Abstract
Oridonin (Orid) has been diffusely applied to remedy dissimilar cancers. Howbeit, the influence of Orid in ischemic heart disease (IHD) remains imprecise. The current study uncovered the functions of Orid in hypoxia-caused apoptosis and autophagy in H9c2 cells. H9c2 cells received hypoxia and Orid manipulation, cell viability, apoptosis, apoptosis-interrelated factors and autophagy-correlative factors were appraised. After the extraordinary vectors transfections, the impacts of miR-214 inhibition on hypoxia-triggered apoptosis and autophagy were investigated. Further, dual luciferase reporter assay was enforced for ascertaining the pertinence between miR-214 and PTEN. PI3K/AKT/mTOR pathway was finally determined using western blot. We found that, Orid significantly alleviated hypoxia-induced apoptosis and autophagy through regulation their associated proteins in H9c2 cells. Up-regulation of miR-214 was found in hypoxia and Orid co-managed cells, meanwhile, repression of miR-214 obviously annulled the modulatory functions of Orid in hypoxia-evoked apoptosis and autophagy. Additionally, PTEN was forecasted to be a firsthand target of miR-214. Besides, we observed that Orid evoked PI3K/AKT/mTOR activation through elevation of miR-214 in hypoxia-managed H9c2 cells. In conclusion, the amusing results corroborated that Orid relieved hypoxia-caused apoptosis and autophagy via adjusting PI3K/AKT/mTOR pathway through enhancement of miR-214 in H9c2 cells.
Introduction
Myocardial ischemia (MI) is a pathological condition in which the decrease blood perfusion of the heart, resulting in a decrease in oxygen supply to the myocardial tissue and producing an impairment of the energy metabolism of the heart [Citation1,Citation2]. With the improvement of people’s living standards, the prevalence of MI is increasing, which has become a common and frequently-occurring disease in the middle-aged and old people [Citation3]. Ischemic heart disease (IHD) is also informed as coronary artery malady, which is the most shared genre of cardiovascular ailment [Citation4]. There are many risk factors inducing IHD, embracing hypertension, diabetes, hypercholesterolemia, smoking, undue alcohol and obesity [Citation5,Citation6]. In 2015, about 110 million people were diagnosed with IHD, and 8.9 million patients were died from the disease, accounting for 15.9% of all deaths, and it has become the most common cause of death worldwide [Citation5]. The current treatments for IHD include medications, anti-platelet therapy and surgery [Citation7]. However, the side effects of these methods are unavoidable. Thus, it is meaningful to delve a neoteric method for healing IHD.
Oridonin (Orid, C20H28O6) is a bioactive natural substance isolated from the Chinese herb Rabdosia Rubescens [Citation8]. It is also known as rubesecensin A, and its main constituent is 7, 20-epoxy-ent-kauranes [Citation9]. Due to the widely pharmacological functions, Orid has been studied in different research fields. Recent studies have been clarified the antitumour effects of Orid on lung cancer, prostate cancer and breast cancer [Citation10–12]. Additionally, Orid is embroiled in modulation of miscellaneous biological processes, encompassing proliferation, apoptosis and autophagy [Citation13]. The diverting research of Li et al. announced that Orid could cause apoptosis and autophagy in prostate cancer cells [Citation14]. Further, Ikezoe et al. revealed that Orid could trigger cell growth inhibition in the assorted cancer cells, indicating that Orid might be an innovative and auxiliary therapy for the heterogeneous malignancies [Citation15]. However, there is still lack of evidence to uncover the effect of Orid on IHD in existing research.
In this study, we focus on exploring the effect of Orid on IHD, and uncovering the underlying mechanism. H9c2 cells served as experimental cell line and received hypoxia treatment. The functions of Orid on hypoxia-caused apoptosis and autophagy of H9c2 cells were investigated. The underlying mechanism was explored through determining PI3K/AKT/mTOR pathway. The study might furnish an innovative therapeutic method for healing IHD.
Materials and methods
Cell culture and treatment
H9c2 cells procured from American Type Culture Collection (ATCC, Rockville, MD) were cultured according to the cell culture instruction. In brief, the 75 cm2 flasks (Coming Incorporated, Corning, NY) were used for sub-culturing H9c2 cells. H9c2 cells were detached from flasks using 0.25% trypsin–EDTA solution. Then, 8.0 mL complete growth medium was padded to the cells and aspirated cells by adopting gently pipetting. The cell suspension was transferred to a new culture flask embracing DMEM (LifeTechnologies, Carlsbad, CA) with 10% FBS (Gibco, Gaithersburg, MD). H9c2 cells were situated an incubator encompassing 5% CO2 and 95% air at 37 °C.
For hypoxia manipulation, H9c2 cells were fostered in hypoxic incubator embracing 94% N2, 5% CO2 and 1% O2 for 12 h. Orid picked up from Sigma (St Louis, MO) and diluted into adequate volume of DMSO to configure different concentrations solutions (1, 5, 10 and 20 μM). H9c2 cells were managed with before-mentioned concentrations of Orid for 12 h.
Cell Counting Kit-8 assay
A kit of Cell Counting Kit-8 (CCK-8, Beyotime, Shanghai, China) was applied for the evaluation of the viability of H9c2 cells after manipulation with hypoxia and Orid. Conforming to the kit instruction, the treated H9c2 cells (5 × 103 cells/well) were trained in 96-well plate, and 10 μL of CCK-8 solution was then supplemented into this culture plate and co-fostered with H9c2 cells for 1 h at 37 °C. For the evaluation of the absorbance at 450 nm, the Microplate Reader equipment (Bio-Rad, Hercules, CA, USA) was then immediately carried out.
Flow cytometry assay
Annexin V-FITC/PI kit (Beckman Coulter, Fullerton, CA) was applied for the administration of the percentage of apoptotic H9c2 cells after manipulation with hypoxia and Orid. Briefly, treated H9c2 cells were gathered and were suspended with PBS on ice. Cells were then re-suspended in binding buffer, in the meantime co-cultivated with 10 μL Annexin V-FITC and 5 μL PI for reacting with H9c2 cells for 15 min with sheltering the light. After this, H9c2 cells were re-suspended in binding buffer again, and a flow cytometer equipment (Beckman Coulter, Fullerton, CA) was adopted for analysing the percentage of apoptotic cells.
Cell transfection
The extraordinary miRNA expression vectors of miR-214 mimic, miR-214 inhibitor and the interrelated control named as NC were synthesized by GenePharma Co. (Shanghai, China). The vectors of miR-214 inhibitor and NC were then exploited to transfect into H9c2 cells. MiR-214 mimic was used in the following experiment of dual luciferase reporter assay. The Lipofectamine 3000 reagent (Invitrogen, Carlsbad, CA, USA) was then implemented for enforcing the cell transfection process in consonance with the reagent specification.
Dual luciferase reporter assay
The PTEN 3’-UTR was amplified by PCR and subsequently inserted into the pmiR-Report vector (Ambion, Austin, TX, USA), and the constructed vector named as Wt-PTEN. The Quick-Change Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA, USA) was utilized for the creation of Mut-PTEN vector. After construction, these vectors were co-transfected with the miR-214 mimic and NC into H9c2 cells via applying Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) for 48 h. The luciferase activity was then appraised through utilizing the Dual Luciferase assay (Promega, Beijing, China) in consonance with the specification.
Reverse transcription quantitative real-time PCR (RT-qPCR)
For the extraction of the total RNA from H9c2 cells which were dealt with hypoxia and Orid, the Trizol reagent (Invitrogen, Carlsbad, CA, USA) was applied in this trial. Twenty-five micrograms template RNA was utilized for synthesizing cDNA via adopting miScript II RT Kit (Qiagen, Valencia, CA, USA). MiR-214 expression was analysed via enforcing RT-qPCR trial through applying SYBR Green PCR Master Mix accompanied by Applied Biosystems 7500 Real-Time PCR System (ABI, Foster City, CA, USA). MiR-214 expression was normalized to the housekeeping gene of U6 snRNA, and the correlative outcomes were reckoned via exploiting the 2−ΔΔCt method [Citation16].
Western blot assay
The H9c2 cells disposed with Orid and hypoxia were harvested for protein analysis through implementing western blot trial. The aforementioned stimulated cells were diffused in RIPA buffer (Beyotime, Shanghai, China) embracing protease inhibitor (Sigma-Aldrich). The BCA Protein Assay Kit (Beyotime, Shanghai, China) was applied for the evaluation of the total protein contents. These protein samples were subsequently thawed and equal amount of protein was loaded in each lane and were disconnected by utilizing SDS-PAGE gels. These above proteins were transferred to a PVDF membrane (Millipore, Bedford, MA, USA), and sealed in 5% BSA for 1 h at 37 °C. The membrane was the probed with rabbit monoclonal antibodies or polyclonal antibodies to Pro-Caspase-3 (ab32150), Cleaved-Caspase-3 (ab2302), Pro-Caspase-9 (ab138412), Cleaved-Caspase-9 (ab2324), Pro-Poly(ADP-ribose) polymerases (PARP, ab74290), Cleaved-PARP (ab32064), p62 (ab155686), Beclin-1 (ab62557), LC3-B (ab48394), PTEN (ab32199), p-PI3K (ab182651), t-PI3K (ab191606), p-AKT (ab38449), t-AKT (ab8805), p-mTOR (ab109268), t-mTOR (ab2732) and β-actin (ab227387, Abcam, Cambridge, UK) overnight at 4 °C. Afterward, the suited second antibody (ab205718, Abcam, Cambridge, UK) was co-fostered with the PVDF membrane for 1 h at indoor temperature. The chemiluminescence reagents (Amersham Biosciences, Uppsala, Sweden) were applied to make bands visible, exposed them to X-ray films, and quantify them via scanning the films exploiting Image-Pro Plus 6.0 software (Bio-Rad).
Statistical analysis
The consequences in the current research were analysed via adopting GraphPad 6.0 statistical software (San Diego, CA, USA). The data of two groups or multiple groups were reckoned via employing Student’s t-test and one-way ANOVA along with Duncan’s post hoc test. Significances were indicated at p < .05. All assays were independently performed three times.
Results
Orid relieves hypoxia-induced cell apoptosis in H9c2 cells
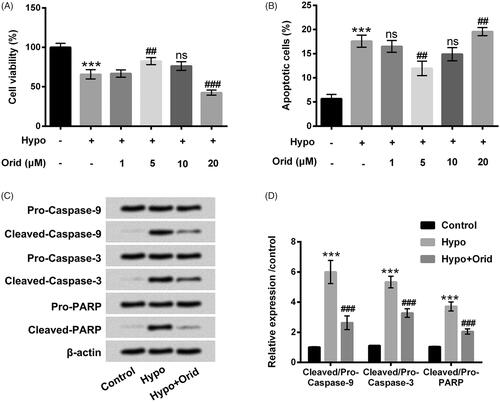
H9c2 cells received hypoxia management to construct a cell injury model, and then the diverse doses of Orid (1, 5, 10 and 20 μM) were applied to stimulate these cells to uncover the regulatory effect of Orid on H9c2 cells apoptosis induced by hypoxia. In , cell viability was significantly restrained in hypoxia-managed H9c2 cells compared to that in untreated cells (p < .001). After stimulation with the diverse doses of Orid, we observed that Orid at 5 μM was recovered the viability of H9c2 cells injured by hypoxia (p < .01). However, Orid at 20 μM was further decreased cell viability of H9c2 cells after manipulation with hypoxia (p < .001). There was a slight declination of cell viability of H9c2 cells triggered by Orid at 10 μM was observed in H9c2 cells as compared with 5 μM Orid-managed group. To explore the effect of Orid on cell apoptosis, flow cytometry and western blot assays were enforced. The percentage of apoptotic H9c2 cells was significantly induced by hypoxia (p < .001), however, substantially diminished by Orid at 5 μM (p < .01, ). After manipulation with Orid at 10 and 20 μM, cell apoptosis of H9c2 cells was obvious expedited as contrasted to 5 μM Orid-managed group (). These outcomes indicated that Orid triggered the growth inhibition in H9c2 cells in a dose-dependent manner. The 5 μM Orid was elected to dispose H9c2 cells in the follow-up trials. Meanwhile, elevations of Cleaved-Caspase-9, Cleaved-Caspase-3 and Cleaved-PARP were discovered in hypoxia-managed cells (p < .001). However, Orid manipulation significantly reversed hypoxia-increased these factors protein levels (p < .001, ). Protein levels of Pro-Caspase-9, Pro-Caspase-3 and Pro-PARP were unaffected by hypoxia and Orid treatment. These results together suggested that Orid at 5 μM could relieve hypoxia-caused cell apoptosis in H9c2 cells.
Figure 1. Effect of Orid on hypoxia-caused cell apoptosis in H9c2 cells. (A) CCK-8 assay was executed for the examination of the viability of H9c2 cells after management with hypoxia and diverse dosages of Orid (1–20 μM); (B) flow cytometry was implemented for the determination of the percentage of apoptotic cells after manipulation with hypoxia and disparate concentrations of Orid (1–20 μM); (C, D) Western blot assay was enforced for the assessment of Pro/Cleaved-Caspase-9, Pro/Cleaved-Caspase-3 and Pro/Cleaved-PARP in hypoxia and Orid (5 μM) co-managed H9c2 cells. ***p < .001: hypoxia group vs. control group; ##p < .01, ###p < .001: hypoxia + Orid group vs. hypoxia group; ns: not significant.
Orid relieves hypoxia-caused cell autophagy in H9c2 cells
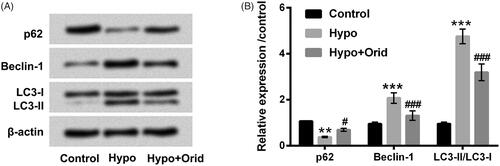
Cell autophagy, like apoptosis, is a very important biological phenomenon and involves in various processes such as biological development and growth. It is well known that p62 and BeclinD1 are important regulators in autophagy. Moreover, microtubule-associated protein 1 light-chain (LC3)-I/II has been widely used to monitor the activity of autophagy. In the present study, to delve the impact of Orid on autophagy in H9c2 cells, we examined these key factors (p62, BeclinD1 and LC3-II) protein levels in hypoxia and Orid treated H9c2 cells. Results in revealed that hypoxia treatment down-regulated p62 protein level (p < .01) and up-regulated Beclin-1 protein level (p < .001). However, the results of hypoxia affected p62 and Beclin-1 protein levels were markedly reversed by Orid manipulation (p < .05 or p < .001). Additionally, we observed that autophagosome marker LC3-II that increased by hypoxia was notably attenuated by Orid management (p < .001). These results stated that Orid could relieve hypoxia-triggered cell autophagy in H9c2 cells.
Figure 2. Effect of Orid on hypoxia-caused cell autophagy in H9c2 cells. (A, B) Western blot assay was executed for the detection of p62, Beclin-1, LC3-I and LC3-II in hypoxia and Orid (5 μM) co-stimulated H9c2 cells. **p < .01, ***p < .001: hypoxia group vs. control group; #p < .05, ###p < .001: hypoxia + Orid group vs. hypoxia group.
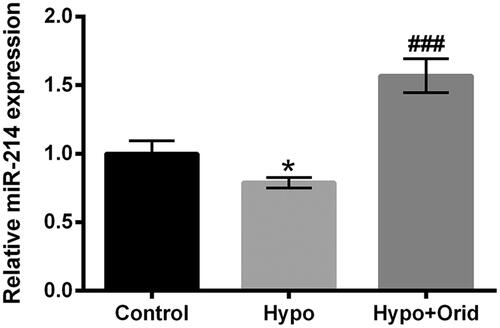
Orid up-regulates miR-214 expression in hypoxia-injured H9c2 cells
The pertinence between Orid and miR-214 in hypoxia-managed H9c2 cells was estimated through applying RT-qPCR trial. The results in presented that hypoxia management significantly restrained miR-214 expression in H9c2 cells (p < .05). Howbeit, the enhancement of miR-214 expression was observed in H9c2 cells co-managed with hypoxia and Orid compared with that in hypoxia alone disposed cells (p < .001). The results indicated that Orid could trigger the elevation of miR-214 in hypoxia-impaired H9c2 cells.
Orid relieves hypoxia-caused cell apoptosis and autophagy by enhancement of miR-214
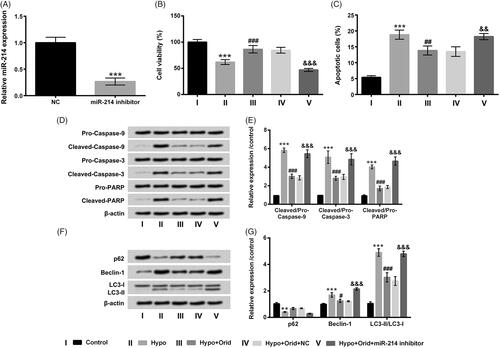
Next, we wanted to further probe whether miR-214 can adjust cell apoptosis and autophagy in H9c2 cells, miR-214 inhibitor was employed to transfect into H9c2 cells to silence miR-214 expression. In , the conspicuous reduction was presented in miR-214 inhibitor-transfected cells (p < .001), which hinting that miR-214 inhibitor was triumphantly transfected into H9c2 cells. Subsequently, the functions of miR-214 inhibitor in cell viability, apoptosis and autophagy in H9c2 cells were further evaluated. We found that the promoting activity of Orid in cell viability (p < .001) and the suppressive activity of Orid in cell apoptosis (p < .01) were obviously abrogated by miR-214 inhibition in hypoxia-managed H9c2 cells (). Additionally, Cleaved-Caspase-9, Cleaved-Caspase-3 and Cleaved-PARP protein levels declined by Orid management were all reversed by miR-214 inhibition (p < .001, ). Outside of these, the functions of Orid in p62, Beclin-1 and LC3-II expression were also inverted by miR-214 inhibition (p < .001, ). All above results indicated that miR-214 might be an momentous regulator of Orid to mitigate hypoxia-caused cell apoptosis and autophagy.
Figure 4. Effect of miR-214 inhibition on hypoxia-caused cell apoptosis and autophagy in H9c2 cells. (A) RT-qPCR assay was enforced for the evaluation of miR-214 expression in H9c2 cells after miR-214 inhibitor and NC vectors transfection; these above-mentioned transfected cells were dealt with hypoxia and Orid (5 μM), (B) CCK-8 assay was administrated for the determination of cell viability in above disposed cells; (C) flow cytometry was enforced for the estimation of cell apoptosis in above disposed cells; (D, E) Western blot assay was implemented for the examination of Pro/Cleaved-Caspase-9, Pro/Cleaved-Caspase-3 and Pro/Cleaved-PARP in above disposed cells; (F, G) Western blot assay was performed for the assessment of p62, Beclin-1, LC3-I and LC3-II in above treated cells. **p < .01, ***p < .001: hypoxia group vs. control group; #p < .05, ##p < .01, ###p < .001: hypoxia + Orid group vs. hypoxia group; &&p < .01, &&&p < .001: hypoxia + Orid + miR-214 inhibitor group vs. hypoxia + Orid + NC group.
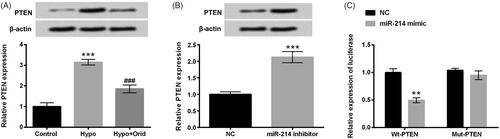
PTEN is a direct target gene of miR-214
PTEN is a newly discovered tumour suppressor gene, belonging to PTP (protein tyrosine phosphatases) gene family. We next explored the mRNA and protein level of PTEN in hypoxia and Orid treated H9c2 cells. Results in showed that PTEN expression in hypoxia-managed H9c2 cells was significantly elevated (p < .001), and PTEN expression in Orid and hypoxia co-stimulated H9c2 cells was predominantly abated (p < .001). Interestingly, the up-regulation of PTEN was observed in miR-214 inhibitor-transfected cells (p < .001, ). Numerous studies confirm that PTEN serves as a straightforward target gene of various miRNAs. However, whether PTEN is a neoteric target gene of miR-214 is still unclear. We then explored the pertinence between PTEN and miR-214 through implementing dual luciferase reporter assay. Results in revealed that the luciferase active in co-transfected with miR-214 mimic and PTEN-Wt significantly repressed as compared with that in co-transfected with NC and PTEN-Wt group (p < .01). Nonetheless, there was no apparent difference in co-transfected with miR-214 and PTEN-Mut cells and in co-transfected with NC and PTEN-Mut cells. These results demonstrated that Orid could down-regulate PTEN expression in hypoxia-impaired H9c2 cells and PTEN was an innovative direct target of miR-214.
Figure 5. PTEN is a neoteric direct target gene of miR-214. Western blot and RT-qPCR were enforced for the examination of PTEN expression in (A) hypoxia and Orid (5 μM) co-managed cells and (B) miR-214 inhibitor and NC vectors transfected cells; (C) dual luciferase reporter assay was applied for the detection of the relationship between miR-214 and PTEN. **p < .01, ***p < .001: hypoxia group vs. control group; miR-214 inhibitor group vs. NC group; Wt-PTEN + miR-214 mimic vs. Wt-PTEN + NC; ###p < .001: hypoxia + Orid group vs. hypoxia group.
Orid activates PI3K/AKT/mTOR pathway via adjusting miR-214 in hypoxia-impaired H9c2 cells
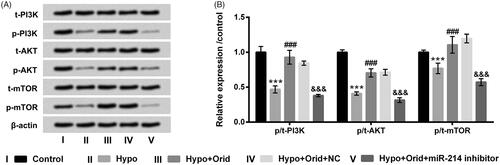
At last, PI3K/AKT/mTOR pathway was appraised to disclose the latent mechanism through carrying out western blot trial. Results in displayed that hypoxia manipulation notably decreased p-PI3K, p-AKT and p-mTOR protein levels in H9c2 cells (p < .001). Orid management ascended p-PI3K, p-AKT and p-mTOR protein levels in hypoxia-disposed H9c2 cells (p < .001). But, when H9c2 cells were transfected with miR-214 inhibitor, the accelerative action of Orid in these above-mentioned proteins was obviously abrogated by miR-214 inhibition (p < .001). The t-PI3K, t-AKT and t-mTOR protein levels had no evident changes in these diverse treated groups. These results hinted that Orid could activate PI3K/AKT/mTOR signalling pathway through mediating miR-214 expression in hypoxia-impaired H9c2 cells.
Figure 6. Effect of Orid on PI3K/AKT/mTOR pathway in hypoxia-managed H9c2 cells. (A, B) H9c2 cells were transfected with miR-214 inhibitor and NC vectors and meanwhile co-managed with hypoxia and Orid (5 μM). Western blot assay was applied for the determination of p/t-PI3K, p/t-AKT and p/t-mTOR in these above disposed cells. ***p < .001: hypoxia group vs. control group; ###p < .001: hypoxia + Orid group vs. hypoxia group; &&&p < .001: hypoxia + Orid + miR-214 inhibitor group vs. hypoxia + Orid + NC group.
Discussion
The current results corroborated that Orid relieved hypoxia-caused apoptosis and autophagy in H9c2 cells. Interesting study revealed that expression level of miR-214 was ascended in hypoxia and Orid co-managed H9c2 cells, and miR-214 inhibition abrogated the regulatory function of Orid in hypoxia-triggered apoptosis and autophagy in H9c2 cells. Additionally, we found that PTEN was hindered by Orid management in hypoxia-impaired cells, and further experiment results stated that PTEN was a direct target of miR-214. Besides, the PI3K/AKT/mTOR signalling pathway was activated by Orid through up-regulating miR-214 expression in hypoxia-treated cells.
In a broad sense, IHD covers several closely related syndromes caused by MI, imbalance in blood supply to the heart and the need for oxygen and nutrients in the heart cells, and it is the leading cause of heart failure [Citation17,Citation18]. Apoptosis is of important pathological significance in the process of MI injury [Citation19]. Once the myocardial cells are damaged, the apoptosis process is initiated, which can decrease the number of myocardial cells and blemish the cardiac function, thereby aggravating MI injury [Citation20]. It is generally known that Caspase family plays a momentous role in the regulation of apoptosis, and activation of Caspase can induce cell apoptosis [Citation21]. Caspase-3, Caspase-9 and PARP are key members of Caspase family, and up-regulations of these protein levels are found in MI injury [Citation22]. Cell autophagy, like apoptosis, is a very important biological phenomenon and involves in various processes, such as biological development and growth [Citation23]. Additionally, in many cardiovascular diseases, most of them are accompanied by changes in autophagy of cardiac myocytes. Hereon, we delved the actions of Orid in hypoxia-caused apoptosis and autophagy in H9c2 cells. Our study found that Orid significantly alleviated hypoxia-triggered apoptosis and autophagy in H9c2 cells. The study indicated that Orid might be an effective agent for healing IHD.
Several studies have demonstrated that miRNAs play vital roles in various diseases, including IHD [Citation24,Citation25]. MiR-214 is an important miRNA, which has been widely corroborated to be embroiled in diverse biological processes. In term of IHD, study from Lu et al. revealed that miR-214 was linked to the severity of IHD [Citation26]. Similarly, Jin et al. found that elevation of miR-214 could be applied to forecast the presence and severity of coronary lesions in IHD patients [Citation27]. However, the functions of miR-214 in hypoxia-caused apoptosis and autophagy in H9c2 cells remain unclear. The study revealed that miR-214 was ascended by Orid management, and miR-214 suppression reversed the inhibitory action of Orid in hypoxia-caused apoptosis and autophagy in H9c2 cells. The results indicated that miR-214 might participate in mediating hypoxia-triggered apoptosis and autophagy in H9c2 cells.
PTEN is a newly discovered tumour suppressor gene, which maps to chromosome 10q23, and has been reported in various cancers [Citation28]. Biological functions for PTEN have been demonstrated in mediating several cell processes, including cell growth, adhesion, metastasis and apoptosis [Citation29,Citation30]. Further, PTEN has been clarified as a direct target of various miRNAs, such as miR-19b, miR-93 and miR-370 [Citation31–33]. In the current study, we appraised PTEN expression in H9c2 cells with Orid and hypoxia co-management or miR-214 inhibitor transfection. We found that PTEN was down-regulated by Orid in hypoxia-treated cells. Moreover, interesting study revealed that miR-214 suppression up-regulated PTEN expression, and we forecasted that PTEN might be a neoteric direct target of miR-214. Whether PTEN is involved in mediating hypoxia-induced apoptosis and autophagy in H9c2 cells is still needed to explore in the future.
PI3K/AKT/mTOR pathway has a wide range of biological effects, which can regulate cell proliferation, apoptosis and autophagy [Citation34]. Previous study has demonstrated that the activated PI3K/AKT/mTOR pathway can inhibit the autophagy process during ischemia injury [Citation35]. Study from Li et al. revealed that Taurine could ease methamphetamine-triggered autophagy and apoptosis in PC-12 cells through modulating mTOR pathway [Citation36]. However, whether Orid relieves hypoxia-induced apoptosis and autophagy via regulating PI3K/AKT/mTOR signalling pathway remain dimness. In this research, we discovered that Orid could activate PI3K/AKT/mTOR pathway through adjusting miR-214 expression in hypoxia-disposed H9c2 cells. The results indicated that PI3K/AKT/mTOR might participate in mediating hypoxia-triggered apoptosis and autophagy.
Taken together, the study demonstrated that Orid could mitigate hypoxia-caused apoptosis and autophagy by activating PI3K/AKT/mTOR pathway via regulation of miR-214 in H9c2 cells. These findings might provide innovative therapeutic strategies for IHD.
Disclosure statement
The authors declare that they have no conflicts of interest with the contents of this article.
References
- Aguilarsanchez Y, Zavalza M, To V, et al. L-type calcium and NCX currents during ischemia and reperfusion in intact mouse hearts. Biophys J. 2016;110:271a–272a.
- He B, Zhao Y, Xu L, et al. The nuclear melatonin receptor RORalpha is a novel endogenous defender against myocardial ischemia/reperfusion injury. J Pineal Res. 2016;60:313–326.
- Boudik F, Reissigova J, Tomeckova M, et al. Myocardial ischemia screening in middle-aged and elderly men. Salud I Ciencia. 2010;17:352–356.
- Jansen MF, Hollander MR, van Royen N, et al. CD40 in coronary artery disease: a matter of macrophages? Basic Res Cardiol. 2016;111:38.
- Wang H, Naghavi M, Allen C, et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–1544.
- Charlson FJ, Moran AE, Freedman G, et al. The contribution of major depression to the global burden of ischemic heart disease: a comparative risk assessment. BMC Med. 2013;11:250–250.
- McCreanor V, Graves N, Barnett AG, et al. A systematic review and critical analysis of cost-effectiveness studies for coronary artery disease treatment. F1000Res. 2018;7:77.
- Luan J, Zhang Y, Yang S, et al. Oridonin: a small molecule inhibitor of cystic fibrosis transmembrane conductance regulator (CFTR) isolated from traditional Chinese medicine. Fitoterapia. 2015;100:88–94.
- Li CY, Wang EQ, Cheng Y, et al. Oridonin: an active diterpenoid targeting cell cycle arrest, apoptotic and autophagic pathways for cancer therapeutics. Int J Biochem Cell Biol. 2011;43:701–704.
- Liu Y, Shi QF, Qi M, et al. Interruption of hepatocyte growth factor signaling augmented oridonin-induced death in human non-small cell lung cancer A549 cells via c-met-nuclear factor-kappaB-cyclooxygenase-2 and c-Met-Bcl-2-caspase-3 pathways. Biol Pharm Bull. 2012;35:1150–1158.
- Ye LH, Li WJ, Jiang XQ, et al. Study on the autophagy of prostate cancer PC-3 cells induced by oridonin. Anat Rec. 2012;295:417–422.
- Wang S, Zhong Z, Wan J, et al. Oridonin induces apoptosis, inhibits migration and invasion on highly-metastatic human breast cancer cells. Am J Chin Med. 2013;41:177–196.
- Shang CH, Zhang QQ, Zhou JH. Oridonin inhibits cell proliferation and induces apoptosis in rheumatoid arthritis fibroblast-like synoviocytes. Inflammation. 2016;39:873–880.
- Li X, Li X, Wang J, et al. Oridonin up-regulates expression of P21 and induces autophagy and apoptosis in human prostate cancer cells. Int J Biol Sci. 2012;8:901–912.
- Ikezoe T, Chen SS, Tong XJ, et al. Oridonin induces growth inhibition and apoptosis of a variety of human cancer cells. Int J Oncol. 2003;23:1187–1193.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(–Delta Delta C(T)) method. Methods. 2001;25:402–408.
- Feigal JP, Boyle SH, Samad Z, et al. Associations between positive emotional well-being and stress-induced myocardial ischemia: well-being scores predict exercise-induced ischemia. J Psychosom Res. 2017;93:14–18.
- Lecour S, Bøtker HE, Condorelli G, et al. ESC working group cellular biology of the heart: position paper: improving the preclinical assessment of novel cardioprotective therapies. Cardiovasc Res. 2014;104:399–411.
- McCully JD, Wakiyama H, Hsieh YJ, et al. Differential contribution of necrosis and apoptosis in myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2004;286:H1923–H1935.
- Wang S, Li Y, Song X, et al. Febuxostat pretreatment attenuates myocardial ischemia/reperfusion injury via mitochondrial apoptosis. J Transl Med. 2015;13:209.
- Ola MS, Nawaz M, Ahsan H. Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Mol Cell Biochem. 2011;351:41–58.
- Zheng C, Wu Z, Tian L, et al. Long noncoding RNA AK12348 is involved in the regulation of myocardial ischaemia-reperfusion injury by targeting PARP and caspase-3. Heart Lung Circ. 2018;27:e51–e58.
- Lee SB, Kim S, Lee J, et al. ATG1, an autophagy regulator, inhibits cell growth by negatively regulating S6 kinase. EMBO Rep. 2007;8:360–365.
- Frost RJ, van Rooij E. miRNAs as therapeutic targets in ischemic heart disease. J Cardiovasc Trans Res. 2010;3:280–289.
- Hu S, Huang M, Li Z, et al. MicroRNA-210 as a novel therapy for treatment of ischemic heart disease. Circulation. 2010;122:S124–S131.
- Lu HQ, Liang C, He ZQ, et al. Circulating miR-214 is associated with the severity of coronary artery disease. J Geriatr Cardiol. 2013;10:34–38.
- Jin Y, Yang CJ, Xu X, et al. MiR-214 regulates the pathogenesis of patients with coronary artery disease by targeting VEGF. Mol Cell Biochem. 2015;402:111–122.
- Wu RC, Li X, Schonthal AH. Transcriptional activation of p21WAF1 by PTEN/MMAC1 tumor suppressor. Mol Cell Biochem. 2000;203:59–71.
- Yamada KM, Araki M. Tumor suppressor PTEN: modulator of cell signaling, growth, migration and apoptosis. J Cell Sci. 2001;114:2375–2382.
- Ma CC, Xiong Z, Zhu GN, et al. Long non-coding RNA ATB promotes glioma malignancy by negatively regulating miR-200a. J Exp Clin Canc Res. 2016;35:1–13.
- Xu J, Tang Y, Bei Y, et al. miR-19b attenuates H2O2-induced apoptosis in rat H9C2 cardiomyocytes via targeting PTEN. Oncotarget. 2016;7:10870–10878.
- Ke Z-P, Xu P, Shi Y, et al. MicroRNA-93 inhibits ischemia-reperfusion induced cardiomyocyte apoptosis by targeting PTEN. Oncotarget. 2016;7:28796–28805.
- Zeng Y, Fu M, Wu GW, et al. Upregulation of microRNA-370 promotes cell apoptosis and inhibits proliferation by targeting PTEN in human gastric cancer. Int J Oncol. 2016;49:1589–1599.
- Liu Z, Wang F, Zhou ZW, et al. Alisertib induces G2/M arrest, apoptosis, and autophagy via PI3K/Akt/mTOR- and p38 MAPK-mediated pathways in human glioblastoma cells. Am J Transl Res. 2017;9:845–873.
- Zhang J, Wang C, Yu S, et al. Sevoflurane postconditioning protects rat hearts against ischemia-reperfusion injury via the activation of PI3K/AKT/mTOR signaling. Sci Rep. 2014;4:7317.
- Li Y, Hu Z, Chen B, et al. Taurine attenuates methamphetamine-induced autophagy and apoptosis in PC12 cells through mTOR signaling pathway. Toxicol Lett. 2012;215:1–7.