?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In this study, sevoflurane (SF) loaded, Fas ligand conjugated reduced graphene oxide (rGO) system is fabricated as a therapeutic agent to target brain ischaemic region. The fluorescence investigation of mice brain denoted that the encapsulated SF in rGOs adsorbed with Fas ligand antibody could be significantly distributed to the ipsilateral side of the ischaemic brain. In addition, the immune-histochemical assay presented that the specific nanoparticles especially deposited in the ischaemic part of the tested mice model. Furthermore, SF encapsulated rGO system exhibited noticeable progress in the brain damage along with neurological deficit post ischaemia with limited dosages in contrast to regular SF. Additionally, Rhodamine labelled nanoparticles were used to find whether Fas ligand antibody has the ability to lead the SF-encapsulated nano rGO to enter the ischaemic part of brain as well as carry out neuro-protection. Overall, these experimental findings suggested that rGOs conjugated Fas ligand system could be treated as an ideal brain targeting drug for cerebral ischemia.
Introduction
Ischaemia is the shortage of oxygen and glucose supply required for cellular metabolism while ischaemic stroke is a serious medical condition that results from occluded cerebral vessel caused by the obstruction of blood supply to a part of the brain. Nowadays, tissue plasminogen activator (tPA) is a barely available healing remedy against acute ischaemic stroke which targets on thrombus in the blood vessels [Citation1]. Although thrombolytic therapy is in use, ischaemic stroke has instigated adult disability in the contemporary period. In order to reduce the hazardous consequences of stroke, researchers persistently continued to explore rather effective recovery ways that could limit the damage in ischaemic patients. In this current scenario, a potent therapeutic remedy should be intended to prevail over the existing drawbacks in the conventional drug delivery system by targeting the brain to reduce the neural tissue damage. Substantial research on neuro-therapeutic agents has focused on the blood–brain barrier (BBB), as most of the molecules possess restricted permeability across the blood-brain barrier system [Citation2,Citation3]. However, competent BBB penetration ability and brain circulation serve as fundamental criteria for brain levelling agents [Citation4,Citation5]. As a consequence, the latest drug design approaches significantly concentrate on the bioavailability and BBB infiltration capability of the drugs [Citation6]. Most effective neurovascular ischaemic therapeutic agents principally target on the ischaemic tissue, thus we estimated that Fas ligand conjugated system could be an appropriate contender for the treatment of brain-targeted cerebral ischaemia [Citation7].
In the recent times, graphene has grabbed the immense interest of researchers because of its wide range of physicochemical properties such as tremendous mechanical strength, strong electron mobility, amazing electronic transport as well as rich electrical conductivity [Citation8–10]. In addition, graphene demonstrated significant applications as nanomaterials [Citation11], bactericidal [Citation12–14], antiviral [Citation15], targeting and therapy [Citation16–18], cancer cell imaging [Citation19], disease diagnosis and drug delivery [Citation11,Citation20,Citation21]. Nanomaterial science has spotted a sparkling path in the advancement of therapeutics, in particular, graphene oxide (GO) was established as a nano-carrier for drug transport in the field of medicine. In comparison with the rest of the carbon materials like carbon nanotubes and fullerenes, GO has the ability to accommodate greater surface area and bear enhanced solvent dispersal potential [Citation22]. In addition, stable colloidal GO suspensions can be prepared due to the presence of hydrogen bonds among H2O molecules and polar functional groups on the GO surface [Citation23]. Interestingly a number of nanoparticles were developed on the surface of GO or GO was reduced to enhance its optical properties. In particular, some literature denoted that reduced graphene oxide (rGO) exhibited considerably improved NIR absorption in contrast to that of GO.
For the preparation of rGO sheets, a great deal of research has endeavored through different synthetic approaches like chemical vapor deposition [Citation24], chemical reduction of GO [Citation25–27] along with micromechanical cleavage. Among them, chemical reduction of GO is regarded to possess the paramount potential because of its economic availability for large-scale synthesis. Moreover, chemically synthesized graphene is found to exhibit hydrophobic nature as well as can readily accumulate through effective pi–pi stacking as well as van der Waals interactions existing between the graphene sheets (GS) [Citation28,Citation29].
Therefore, it is apparent that the evasion of graphene sheets aggregation would be of immense significance in order to uphold the characteristic properties in distinctive graphene sheets. Consequently, it is believed that functionalization would be an appropriate approach to seize graphene agglomeration, by making use of both covalent and non-covalent methods. In the recent times, a unique, non-toxic, and environmentally benign method for the synthesis of graphene sheets has been developed by making use of L-ascorbic acid (AA), casein, and glucose as reducing and functionalizing agents [Citation30]. However, usage of some reducing sugars and numerous plant extracts has been earlier reported in the preparation of various nanomaterials [Citation31–34]. In particular, plant extracts of pomegranate [Citation35], Terminalia bellirica [Citation31], and Terminalia chebula [Citation36] have been also employed in the preparation of graphene sheets.
Plant extracts comprise of several polyphenols, like tannic acid and gallic acid, which play a crucial role as capping and reducing agents throughout the synthesis of silver and gold nanoparticles. Consequently, these polyphenols are expected to serve as reducing agents for GO reduction and switch to quinone forms. Similarly, SF is used in the present work to convert GO to rGO during the preparation of Fas ligand-conjugated rGO loaded with SF (SF/Fas/rGO).
According to our hypothesis, rGO nanoparticles could sustain the shielding effect of drug candidate in in vivo treatment of cerebral ischaemia. As an enhancement to this concept, SF was rGO encapsulated and Fas ligand antibody conjugated in order to carry out extended exploration about its efficiency against focal cerebral ischaemic reperfusion injury in animal models.
Experimental section
Chemicals and animals
All regents and used chemicals were bought from “Sigma-Aldrich Chemical Co”. In this current study, adult wild C57BL/6J male mice models were employed. The rhodamine, which is taken as control along with rhodamine–labelled rGOs, SF loaded Fas ligand conjugated antibody (30 mg per each gram body weight) were administered intravenously into the animal models. The mice were accommodated at 12 h (light and dark cycle) and water and food was made accessible ad libitum. In addition, all animal handling protocols, as well as experimental procedures, were done in compliance as per the guidelines of National Institute of Health (NIH, USA).
Fabrication of Fas ligand-conjugated rGO loaded with SF
To prepare Fas modified SF loaded rGO, the following experiments were performed. Graphene oxide (1 mg) was dispersed ultrasonically in (5 mmol/L) 2–(N–Morpholino) ethane sulfonic acid buffer solution, whose pH should be about 4.0. To activate the GO, MES buffer with (4 mg/mL) EDC and (6 mg/mL) NHS was further mixed with GO–dispersed MES suspension. Initially, the mixture was allowed to stir at 15 °C for about 30 min, followed by centrifugation and washing thrice with (20 mmol/L) phosphate buffer with the intent to wash out excess reagents. Activated GO was redispersed in PBS to interact with (50 μg) Fas ligand for GO modification. An electronic shaker was used to mix the samples for 2 h at 15 °C. Leftover GO active sites were obstructed using 2% BSA in PBS buffer for about 30 min. Later, the obtained solution was subjected to centrifugation at 16,000 rpm for about 10 min to eliminate any unreacted molecules. 20 mg of Fas/rGO was diffused in 16 ml of deionized water, followed by the addition of 10 mg SF with constant stirring for 24 h at room temperature in the absence of light. Thus, formed product (SF/Fas/rGO) was allowed to centrifuge followed by thorough washing by making use of deionized water and then vacuum dried. All supernatants were collected together.
Loading of SF on Fas conjugated rGO
The content of SF in Fas/rGO was measured by recording absorbance at nearly 228 nm by means of a UV spectrophotometer. The drug loading efficiency was determined by using a centrifugal-ultrafiltration technique. 0.5 ml of SF/Fas/rGO suspension was inoculated into centrifugal–ultrafiltration tube and subjected to centrifugation at 10,000 rpm for about 20 min. The concentration of SF in the obtained ultra–filtrate was calculated. Fas/rGO drug loading could be determined by means of the below equation:
(1)
(1)
Measurement of drug encapsulation efficiency of SF
An ultraviolet spectrophotometer was used to determine the content of SF in the SF/Fas/rGO by recording the absorbance at 227 nm. Centrifugal-ultra filtration method was used to measure the drug encapsulation efficacy. ∼0.5 ml of SF/Fas/rGO solution was inoculated into the centrifugal ultrafiltration tube followed by centrifugation at 10,000 rpm for about 20 min duration. The SF concentration in the ultrafiltrate suspension was estimated by using the following equation in order to calculate the drug loading percentage (DL %) and encapsulation efficiency (EE %) of the lipid nanoparticles:
(2)
(2)
While Mc stands for the mass of SF in ultrafiltrate and M0 represents the charged amount of SF in SF/Fas/rGO.
In vitro drug release study
In vitro drug release tests were performed by using (pH 7.4) phosphate buffer saline as a suspension medium. (6 ml) SF/Fas/rGO suspension was added into a (30 ml) PBS solution bearing plastic tube. This tube was later kept on an incubator shaker with a pre-maintained temperature of 50 °C at 60 rpm. At regular time gaps, 1 ml of medium was collected and its concentration was estimated by means of an ultraviolet spectrophotometer in relation to the standard graph. All these drug release experiments were repeated thrice.
Preparation of cerebral ischaemia model
All the mice were firstly sedated with 3% isoflurane and further maintained at 1.5% isoflurane by using a facial mask for the frontal side of the mice heads. Then, a neck incision was made laterally and left collective carotid artery was interpreted to ligate its peripheral branch. Middle cerebral artery (MCA) was occluded by inserting an 11 mm nylon surgical thread intraluminally and cranially starting from the carotid junction. Ischaemic reperfusion was permitted by removal of the thread after 45 min of occlusion. Throughout the surgical procedure, the rectal temperature was carefully monitored while a heating pad is used to maintain the body temperature at 37 °C. Sham-operated group mice endure similar procedure excluding MCA occlusion. The neurological scores were recorded for 24 h. For pharmacological experiments, the mice were lethally sedated after 23 h of reperfusion and brain slices were collected for the quantification of infarct area by making use of crystal violet staining. Image J software was used to evaluate the scanned digital images of the infarct area of mice in terms of percentage.
Drug administration and animal group division
All the experimental rats were randomly distributed into five groups. Sham-operated group, vehicle group, SF-treated group (10 mg/kg), SF-loaded rGO treated group (SF/rGOs, 5 mg/kg), SF-loaded Fas ligand–rGO treated groups (SF/FL/rGOs, 1 mg/kg or 5 mg/kg). An hour prior to the inception of MCAO, the drugs were injected intravenously into the animal at the rate of 0.1 ml volume per 10 g of body weight.
Immuno-histochemical labelling and analysis
All the experimental mice were sedated and perfusion-fixed transcardially with 4% paraformaldehyde in PBS. A Vibrating microslicer (VT1000S; Leica, Wetzlar, Germany) is used to prepare 35 mm thickness coronal brain sections by cutting posterior or anterior to the bregma [Citation37]. Later, the obtained brain sections were incubated for overnight at 4 °C with antibodies against OX-42, 1:300 and Fas ligand, 1:200 further were exposed to fluorescent secondary antibodies. Excluding the primary antibodies, negative staining controls for all the immuno-histochemical methods were carried out. Previously reported procedure was followed to prepare Fluoro-Jade B stained brain slices [Citation38]. The slices were dipped in 0.06% KMNO4 solution for almost 10 min followed by rinsing in double distilled water for about a minute. Later, these slices were later immersed in Fluoro-Jade B staining solution (0.0001%) for about 30 min. Subsequent to staining, the slices were rinsed thrice in distilled water for 1 min. Later, the slices were mounted on top of glass slides. Zeiss–LSM, 510 confocal microscope (Caril Zeiss, Jena, Germany) is used to detect the Immunofluorescence.
Characterization
A DU640, Beckman-Counter ultraviolet-visible spectrophotometer is used for optical absorption measurements. Zetasizer Nano-ZS9 was used to calculate the Zeta potential by means of dynamic light scattering measurements. GO samples were made to drip over freshly-cleaved mica by means of a pipette and air dried for calculating AFM. AFM (CSPM 4000), which is armed with silicon cantilever was used to scan the surface topographic aspects in contact mode. HR-TEM photographs were captured using a JEM-2100 microscope, which is functioned at 200 kV. UV–3600, UV–Vis–NIR spectrophotometer was used to obtain the absorption spectra. A Microcon YM-10, MWCO 3000 centrifuge was used for centrifugation of samples during experiments.
Results and discussion
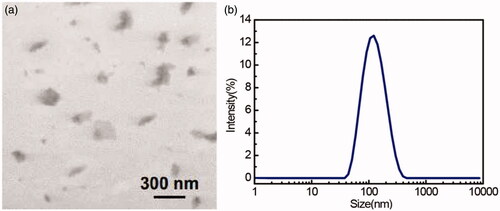
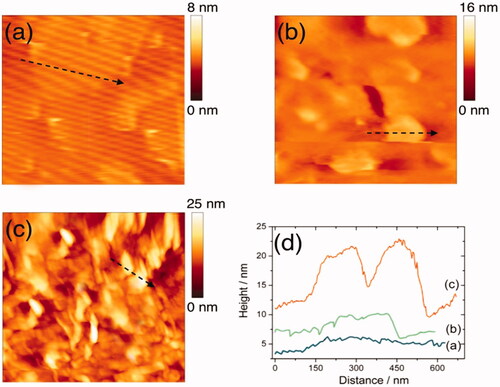
, showed the TEM image and DLS size distribution of fabricated graphene sheets, which showed the thin transparent graphene sheets with an average size of about 100 nm. AFM method was used to determine different stages of the GO surface modifications (Shown in . Lee et al. [Citation39] and Hosseini et al. [Citation40] have earlier reported that an increase in the GO height will be recorded followed by SF treatment and antibody conjugation. However, these experimental studies were taken as reference to operate AFM in order to perform surface morphology characterization of simple GO, SF–activated GO, as well as FL–conjugated GO so as to confirm the occurrence of SF coupling process with the incidence of antibodies on GO surface (). Followed by SF activation, GO has recorded a significant increase in the thickness of ∼4 nm. Subsequently, a 10 nm increase in the FL-conjugated GO height was observed as indicated by the height profile (). Additionally, few bright bands were recorded on the sample as reported by Hosseini et al. experiments [Citation40]. These bright bands resulted because of the FL-antibody deposition on the GO surface (also confirmed from the zeta potential analysis as shown in ). From these surface morphology findings, it was obvious that the FL-antibody has been efficiently capped over the GO surface.
Figure 2. AFM images of bare GO (a), EDC-NHS activated GO (b), antibody conjugated GO (c) on freshly cleaved mica, and corresponding height profiles of the line scans (arrows shown in a,b,c) (d).
Encapsulation efficiency and drug loading percentages
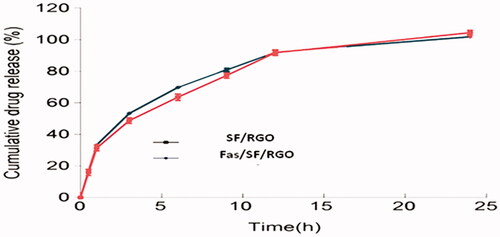
In this experiment, the solvent diffusion method is used to prepare SF and SF loaded rGO (SF/rGOs). The SF/FL/rGO encapsulation efficiency percentage and drug loading percentage has presented not much variation between the SF and SF/FL/rGOs as shown in . However, no significant variations were observed between the in vitro results of the SF and SF/FL/rGOs (.
Table 1. Encapsulation efficiency percentage and drug loading percentage of SF/FL/rGO.
Allocation of FL/SF/rGO in the ischaemic brain
The drug encapsulated rGOs accumulation in the ischaemic brain is further assessed in vivo. In vivo rat model was already used to investigate the SF preconditioning, which has been employed for its time-course neuroprotection in the cerebral ischaemia [Citation41]. Herein, specific accumulation of the drug in the brain ischaemic region with and without Fas ligand conjugation was compared. Intracellular fluorescence tracking imaging technique is employed to study the in vivo cooperative rGOs–Fas ligand specificity in the ischaemic region (). The drug deposition after 24 h to intravenous injection endured significantly in brain regions in both cases. The image analysis after 24 h to rGO intravenous injection has presented fluorescence in both ipsilateral and contralateral sides of the brain in contrast to that of control mice (). In particular, SF encapsulated rGO conjugated with Fas ligand (Fas/SF/rGO) recorded the greatest deposition in the ipsilateral ischaemic brain region, while the highest fluorescence intensity was particularly recorded in the ischaemic region of the brain (). Therefore, this study signified that the conjugation of rGOs and Fas ligand would comprise a perfect ischaemic targeting drug distribution model for cerebral ischaemia. Markedly, the (Fas/SF/rGO) ligand has been, particularly, transported to the ipsilateral ischaemic brain region, instead of being distributed to the entire brain region. This might be attributed to the targeting ability of the Fas ligand towards the specific injured molecules in the ischaemic brain, thus laying the foundation for more efficient injured region distribution and therapy against ischaemic brain damage [Citation38,Citation42]. Therefore, it is anticipated that the multifunctional nanomedicine advancements essentially be competent in treating diseases along with assisting imaging in the efficient targeted drug distribution [Citation4,Citation43].
Neurodegenerative effect of FL/rGO
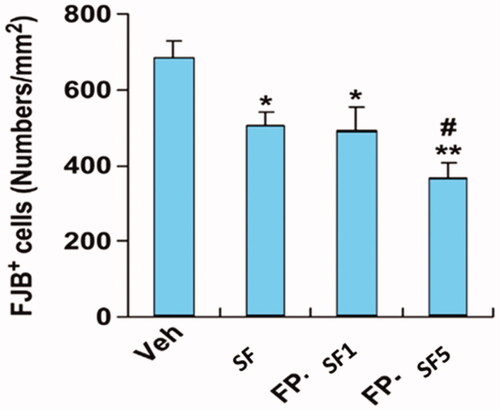
Fluoro-Jade B is used to estimate the neuronal damage at 24 h followed by MCAO (. The count of Fluoro-Jade B-positive cell lines in the vehicle group animals has progressively increased in the ipsilateral brain portion (; ). While Fluoro-Jade B signal staining intensity evidently diminished after the intravenous injection of rGO/SF and FL/SF/rGOs (). Above all, our experimental results denoted that the neuronal degenerative inhibitory efficacy of SF encapsulated FL/rGOs (5 mg/kg) was more significant than that of the regular SF (10 mg/kg) treatment.
Figure 4. Immunoblot images representing corresponding signals of neurovascular damage measured 24 h after MCAO with nano conjugates treatment. Mice on Akt and ERK signalling.
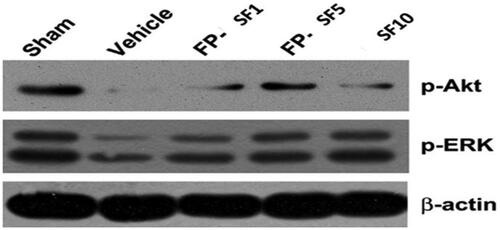
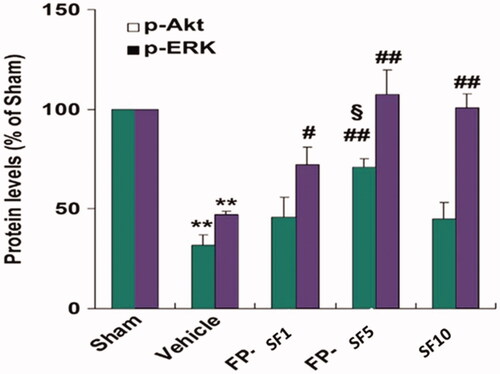
Figure 5. Quantified data for the changes of protein expression after vehicle, regular SF (10 mg/kg), and FP-SF (1 or 5 mg/kg) treatment in MCAO mice (Right panel). Immunodetection of β-lactin was used as a loading control. The data values are expressed as the mean SEM (n = 4). **p < .01 versus sham mice; #p < .05, ##p < .01 versus vehicle mice; §p<.05 versus regular SF treated mice, FP-SF, and SF encapsulated Fas ligand/rGO nanoconjugate.
Neuro-protection ability of Fas ligand/rGO-nanoconjugate
The activation of MMP or calpain induced deprivation of tight-junction proteins along with the subsequent proliferation in the BBB leakage play a fundamental role in the pathogenesis of several neurological diseases [Citation44,Citation45]. Here, a raise of metabolites of spectrin and MMP–9 in MCAO mice along with the ischaemia stimulated decline in the survival protein Akt (Ser 473) and ERK (Thr202/Tyr204) phosphorylation (; ) were monitored. In comparison, we have observed that both SF (10 mg/kg) and Fas/SF/rGOs (SF 1, 5 mg/kg) momentarily affected the suppression of survival signalling. The obtained results displayed that (5 mg/kg) SF/FL/rGOs were more potent on calpain activation and Akt dephosphorylation when compared to that of regular SF treatment. This current neuroprotective mechanism of Fas ligand/rGO encapsulated SF is associated with the defense of neurovascular components.
Figure 6. Effect of SF conjugated Fas ligand/rGO nanoconjugate on Fluoro-Jade B staining. The number of Fluoro-Jade B-stained cells was scored and quantified. The graph is the average values of brain sections after vehicle, regular SF (10 mg/kg), and FP-SF (1 or 5 mg/kg) treatment following MCAO. The data are expressed as the mean ± SEM (n = 8). *p < .05, **p < .01 versus vehicle mice; #p < .05 versus regular SF treated mice. FP-SF, SF encapsulated Fas ligand/rGO-nanoconjugate.
In the current study, rGOs encapsulated Fas ligand antibody presented huge deposition in the ischaemic part of the brain. In addition, SF encapsulated rGOs conjugated Fas ligand antibody depicted notable recovery from brain damage and in neurological dearth post ischaemia. In general, these experimental findings represent that rGOs encapsulated Fas ligand antibody possesses the promising potential for progressive applications in the treatment of brain diseases.
Conclusions
In conclusion, we successfully fabricated the Fas ligand conjugated SF loaded rGO system and characterized using microscopic techniques. Also, we showed that the conjugation of nanoparticles to antibodies or other ligands could significantly deliver the drug by targeting the ischaemic region, thus presenting a challenging path to explore novel ways in the treatment of cerebral ischaemia.
suppli-info_new-cerebral.docx
Download ()Disclosure statement
No potential conflict of interest was reported by the authors.
References
- The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischaemic stroke. N Engl J Med. 1995;333:1581–1588.
- Neuwelt E, Abbott NG, Abrey L, et al. Strategies to advance translational research into brain barriers. Lancet Neurol. 2008;7:84–96.
- Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. Neurotherapeutics 2005;2:3–14.
- Wang C, Cheng L, Liu Z. Drug delivery with upconversion nanoparticles for multi functional targeted cancer cell imaging and therapy. Biomaterials 2011;32:1110–1120.
- Lu RM, Chang YL, Chen MS, et al. Single chain anti-c-Met antibody conjugated nanoparticles for in vivo tumor-targeted imaging and drug delivery. Biomaterials 2011;32:265–274.
- Orive G, Anitua E, Pedraz JL, et al. Biomaterials for promoting brain protection, repair and regeneration. Nat Rev Neurosci. 2009;10:682–692.
- Liu L, Guo K, Lu J, et al. Biologically active core/shell nanoparticles self-assembled from cholesterol-terminated PEG TAT for drug delivery across the blood-brain barrier. Biomaterials 2008;29:1509–1517.
- Geim AK, Novoselov KS. The rise of grapheme. Nature Mater. 2007;6:183–191.
- Akhavan O, Ghaderi E, Rahighi R. Toward single-DNA electrochemical biosensing by graphene nanowalls. ACS Nano. 2012;6:2904–2916.
- Lee C, Wei X, Kysar JW, et al. Measurement of the elastic properties and intrinsic strength of monolayer grapheme. Science 2008;321:385–388.
- Mohanty N, Berry V. Graphene-based single-bacterium resolution bio-device and DNA transistor interfacing graphene derivatives with nanoscale and microscale biocomponents. Nano Lett. 2008;8:4469–4476.
- Akhavan O, Ghaderi E. Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano. 2010;4:5731–5736.
- Akhavan O, Ghaderi E. Escherichia coli bacteria reduce graphene oxide to bactericidal graphene in a self-limiting manner. Carbon 2012;50:1853–1860.
- Akhavan O, Ghaderi E, Esfandiar A. Wrapping bacteria by graphene nanosheets for isolation from environment, reactivation by sonication, and inactivation by near-infrared irradiation. J Phys Chem B. 2011;115:6279–6288.
- Akhavan O, Choobtashani M, Ghaderi E. Protein degradation and RNA efflux of viruses photocatalyzed by graphene tungsten oxide composite under visible light irradiation. J Phys Chem C. 2012;116:9653–9659.
- Robinson JT, Tabakman SM, Liang Y, et al. Ultrasmall reduced grapheme oxide with high near-infrared absorbance for photothermal therapy. J Am Chem Soc. 2011;133:6825–6831.
- Yang K, Wan J, Zhang S, et al. The influence of surface chemistry and size of nanoscale graphene oxide on photothermal therapy of cancer using ultra-low laser power. Biomaterials 2012;33:2206–2214.
- Yang K, Zhang S, Zhang G, et al. Graphene in mice: ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano Lett. 2010;10:3318–3323.
- Yang K, Hu L, Ma X, et al. Multimodel imaging guided photothermal therapy using functionalized graphene nanosheets anchored with magnetic nanoparticles. Adv Mater. 2012;24:1868–1872.
- Sun X, Liu Z, Welsher K, et al. Nano-graphene oxide for cellular imaging and drug delivery. Nano Res. 2008;1:203–212.
- Zhang L, Xia J, Zhao Q, et al. Functional graphene oxide as a nanocarrier for controlled loading and targeted delivery of mixed anticancer drugs. Small 2010;6:537–544.
- Bussy C, Ali-Boucetta H, Kostarelos K. Safety considerations for graphene: lessons learnt from carbon nanotubes. Acc Chem Res. 2013;46:692–701.
- Shih CJ, Lin S, Sharma R, et al. Understanding the pH-dependent behavior of graphene oxide aqueous solutions: a comparative experimental and molecular dynamics simulation study. Langmuir 2012;28:235–241.
- Eizenberg M, Blakely JM. Carbon monolayer phase condensation on Ni (111). Surf Sci. 1979;82:228–236.
- Bagri A, Mattevi C, Acik M, et al. Structural evolution during the reduction of chemically derived graphene oxide. Nature Chem. 2010;2:581–587.
- Park S, Ruoff RS. Chemical methods for the production of graphenes. Nat Nanotechnol. 2009;4:217–224.
- Stankovich S, Dikin DA, Piner RD, et al. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007;45:1558–1565.
- Wang G, Yang J, Park J, et al. Facile synthesis and characterization of graphene nanosheets. J Phys Chem C. 2008;112:8192–8195.
- Si Y, Samulski ET, Hill C, et al. Synthesis of water soluble graphene. Nano Lett. 2008;8:1679–1682.
- Paredes JI, Villar-Rodil S, Fernandez-Merino MJ, et al. Environmentally friendly approaches toward the mass production of processable graphene from graphite oxide. J Mater Chem. 2011;21:298–306.
- Maddinedi SB, Mandal BK. Biofabrication of reduced graphene oxide nanosheets using Terminalia bellirica fruit extract. Current Nanosci. 2015;12:94–102.
- Farnosh T, Masoud SN, Fatemeh M. Green synthesis of flower-like CuI microstructures composed of trigonal nanostructures using pomegranate juice. Mater Lett. 2013;100:133–136.
- Farnosh T, Masoud SN, Davood G, et al. Application of glucose as a green capping agent and reductant to fabricate CuI micro/nanostructures. Mater Res Bull. 2014;49:14–20.
- Samira M, Faezeh S, Masoud SN. Sol–gel auto combustion synthesis of BaFe12O19nanoceramics by using carbohydrate sugars as a novel reducing agent. Adv Powder Technol. 2015;26:1348–1354.
- Farnosh T, Masoud SN, Alireza B, et al. Green synthesis and characterization of graphene nanosheets. Mater Res Bull. 2015;63:51–57.
- Maddinedi SB, Mandal BK, Vankayala R, et al. Bioinspired reduced graphene oxide nanosheets using Terminalia chebula seeds extract. Spectrochim Acta A Mol Biomol Spectrosc. 2015;145:117–124.
- Han F, Chen YX, Lu YM, et al. Regulation of the ischemia-induced autophagy-lysosome processes by nitrosative stress in endothelial cells. J Pineal Res. 2011;51:124–135.
- Lu YM, Tao RR, Huang JY, et al. P2X7 signaling promotes microsphere embolism-triggered microglia activation by maintaining elevation of Fas ligand. J Neuroinflamm. 2012;9:172.
- Lee JS, Joung H, Kim M, et al. Graphene-based chemiluminescence resonance energy transfer for homogeneous immunoassay. ACS Nano. 2012;6:2978–2983.
- Hosseini S, Ibrahim F, Djordjevic I, et al. Synthesis and processing of ELISA polymer substitute: the influence of surface chemistry and morphology on detection sensitivity. Appl Surf Sci. 2014;317:630–638.
- Jean-Laurent C, Lionel JV, Chahrazad M. Sevoflurane preconditioning against focal cerebral ischemia. Anesthesiology 2009;110:1271–1278.
- Shioda N, Han F, Moriguchi S, et al. Constitutively active calcineurin mediates delayed neuronal death through Fas-ligand expression via activation of NFAT and FKHR transcriptional activities in mouse brain ischemia. J Neurochem. 2007;102:1506–1517.
- Lu RM, Chang YL, Chen MS, et al. Single chain anti-c-Met antibody conjugated nanoparticles for in vivo tumor-targeted imaging and drug delivery. Biomaterials 2011;32:3265–3274.
- Zhang GS, Tian Y, Huang JY, et al. Theg-Secretase blocker DAPT reduces the permeability of the blood-brain barrier by decreasing the ubiquitination and degradation of occluding during permanent brain ischemia. CNS Neurosci Ther. 2013;19:53–60.
- Tai SH, Chen HY, Lee EJ, et al. Melatonin inhibits post ischaemic matrix metalloproteinase-9 (MMP-9) activation via dual modulation of plasminogen/plasmin system and endogenous MMP inhibitor in mice subjected to transient focal cerebral ischemia. J Pineal Res. 2010;49:332–341.