) were transfected into NSCs using Lipofectamine 2000 reagent (Invitrogen).
Western blot
To examine whether the expression of interest protein was altered, Western blot assay was performed. Protein was obtained from NSCs using RIPA lysis buffer (Solarbio, Beijing, China) in the presence of protease inhibitors (Thermo Fisher Scientific, Waltham, MA, USA). The BCA™ Protein Assay Kit (Pierce) was applied for determination of protein concentrations. The Western blot system was constructed using a Bio-Rad Bis-Tris Gel system following the manufacturer’s instructions. The separated proteins were transferred onto polyvinylidene difluoride membrane (Millipore, Bedford, MA, USA) and then incubated with the indicated antibodies at 4 °C overnight. Primary antibodies were prepared in 5% bovine serum albumin (Thermo Fisher Scientific). Then, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody. Detection was performed by capturing the signals and analysing the intensity of the protein bands using Image Lab™ Software (Bio-Rad).
Statistical analysis
All data are shown as mean ± standard deviation (SD) of three times experiments. Statistical analysis was performed using Graphpad 6.0 statistical software (GraphPad, San Diego, CA, USA). The p values were calculated using a one-way analysis of variance and student’s t-test. * (p < .05), ** (p < .01) and *** (p < .001) were all treated as significant difference.
Results
AP alleviated hypoxia-induced NSCs apoptosis and autophagy
Firstly, we detected the viability of NSCs treated with AP at various concentrations (0, 10, 30, 50, 70 and 100 μM). Results revealed that AP at the concentration ≤ 50 μM, conferred no significant (p > .05) effects on viability of NSCs (). However, when the concentration of AP was above 70 μM, the cell viability was significantly increased (70 and 100 μM, both p < .05, ). We have to choose the highest concentration of AP meanwhile this concentration has no obvious influence on viability of NSCs. Hence, AP at the concentration of 50 was chosen in the following experiments.
Figure 1. Angelica polysaccharide (AP) alleviated hypoxia-induced decrement in cell viability. (A) NSCs cells were treated with 10–100 μM of AP. Cell viability was assessed using Cell Counting Kit-8 (CCK-8). NSCs were pre-incubated with 50 μM of AP before submitted to O2 (1%). (B) Cell viability was examined using CCK-8 assay. (C) Apoptotic cells were observed by flow cytometry. (D and E) Total cell lysate was extracted and apoptosis-associated proteins were measured through Western blotting analysis. (F and G) Autophagy related factors were detected by Western blot. Data were presented as mean ± standard deviation (SD). n = 3. *p < .05, ** p < .01, *** p < .001.
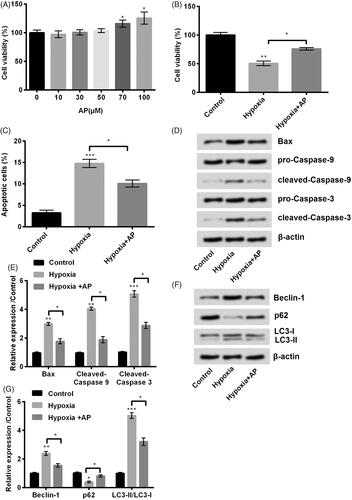
Subsequent experiments were performed to detect the effects of AP on hypoxia-stimulated cells. Interestingly, we found that the viability of NSCs was significantly inhibited under hypoxia environment (p < .01) while increased by AP pre-incubation compared with hypoxia treatment alone (p < .05, ). Furthermore, NSCs pre-treated with AP showed resistance (p < .001) to hypoxia-induced apoptosis (p < .05, ). In line with the results observed by flow cytometry, AP-treated NSCs displayed reduced expression of Bax (p < .05), cleaved-Caspase-3 (p < .05) and cleaved-Caspase-9 (p < .05) which were up-regulated by hypoxia (p < .01 or p < .001) (). In addition, a former study proved that oxygen-glucose deprivation (OGD) induces autophagy process in NSCs which plays important roles in neonatal hypoxia progress [Citation13]. As reported, Beclin-1, p62, light chain-3 (LC3)-I and LC3-II are important regulators for autophagy [Citation14,Citation15]. To validate the protective roles of AP against hypoxia-induced autophagy in NSCs, we next examined the levels of Beclin-1, p62, LC3-I and LC3-II in NSCs after pre-treated with AP and stimulated under hypoxia circumstance. Results from Western blot showed that hypoxia obviously downregulated the expression of p62 (p < .05) while increased the level of Beclin-1 (p < .01) and ratio of LC3-II to LC3-I (p < .001) (). NSCs pre-treated with AP were resistant to hypoxia-induced autophagy as evidenced by the increased expression of p62 (p < .05), the decreased production of Beclin-1 (p < .05) and the reduced ratio of LC3-II to LC3-I (p < .05) (). Taken all together, AP possessed the ability to postpone hypoxia-induced apoptosis and autophagy as well as the decrease in cell viability of NSCs.
AP activated mTOR and Notch cascades in hypoxia-treated NSCs
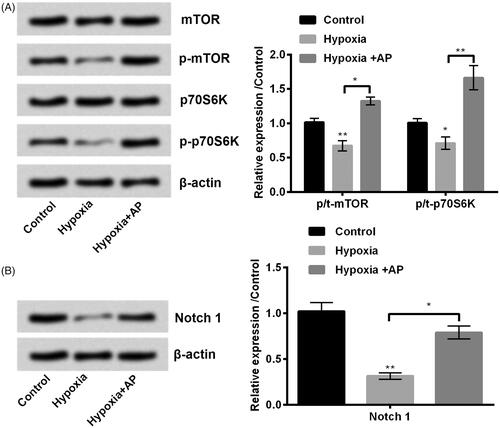
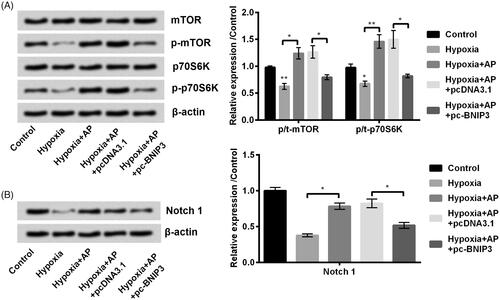
Mammalian target of rapamycin (mTOR) and Notch signalling pathways are reported as the key signalling transduction cascades in hypoxic ischemic brain injury [Citation16–18]. We also examined the AP’s ability to modulate mTOR and Notch. Results showed that hypoxia obviously decreased the phosphorylation of mTOR (p < .01) and p70S6K (p < .05), while AP facilitated the phosphorylation process (p < .05 or p < .01) in hypoxia-exposed NSCs (). Consistently, we observed the phosphorylated Notch1 was repressed in hypoxia-treated NSCs (p < .01), and AP obviously up-regulated the phosphorylated expression of Notch 1 (p < .05) (). Above information indicated that AP restored the activation of mTOR and Notch1 pathways which were blocked by hypoxia.
Figure 2. Angelica polysaccharide (AP) activated mammalian target of rapamycin (mTOR) and Notch signal pathways which were originally blocked by hypoxia treatment. NSCs were pre-incubated with 50 μM of AP before submitted to O2 (1%). The phosphorylation ratio of mTOR, p70S6K (A) and Notch1 (B) was analysed by Western blot. Data were presented as mean ± standard deviation (SD). n = 3. *p < .05, ** p < .01.
Hypoxia-induced BNIP3 was down-regulated by AP
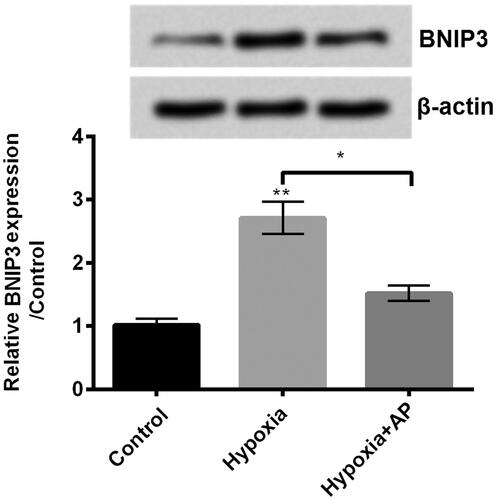
A previous study has proved that BNIP3 has potential roles in regulating hypoxia-induced injury in NSCs [Citation10]. Herein, we wanted to explore the modulatory activity of AP on BNIP3 expression in hypoxia-treated NSCs. In our study, we found that BNIP3 at protein (up) and mRNA (down) levels was significantly enhanced in hypoxia-treated NSCs (p < .01), while BNIP3 expression was decreased in pre-treated NSCs before stimulated by hypoxia (p < .05, ). This finding suggested that BNIP3 might be involved in the protective effect of AP against hypoxia-caused injury.
Figure 3. Angelica polysaccharide (AP) negated the up-regulatory effect of hypoxia on the expression of Bcl2/adenovirus EIB 19kD-interacting protein 3 (BNIP3). NSCs were pre-incubated with 50 μM of AP before submitted to O2 (1%). The expression of BNIP3 was detected by Western blot (up) and qRT-PCR (down). Data were presented as mean ± standard deviation (SD). n = 3. *p < .05, ** p < .01.
AP postponed hypoxia-evoked damages of NSCs via downregulation of BNIP3
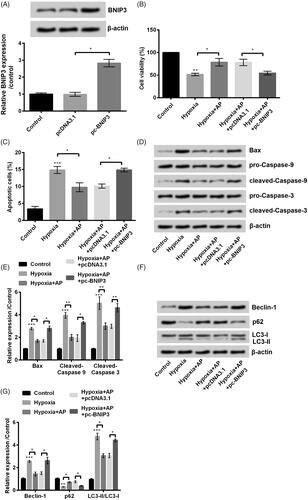
To identify the functions of BNIP3 during the protective process, we transfected pc-BNIP3 and their corresponding NC pcDNA3.1 into NSCs. BNIP3-overexpressed NSCs were efficiently (p < .05) constructed as shown in . Then, we detected the effects of AP on viability, apoptosis and autophagy of BNIP3-overexpressed NSCs under hypoxia environment. Interestingly, we found that BNIP3 overexpression totally reversed the protective effects of AP against hypoxia-induced damages in AP-treated NSCs. In detail, BNIP3-overexpressed NSCs were sensitive to hypoxia-caused damages as evidenced by the decreased viability (p < .05, ) and increased apoptosis (p < .05, ). By the way, Bax, cleaved-Caspase-3 and cleaved-Caspase-9 were all up-regulated in BNIP3- overexpressed NSCs compared with that in pcDNA3.1-transfected cells (). Next, we investigated whether the suppression effects of AP against hypoxia-induced autophagy is dependent on BNIP3. After pre-incubated with AP and stimulated under hypoxia circumstance, BNIP3-overexpressed NSCs showed increased expression of Beclin-1 (p < .05), enhanced ratio of LC3-II to LC3-I (p < .05) and decrement in p62 (p < .05) compared with its NC (p < .05) (). Taken together, overexpression of BNIP3 impaired the protective effects led by AP against hypoxia-caused injury of NSCs, which further suggested that the protective effects of AP on NSCs was through downregulation of BNIP3.
Figure 4. The anti-hypoxia effects of Angelica polysaccharide (AP) in NSCs was through downregulation of Bcl2/adenovirus EIB 19kD-interacting protein 3 (BNIP3). (A) The expression of BNIP3 was altered through transfection with pc-BNIP3 and was detected by Western blot (up) and qRT-PCR (down). NSCs were transfected with BNIP3 and then pre-incubated with 50 μM of AP before submitted to O2 (1%). (B) Cell Counting Kit-8 (CCK-8) assay was carried out to assess cell viability. (C) Flow cytometry was used to observe apoptotic cells. (D and E) Apoptosis-associated proteins were quantified by Western blot. (F and G) Proteins involved in autophagy were detected by Western blot. Data were presented as mean ± standard deviation (SD). n = 3. *p < .05, ** p < .01, *** p < .001.
AP activated mTOR and Notch signalling cascades through downregulation of BNIP3
Similarly as what we performed in , further experiments were performed to check whether BNIP3 mediated the activation of mTOR and Notch pathways. Interestingly, we found that BNIP3 overexpression negated the up-regulatory effects of AP on the phosphorylation of mTOR (p < .05), p70S6K (p < .05) and Notch1 (p < .05, ). In a word, AP might trigger the activation of mTOR and Notch signalling pathways through downregulation of BNIP3.
Figure 5. Angelica polysaccharide (AP) activated mammalian target of rapamycin (mTOR) and Notch1 pathways through downregulation of Bcl2/adenovirus EIB 19kD-interacting protein 3 (BNIP3). NSCs were transfected with BNIP3 and then pre-incubated with 50 μM of AP before submitted to O2 (1%). The phosphorylation ratio of mTOR, p70S6K (A) and Notch1 (B) was detected by Western blot. Data were presented as mean ± standard deviation (SD). n = 3. * p < .05, ** p < .01.
Discussion
Neonatal hypoxia–ischemia is a catastrophic paediatric encephalopathy which causes serious problems in the children growth and also burdened to the family [Citation1]. Our study was designed to looking for a possible breakthrough in Chinese traditional medicine. AP, as a Chinese medicine component extracted from Angelica sinensis, possesses strong anti-oxidative activities [Citation19]. Meanwhile, hypoxia of NSCs is one common reason that causes this disease. Therefore, we constructed cell model in the approach of hypoxia treatment. The current study explored the protective activity of AP against hypoxia-introduced injuries in NSCs. Our results demonstrated that AP moderated hypoxia-induced apoptosis and maintained viability of NSCs to some extent. In addition, further experiment provided a potential mechanism about regulating effects from AP on hypoxia-induced injury. It was BNIP3 that AP achieved its protective effects in hypoxia-treated NSCs.
Amount of studies have corroborated the protective functions of AP in various kinds of injuries [Citation20,Citation21]. Preeminently, AP treatment elevates the activity of antioxidant enzymes in myocardial ischemia-reperfusion rats [Citation22]. In H2O2-caused chrondrocyte injuries, AP maintains the activity of superoxide dismutase and catalase and represses the production of malondialdehyde [Citation23]. Consistently, AP blocks the generation of reactive oxygen species, release of lactic dehydrogenase and accumulation of malondialdehyde in H2O2-treated H9c2 cells [Citation12]. Because of the significant antioxidant property, AP gains considerable attention and becomes more popular in the treatment and prevention of oxidation-associated diseases [Citation8,Citation12,Citation24]. Particularly, some studies have proved that AP achieves its protective effects via melioration of lipid peroxidation and oxidative stress as well as inhibiting apoptosis [Citation23,Citation25]. In our study, analogously, results showed that AP-treated NSCs were resistant to hypoxia-induced apoptosis and showed the enhanced cell viability, which was consistent with the previous studies.
In addition, as we concerned, Bax, cleaved-Caspase-3 and cleaved-Caspase-9 are apoptotic proteins, which are closely involved in the progression of apoptosis [Citation23]. The downregulation of these related factors stood the same line with the result that AP could inhibit apoptosis to some extent. On the other side, cell autophagy is a vital biological process which participated in multiple cell activities in order to maintain intracellular homeostasis [Citation26]. Besides, Beclin-1, p62, LC3-I and LC3-II are crucial autophagy related factors [Citation27,Citation28]. The decreasing accumulated level of Beclin-1 and LC3-II while increasing level of p62 was validated that AP abated cell autophagy to some extent. Above all, our study has proved the protective functions of AP in hypoxia-stimulated NSCs.
Further experiments were performed to determine whether key signalling cascades were implicated in this process. As we have referred, AP, as a regular Chinese medicine component, it might access functions via modulating signalling pathways. mTOR and Notch pathways have been reported to participate in the regulating cascade system in hypoxic ischemic brain injury [Citation16–18]. In our study, we found that AP activated mTOR and Notch pathways inactivated by hypoxia treatment, which indicated that the activation of mTOR and Notch were of benefit in protecting effects. Similarly, the report that protective effects are inhibited when mTOR is blocked [Citation29], suggesting that mTOR pathway exerts crucial role in protecting cells from injuries.
Furthermore, the underlying mechanism by which AP achieved its functions was determined. As we have introduced, BNIP3 was observed to exert vital roles in hypoxia-induced injury in NSCs [Citation10], which motivated us to explore further about whether BNIP3 was also associated with the regulating process of AP against hypoxia-caused damages. As expected, BNIP3 was enhanced in NSCs under a hypoxia environment while was suppressed after pre-administration with AP, which indicated that BNIP3 might participate. Our results were in line with a previous report, BNIP3 is upregulated by the treatment of hypoxia in keratinocyte [Citation30].
To further identify the role of BNIP3, BNIP3-overexpressed NSCs were constructed. Interestingly, BNIP3 overexpression broken all the protective effects presented in cell viability, apoptosis and autophagy. Previous study has proved that overexpression of BNIP3 upregulates LC3 expression and induces the formation of autophagosomes in acute kidney injury [Citation31], which means that overexpression of BNIP3 promotes autophagy. That study result is consistent with our result. Then cascade reaction might be AP downregulated BNIP3 expression and then BNIP3 expression inhibited autophagy. However, the effects of BNIP3 in our study was inhibiting cell growth which was on the opposite with the previous study that BNIP3 promotes cell motility and migration in keratinocyte [Citation30]. Abovementioned information implied that the protective activity of AP against hypoxia-introduced apoptosis might be mediated by down-regulation of BNIP3. This is also the first time that we observed the possible regulating approach that between AP and BNIP3.
Similarly, we detected whether BNIP3 was also joined the signalling pathways. As what it shown in the results, overexpression of BNIP3 inactivated mTOR and Notch signalling pathways. Previous study referred about the possible relationship that miR-210 could protect PC12 cells by targeting BNIP3 as well as mTOR signalling pathway [Citation32]. However, in their study, the modulatory association of BNIP3 to mTOR pathway is not well elucidated. The result in our study cleared a modulatory role of BNIP3 on mTOR and also provided another explanation that AP regulated hypoxia-induced injury in NSCs. Moreover, we also observed BNIP3 with Notch signalling pathway: overexpression of BNIP3 with inactivation of Notch pathway. However, in malignant glioma cells, inhibition of BNIP3 is along with inactivation of Notch pathway [Citation33]. Therefore, the possible relationship between BNIP3 and signalling pathway are different in injury cells and in cancers.
In conclusion, we found that AP, as a traditional Chinese medicine component, conferred a protective effect in prevention of hypoxia-introduced damages in NSCs by maintaining viability, inhibiting apoptosis and autophagy. Furthermore, we also obtained the underlying mechanism that the functions of AP in all these biological activities were all through regulating BNIP3. Our study shed a light on the approach using traditional Chinese medicine in the treatment of neonatal hypoxia–ischemia damage.
Disclosure statement
The authors declare no conflict of interest.
References
- Miguel PM, Schuch CP, Rojas JJ, et al. Neonatal hypoxia-ischemia induces attention-deficit hyperactivity disorder-like behavior in rats. Behav Neurosci. 2015;129:309–320.
- Vasquez-Vivar J, Shi Z, Luo K, et al. Tetrahydrobiopterin in antenatal brain hypoxia-ischemia-induced motor impairments and cerebral palsy. Redox Biol. 2017;13:594–599.
- Chicha L, Smith T, Guzman R. Stem cells for brain repair in neonatal hypoxia-ischemia. Childs Nerv Syst 2014;30:37–46.
- Sun K, Fan J, Han J. Ameliorating effects of traditional Chinese medicine preparation, Chinese materia medica and active compounds on ischemia/reperfusion-induced cerebral microcirculatory disturbances and neuron damage. Acta Pharm Sinica B. 2015;5:8–24.
- Bian H, Shan H, Chen T. Resveratrol ameliorates hypoxia/ischemia-induced brain injury in the neonatal rat via the miR-96/Bax axis. Childs Nerv Syst. 2017;33:1937–1945.
- Xie Y, Zhang H, Zhang Y, et al. Chinese angelica polysaccharide (CAP) alleviates LPS-induced inflammation and apoptosis by down-regulating COX-1 in PC12 cells. Cell Physiol Biochem. 2018;49:1380–1388.
- Li R, Yin F, Guo Y, et al. Angelica polysaccharide protects PC-12 cells from lipopolysaccharide-induced injury via down-regulating microRNA-223. Biomed Pharmacother. 2018;108:1320–1327.
- Pan H, Zhu L. Angelica sinensis polysaccharide protects rat cardiomyocytes H9c2 from hypoxia-induced injury by down-regulation of microRNA-22. Biomed Pharmacother. 2018;106:225–231.
- Lei T, Li H, Fang Z, et al. Polysaccharides from Angelica sinensis alleviate neuronal cell injury caused by oxidative stress. Neural Regen Res. 2014;9:260–267.
- Walls KC, Ghosh AP, Ballestas ME, et al. bcl-2/Adenovirus E1B 19-kd interacting protein 3 (BNIP3) regulates hypoxia-induced neural precursor cell death. J Neuropathol Exp Neurol. 2009;68:1326–1338.
- Yang J, Shao X, Jiang J, et al. Angelica sinensis polysaccharide inhibits proliferation, migration, and invasion by downregulating microRNA-675 in human neuroblastoma cell line SH-SY5Y. Cell Biol Int. 2018;42:867–876.
- Niu X, Zhang J, Ling C, et al. Polysaccharide from Angelica sinensis protects H9c2 cells against oxidative injury and endoplasmic reticulum stress by activating the ATF6 pathway. J Int Med Res. 2018;46:1717–1733.
- Carloni S, Buonocore G, Balduini W. Protective role of autophagy in neonatal hypoxia-ischemia induced brain injury. Neurobiol Dis. 2008;32:329–339.
- Maejima Y, Isobe M, Sadoshima J. Regulation of autophagy by Beclin 1 in the heart. J Mol Cell Cardiol. 2016;95:19–25.
- Liu H, Dai C, Fan Y, et al. From autophagy to mitophagy: the roles of P62 in neurodegenerative diseases. J Bioenerg Biomembr. 2017;49:413–422.
- Lechpammer M, Wintermark P, Merry KM, et al. Dysregulation of FMRP/mTOR signaling cascade in hypoxic-ischemic injury of premature human brain. J Child Neurol. 2016;31:426–432.
- Lechpammer M, Tran YP, Wintermark P, et al. Upregulation of cystathionine beta-synthase and p70S6K/S6 in neonatal hypoxic ischemic brain injury. Brain Pathol. 2017;27:449–458.
- Braccioli L, Vervoort SJ, Adolfs Y, et al. FOXP1 promotes embryonic neural stem cell differentiation by repressing Jagged1 expression. Stem Cell Reports. 2017;9:1530–1545.
- Ji P, Wei Y, Xue W, et al. Characterization and antioxidative activities of polysaccharide in Chinese angelica and its processed products. Int J Biol Macromol. 2014;67:195–200.
- Wang K, Song Z, Wang H, et al. Angelica sinensis polysaccharide attenuates concanavalin A-induced liver injury in mice. Int Immunopharmacol. 2016;31:140–148.
- Xia JY, Fan YL, Jia DY, et al. [Protective effect of Angelica sinensis polysaccharide against liver injury induced by D-galactose in aging mice and its mechanisms]. Zhonghua Gan Zang Bing za Zhi 2016;24:214–219.
- Zhang S, He B, Ge J, et al. Extraction, chemical analysis of Angelica sinensis polysaccharides and antioxidant activity of the polysaccharides in ischemia-reperfusion rats. Int J Biol Macromol. 2010;47:546–550.
- Zhuang C, Xu NW, Gao GM, et al. Polysaccharide from Angelica sinensis protects chondrocytes from H2O2-induced apoptosis through its antioxidant effects in vitro. Int J Biol Macromol. 2016;87:322–328.
- Xiao H, Xiong L, Song X, et al. Angelica sinensis polysaccharides ameliorate stress-induced premature senescence of hematopoietic cell via protecting bone marrow stromal cells from oxidative injuries caused by 5-fluorouracil. Int J Mol Sci. 2017;18:E2265.
- Cao P, Sun J, Sullivan MA, et al. Angelica sinensis polysaccharide protects against acetaminophen-induced acute liver injury and cell death by suppressing oxidative stress and hepatic apoptosis in vivo and in vitro. Int J Biol Macromol. 2018;111:1133–1139.
- Li Z, Yuan Y, Meng Y, et al. Autophagy upregulation ameliorates cell injury in Sequestosome 1 knockout podocytes in vitro. Biochem Biophys Res Commun. 2017;490:98–103.
- Lamark T, Svenning S, Johansen T. Regulation of selective autophagy: the p62/SQSTM1 paradigm. Essays Biochem. 2017;61:609–624.
- Bai X, Liu S, Yuan L, et al. Hydrogen-rich saline mediates neuroprotection through the regulation of endoplasmic reticulum stress and autophagy under hypoxia-ischemia neonatal brain injury in mice. Brain Res. 2016;1646:410–417.
- Zeng Q, Fu Q, Wang X, et al. Protective effects of sonic hedgehog against ischemia/reperfusion injury in mouse skeletal muscle via AKT/mTOR/p70S6K signaling. Cell Physiol Biochem. 2017;43:1813–1828.
- Zhang J, Zhang D, Yan T, et al. BNIP3 promotes the motility and migration of keratinocyte under hypoxia. Exp Dermatol. 2017;26:416–422.
- Ishihara M, Urushido M, Hamada K, et al. Sestrin-2 and BNIP3 regulate autophagy and mitophagy in renal tubular cells in acute kidney injury. Am J Physiol Renal Physiol. 2013;305:F495–F509.
- Luan Y, Zhang X, Zhang Y, et al. MicroRNA-210 protects PC-12 cells against hypoxia-induced injury by targeting BNIP3. Front Cell Neurosci. 2017;11:285.
- Du Y, Li J, Xu T, et al. MicroRNA-145 induces apoptosis of glioma cells by targeting BNIP3 and Notch signaling. Oncotarget 2017;8:61510–61527.
