Abstract
Background/aim
Pressure ulcers are a disastrous health issue in which inflammation is involved. Emodin possesses biological properties in inflammation. Our study investigated functions of emodin in lipopolysaccharide (LPS)-treated HaCaT cells.
Methods
LPS was used to induce cell inflammation. MTT and flow cytometry were applied for cell viability and apoptosis assays, respectively. Moreover, apoptotic proteins were detected by western blot. Similarly, inflammatory factors and signalling related proteins were also determined by western blot.
Results
Emodin increased cell viability and diminished apoptosis in LPS-treated HaCaT cells. Moreover, cleaved-PARP, cleaved-caspase-3 and cleaved-caspase-9 were all downregulated by emodin. Furthermore, inflammatory factors IL-1β, IL-6, Cox-2 and iNOS were inhibited by emodin in LPS-treated cells. In addition, emodin decreased phosphorylation of p65 and IκBα and the level of PTEN while enhanced phosphorylation of PI3K and AKT. Importantly, emodin increased expression of miR-21 suppressed by LPS and miR-21 downregulation negated the protective functions of emodin.
Conclusions
Emodin promoted cell growth presented by increasing viability and blocking apoptosis process with inflammation inhibition. The protective activity of emodin was mediated by miR-21 up-regulation.
Introduction
Pressure ulcers are a disastrous health issue which occurs due to long-term pressure or shear that caused festering because of sustained ischemia, hypoxia and malnutrition [Citation1,Citation2]. Pressure ulcers commonly happen to the range of senior patients who are immobile or limited mobility or suffer from nerve damages [Citation3,Citation4]. Pressure ulcers treatment brings great pains as well as financial burden to patients [Citation2], although various kinds of approaches and strategies are advanced [Citation5]. Importantly, because the progression of ulcers often goes with inflammation, therefore, how to alleviate inflammation is extremely crucial [Citation6]. Herein, it is of great significance to find appropriate anti-inflammatory drugs for the clinical treatment of pressure ulcers.
Traditional Chinese medicine is commonly used in treating pressure ulcers in a long history [Citation7]. Among them, Rheum palmatum L. (Rhubarb) is widely applied as a kind of purgative medicine in China [Citation8]. In the last few years, studies have confirmed the anti-inflammatory [Citation9] and antimicrobial efficacies of Rhubarb [Citation10]. As a main active component in Rhubarb, 3-methyl-1,6,8-trihydroxyanthraquinone (emodin) confers an anti-inflammatory activity to animal models against multiple types of inflammations, for instance, arthritis [Citation11], lung inflammation [Citation12] and acute pancreatitis [Citation13]. Moreover, emodin protects against jejunum injury via reducing inflammation response in rats [Citation14]. Combined with what we have known that aberrant response of inflammation is one crucial step in the development of pressure ulcers, we explored whether emodin could exert an anti-inflammatory role in pressure ulcers.
On the other side, microRNAs (miRNA or miR) which participated in a considerable number of cellular biological activities draw attentions in understanding its modulatory mechanisms and application for the treatment of multiple diseases [Citation15]. For example, previous literatures demonstrated that miR-885-3p, miR-135b and miR-491-5p are involved in pressure ulcers [Citation16–18]. Among these experimental validated miRNAs, miR-21 attracts our attention because of its significant roles in inflammation. For example, miR-21 fortifies anti-inflammatory functions of macrophage in response to peritonitis [Citation19]. Additionally, the expression of miR-21 could be treated as switch to balance anti-inflammation and pro-inflammation [Citation20]. Based on the abovementioned reports, we speculated that miR-21 might alleviate LPS-triggered over-inflammation.
Our study investigated the roles of emodin to determine whether it has positive effects in anti-inflammation as well as the mechanism behind. We hope the outcome from our data provides a novel insight into the treatment of pressure ulcers.
Materials and methods
Cell culture and treatment
HaCaT cells were obtained from Procell Life Science & Technology (Wuhan, China). Cells were cultured in Eagle's minimum essential medium (MEM) (Procell, Wuhan, China) in addition with 15% foetal bovine serum (FBS; Procell, Wuhan, China). The cell culture environment was set as an atmosphere containing 5% CO2 and 95% air at 37 °C. Lipopolysaccharide (LPS) was purchased from Sigma-Aldrich (St. Louis, MO) and HaCaT cells were treated by LPS (0, 1, 5, 10 and 15 μg/mL) for 6 h. In addition, emodin was also provided by Sigma-Aldrich (St. Louis, MO). Emodin was dissolved in dimethylsulphoxide (Sigma-Aldrich, St. Louis, MO) and diluted it into 100 mM as a stock reagent. Next, 100 mM emodin was prepared with medium into the concentration of 0–20 μM, which was applied to pre-treat HaCaT cells for 12 h before LPS treatment.
MTT assay
To examine viability of HaCaT cells, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) colorimetric assay (Invitrogen, Carlsbad, CA) was performed. Shortly, the cells were grown in a 96-well plate. After treatment, MTT reagent was added and incubated with the cells for 4 h at 37 °C. Absorbance of the culture was detected under Multi-Mode Microplate Reader (Molecular devices, San Jose, CA) at 490 nm.
Apoptosis assay
Propidium iodide (PI) and fluorescein isothiocyanate (FITC)-conjugated Annexin V kit (Beyotime, Shanghai, China) were used for detecting cell apoptosis. The suspended cells were gently mixed and the HaCaT cell suspension was collected, centrifuged for 5 min and the suspension was removed. The HaCaT cells were suspended in a labelled buffer. FITC-conjugated Annexin V staining reagent was added and reacted in the darkness for 15 min. Flow cytometry FACScan (Beckman Coulter, Fullerton, CA) was used for detecting cell apoptosis.
RNA extraction and qRT-PCR
Total RNA extraction was performed according to the instructions of the Trizol kit (Sangon Biotech, Shanghai, China). Reverse transcription reaction kit (Sangon Biotech, Shanghai, China) was used according to the instructions. The reaction product was placed at –20 °C for preservation or immediate amplification. The process of PCR was carried out using SYBR Green qPCR kit (TaKaRa Biotechnology, Kyoto, Japan) by ABI 7500 PCR instrument (Applied Biosystems, Foster City, CA). U6 was the internal control, and the 2−ΔΔCT method was used for calculating mRNA expression [Citation21].
Western blot
RIPA lysis (Beyotime, Shanghai, China) was added to the collected cells. The supernatant was collected after centrifugation for 12 min (1500×g, 4 °C). BCA™ protein assay kit (Pierce, Appleton, WI) was exploited to determine protein content. The proteins were separated in 10–12% sodium dodecyl sulphate polyacrylamide gel electrophoresis and transferred onto nitrocellulose membrane (Millipore, Bedford, MA). Primary antibodies (Abcam, Cambridge, UK; Cell Signaling Technology, Danvers, MA) were added after 5% skimmed milk seal for 1.5 h at room temperature. After washed in PBS three times, the target proteins were probed with secondary antibodies (Cell Signaling Technology, Danvers, MA) for 1 h at room temperature. Image Lab™ Software (Bio-Rad, Hercules, CA) was used to detect the western blot and Odyssey 3.0 software (LI-COR Biosciences, Lincoln, NE) was used to quantify the intensity of the bands.
Transfection
miR-21 inhibitor and the negative control inhibitor (NC inhibitor) were synthesized by Life Technologies (Gaithersburg, MD). miR-21 inhibitor and NC inhibitor were transduced into HaCaT cells using Lipofectamine 3000 reagent (Invitrogen, Carlsbad, CA) with reference to the manufacturer’s protocol.
Statistical analysis
Results were shown as mean ± standard deviation (SD). Statistical analyses were performed by Graphpad 6.0 statistical software (GraphPad, San Diego, CA). p Value was detected by a one-way analysis of variance and Student’s t test. If p < .05, it is treated as significant difference.
Results
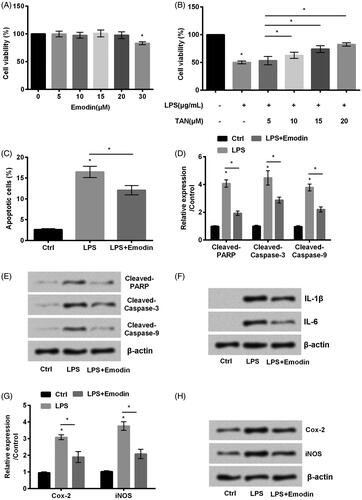
LPS-induced HaCaT cell inflammation
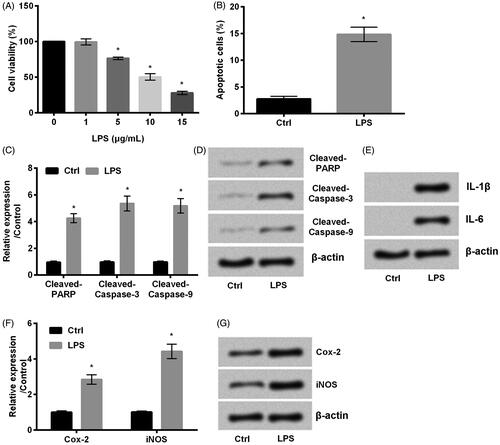
The first set of experiments was set out to induce HaCaT cell injury with LPS. As we expected, cell viability was inhibited and apoptosis was enhanced by LPS (p < .05, ). Furthermore, apoptotic proteins cleaved-PARP, cleaved-caspase-3 and caspase-9 were detected. Interestingly, lined with what we found in , the accumulated levels of these three proteins were increased by LPS (p < .05, ). On the other side, because the interleukin-1β (IL-1β), IL-6, Cox-2 and inducible nitric oxide synthase (iNOS) are inflammatory related factors [Citation22,Citation23], we next examined the alteration. Our result demonstrated that LPS upregulated IL-1β and IL-6 (). Meanwhile, expression of Cox-2 and iNOS was also upregulated by LPS (p < .05, ). Taken together, HaCaT cell inflammation was induced by the treatment of LPS.
Figure 1. LPS stimulates HaCaT cell inflammation. (A) Cell viability, (B) cell apoptosis were detected by MMT assay and flow cytometry, respectively. (C,D) The apoptotic proteins cleaved-PARP, cleaved-caspase-3 and cleaved-caspase-9, (E) the accumulated levels of IL-1β and IL-6, (F, G) Cox-2 and iNOS were all examined by western blot. Results shown as mean ± SD and *p < .05 indicated significant difference.
LPS-induced inflammation was alleviated by emodin
Emodin widely detected in Chinese herbal medicine, was reported to have functions in several different diseases [Citation24]. Hence, our experiments were designed to check whether emodin worked in LPS-induced HaCaT cell injury. It can be seen from that emodin at the concentration of 20 μM did not influence the viability of HaCaT cells but enhanced cell viability in LPS-stimulated cells to the greatest extent (p < .05, ). Next, we observed that emodin decreased apoptosis (p < .05, ) and inhibited these apoptotic proteins cleaved-poly (ADP-ribose) polymerase (PARP), cleaved-caspae-3 and cleaved-caspase-3 expression (p < .05, ). In addition, the inflammation related factors IL-1β and IL-6 () as well as Cox-2 and iNOS (p < .05, ). The above data revealed that emodin abated LPS-induced HaCaT cell inflammation.
Figure 2. Emodin abated HaCaT cell inflammation induced by LPS. (A,B) Cell viability and (C) cell apoptosis were accessed by MTT assay and flow cytometry, respectively. (D,E) The accumulated levels of apoptosis related proteins cleaved-PARP, cleaved-caspase-3 and cleaved-caspase-9, (F) the expression of IL-1β and IL-6 and (G,H) Cox-2 and iNOS were detected by western blot.
NF-κB signal pathway was blocked and PTEN/PI3K/AKT signal was inactivated by emodin
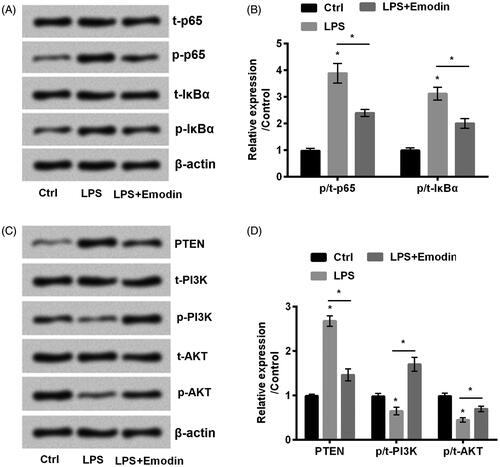
NF-κB and PTEN/PI3K/AKT signal pathways were involved in pressure ulcers [Citation25,Citation26]. Our data revealed that LPS strengthened the phosphorylation of p65 and IκBα (p < .05, ). On the other side, LPS administration promoted PTEN while inhibited phosphorylation of PI3K and AKT compared with control (p < .05, ). However, the administration of emodin reversed the expression results led by LPS. These results suggested that emodin inactivated NF-κB and activated PTEN/PI3K/AKT signal pathways in LPS-stimulated HaCaT cells.
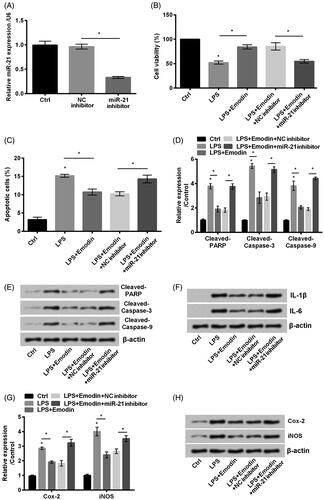
miR-21 expression was upregulated by emodin
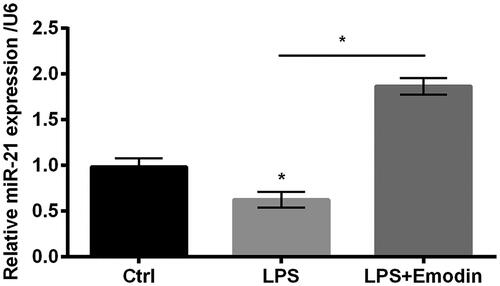
It is apparent that miR-21 is closely involved in the progression of inflammation [Citation20]. It was obviously shown in that LPS diminished miR-21 expression while emodin enhanced expression of miR-21. This crucial data revealed that miR-21 might participate in the alleviating effects by emodin in LPS-stimulated HaCaT cells.
Emodin abated LPS-induced HaCaT cells inflammation via upregulating miR-21
To validate whether miR-21 worked in the protective process mediated by emodin against LPS-caused injuries, miR-21 inhibitor was transfected into HaCaT cells to block miR-21 expression (p < .05, ). Interestingly, we found that transfection with miR-21 inhibitor diminished cell viability (p < .05, ) and accelerated cell apoptosis in LPS-treated HaCaT cells with emodin administration (p < .05, ). At the same time, this treatment increased cleaved-PARP, cleaved-caspase-3 and cleaved-caspase-9 (p < .05, ). In addition, it facilitated expression of IL-1β and IL-6 (p < .05, ) as well as Cox-2 and iNOS (). These results informed us that transfection with miR-21 inhibitor which induced miR-21 silence reversed the effects led by emodin, which implied that the alleviating effects of emodin was via upregulation of miR-21.
Figure 5. miR-21 downregulation aggravated LPS-induced inflammation in HaCaT cells. (A) miR-21 inhibitor was transduced into HaCaT cells. miR-21 level was evaluated by qRT-PCR. (B) Cell viability and (C) cell apoptosis were detected by MTT assay and flow cytometry, respectively. (D,E) The expression of apoptosis associated factors cleaved-PARP, cleaved-caspase-3 and cleaved-caspase-9, (F) the accumulated levels of inflammatory factors IL-1β and IL-6 and (G,H) Cox-2 and iNOS were detected by western blot. Results were shown as mean ± SD and *p < .05 indicated significant difference.
Emodin blocked NF-κB and activated PTEN/PI3K/AKT signal pathways via upregulation of miR-21
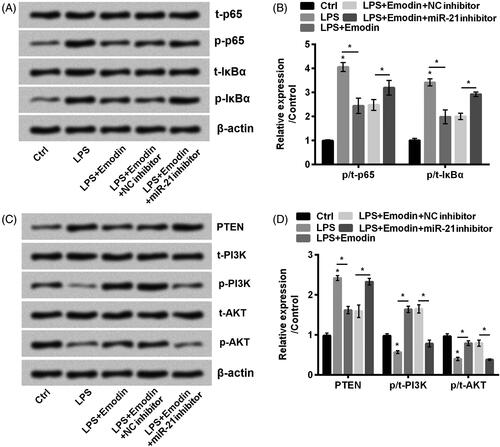
Similarly, we determined the effects of transfection with miR-21 inhibitor and we found it increased phosphorylation of p65 and IκBα (p < .05, ). In addition, it accelerated PTEN expression and meanwhile diminished the phosphorylated expression of PI3K and AKT in LPS-treated HaCaT cells with emodin administration (p < .05, ). To sum results up, results demonstrated that miR-21 downregulation impaired the effects of emodin which suggested that effects of emodin were achieved through upregulation of miR-21.
Discussion
In the occurrence and development of pressure ulcers, the aberrant inflammation was one of the main problems [Citation6]. Inflammatory response is a kind of self-protection and defence mechanism in the face of stimulation. But uncontrolled inflammation can cause tissue damage and even injuries [Citation27]. Our study established an in vitro cell model of pressure ulcers by using LPS to stimulate HaCaT cells. It can be presented in our study that emodin mitigated LPS-induced inflammation via upregulation of miR-21 as well as blunting NF-κB and activated PTEN/PI3K/AKT signal pathways.
LPS is a commonly used stimulus in the field of biomedical experiments [Citation28]. Experimentally, cell viability and cell apoptosis are two vital aspects for evaluating cell growth [Citation29]. HaCaT cells showed decreasing cell viability and increasing apoptosis after LPS treatment, which indicated that LPS brought great effects in cell growth. In addition, cleaved-PARP, cleaved-caspase-3 and cleaved-caspase-9 are three crucial factors [Citation30–32]. Western blot results demonstrated that these three factors are all positively regulated by LPS and down-regulated by emodin, which validated the inhibitory effects of emodin on apoptosis.
On the other side, IL-1β and IL-6 were inflammatory factors. Interestingly, we observed that LPS improved the accumulated levels of IL-1β and IL-6. Moreover, Cox-2 and iNOS are also inflammation associated factors and LPS administration also enhanced these two factors. Next the roles of emodin were investigated. Interestingly, it revealed a positive effect of emodin against over-inflammation evidenced by increase in viability and inhibition of apoptosis as well as inflammatory factor expression. Previous literature pointed out that emodin has its effects not only in cancers but also in inflammations [Citation33]. For example, emodin abates LPS-induced inflammation in RAW264.7 macrophages during which emodin mitigates iNOS, IL-6 and IL-1β genes expression [Citation34], which lined with our own data. Similar results were also observed in the research from Meng et al., that emodin alleviates the accumulated levels of LPS-induced pro-inflammatory cytokines [Citation35].
In addition, it can be shown clearly from our results that emodin blocked NF-κB and activated PTEN/PI3K/AKT signalling. A former literature pointed out that emodin diminishes LPS-induced acute lung inflammation via the inactivation of NF-κB in mice [Citation36], which is consistent with our own study. Moreover, emodin exhibits an anti-apoptotic effect in K562 cells through regulating PTEN/PI3K/AKT signalling pathway [Citation37], which shed a light on that PTEN/PI3K/AKT signalling pathway was involved in emodin-mediated protective process. In addition, miR-21 was widely reported in the process of inflammation [Citation38]. Previous literature presented that miR-21 diminishes apoptosis via activating PTEN/AKT signalling in vitro in cortical neurons [Citation39], which is consistent with our own results. As a consequence, we considered miR-21 as a mediator of emodin in modulation of transduction cascades.
In the end, we also found that emodin inactivated NF-κB and activated PTEN/PI3K/AKT signalling via miR-21. What it was shown in the previous literature that NF-κB signalling brings effects in apoptosis in cardiomyocytes via modulating miR-21 expression in the stress of oxidative [Citation40]. Moreover, miR-21 reveals functions in enhancing cell growth and metastasis with the involvement of PTEN/PI3K/AKT signalling in oesophageal cancer [Citation41]. All these reports previous was lined with what we obtained from our study. Actually, the anti-inflammatory mechanisms of emodin were ascribed to its regulatory role in miR-21 expression in our studies. Consistently, a numerous studies revealed that the protective roles of emodin are mediated by miRs-regulated associated signalling transduction cascades [Citation42–44]. As a whole, the modulatory mechanisms of emodin are complex and are required to be further explored, particularly when miRs are implicated.
In conclusion, we found that emodin promoted cell growth which is presented by increasing cell viability and apoptosis whereas diminished inflammation which were evidenced by inhibiting expression of inflammatory factors IL-1β, IL-6, Cox-2 and iNOS via upregulation of miR-21 as well as modulating NF-κB and PTEN/PI3K/AKT signalling. Our results afford a basic foundation for researching the roles of emodin in the treatment of pressure ulcers. However, clinical practices are still needed in the further study.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- McInnes E, Jammali-Blasi A, Bell-Syer SE, et al. Support surfaces for pressure ulcer prevention. Cochrane Database Syst Rev. 2015;3:CD001735.
- Demarre L, Van Lancker A, Van Hecke A, et al. The cost of prevention and treatment of pressure ulcers: a systematic review. Int J Nurs Stud. 2015;52:1754–1774.
- Allman RM. Pressure ulcer prevalence, incidence, risk factors, and impact. Clin Geriatr Med. 1997;13:421–436.
- Moore ZE, Cowman S. Risk assessment tools for the prevention of pressure ulcers. Cochrane Database Syst Rev. 2008;3:CD006471.
- Niu J, Han L, Gong F. Therapeutic effect of external application of ligustrazine combined with holistic nursing on pressure sores. Med Sci Monit. 2016;22:2871–2877.
- Donato-Trancoso A, Monte-Alto-Costa A, Romana-Souza B. Olive oil-induced reduction of oxidative damage and inflammation promotes wound healing of pressure ulcers in mice. J Dermatol Sci. 2016;83:60–69.
- Zhang QH, Sun ZR, Yue JH, et al. Traditional Chinese medicine for pressure ulcer: a meta-analysis. Int Wound J. 2013;10:221–231.
- Cao YJ, Pu ZJ, Tang YP, et al. Advances in bio-active constituents, pharmacology and clinical applications of rhubarb. Chin Med. 2017;12:36.
- Zhang RZ, Qiu H, Wang N, et al. Effect of Rheum palmatum L. on NF-kappaB signaling pathway of mice with acute liver failure. Asian Pac J Trop Med. 2015;8:841–847.
- Aly MM, Gumgumjee NM. Antimicrobial efficacy of Rheum palmatum, Curcuma longa and Alpinia officinarum extracts against some pathogenic microorganisms. Afr J Biotechnol. 2011;10:12058–12063.
- Hwang JK, Noh EM, Moon SJ, et al. Emodin suppresses inflammatory responses and joint destruction in collagen-induced arthritic mice. Rheumatology (Oxford, England). 2013;52:1583–1591.
- Nemmar A, Al-Salam S, Yuvaraju P, et al. Emodin mitigates diesel exhaust particles-induced increase in airway resistance, inflammation and oxidative stress in mice. Respir Physiol Neurobiol. 2015;215:51–57.
- Wu L, Cai B, Zheng S, et al. Effect of emodin on endoplasmic reticulum stress in rats with severe acute pancreatitis. Inflammation. 2013;36:1020–1029.
- Chen YK, Xu YK, Zhang H, et al. Emodin alleviates jejunum injury in rats with sepsis by inhibiting inflammation response. Biomed Pharmacother = Biomed Pharmacother. 2016;84:1001–1007.
- Vishnoi A, Rani S. MiRNA biogenesis and regulation of diseases: an overview. Methods Mol Biol (Clifton, NJ). 2017;1509:1–10.
- Ji X, Mao J, Zhou S. Rs739837 polymorphism in MiR-885-3p binding site within 3′-untranslated region of vitamin D receptor is associated with a decreased risk of pressure ulcers. Cell Physiol Biochem. 2017;44:2129–2137.
- Yang R, Han L, Zeng X. A functional polymorphism at miR4915p binding site in the 3'UTR of MMP9 gene confers increased risk for pressure ulcers after hip fracture. Oncol Rep. 2018;39:2695–2702.
- Wei S, Wang H. Ligustrazine promoted hypoxia-treated cell growth by upregulation of miR-135b in human umbilical vein endothelial cells. Exp Mol Pathol. 2019;106:102–108.
- Barnett RE, Conklin DJ, Ryan L, et al. Anti-inflammatory effects of miR-21 in the macrophage response to peritonitis. J Leukocyte Biol. 2016;99:361–371.
- Sheedy FJ. Turning 21: induction of miR-21 as a key switch in the inflammatory response. Front Immunol. 2015;6:19.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(–Delta Delta C(T)) method. Methods (San Diego, Calif). 2001;25:402–408.
- Wilms H, Sievers J, Rickert U, et al. Dimethylfumarate inhibits microglial and astrocytic inflammation by suppressing the synthesis of nitric oxide, IL-1beta, TNF-alpha and IL-6 in an in-vitro model of brain inflammation. J Neuroinflammation. 2010;7:30.
- Chen L, Teng H, Fang T, et al. Agrimonolide from Agrimonia pilosa suppresses inflammatory responses through down-regulation of COX-2/iNOS and inactivation of NF-kappaB in lipopolysaccharide-stimulated macrophages. Phytomedicine. 2016;23:846–855.
- Dong X, Fu J, Yin X, et al. Emodin: a review of its pharmacology, toxicity and pharmacokinetics. Phytother Res. 2016;30:1207–1218.
- Romana-Souza B, Dos Santos JS, Monte-Alto-Costa A. Caffeic acid phenethyl ester promotes wound healing of mice pressure ulcers affecting NF-kappaB, NOS2 and NRF2 expression. Life Sci. 2018;207:158–165.
- Jere SW, Houreld NN, Abrahamse H. Role of the PI3K/AKT (mTOR and GSK3beta) signalling pathway and photobiomodulation in diabetic wound healing. Cytokine Growth Factor Rev. 2019. doi:10.1016/j.cytogfr.2019.03.001
- Zhu T, Zhang W, Feng SJ, et al. Emodin suppresses LPS-induced inflammation in RAW264.7 cells through a PPARgamma-dependent pathway. Int Immunopharmacol. 2016;34:16–24.
- Aizawa F, Nishinaka T, Yamashita T, et al. Astrocytes release polyunsaturated fatty acids by lipopolysaccharide stimuli. Biol Pharm Bull. 2016;39:1100–1106.
- Yang N, Liang Y, Yang P, et al. Propofol inhibits lung cancer cell viability and induces cell apoptosis by upregulating microRNA-486 expression. Braz J Med Biol Res = Rev Brasil Pesquisas Med Biol. 2017;50:e5794.
- Casao A, Mata-Campuzano M, Ordas L, et al. Cleaved PARP-1, an apoptotic marker, can be detected in ram spermatozoa. Reprod Domest Anim. 2015;50:688–691.
- Blanco J, Tomas HS, Garcia T, et al. Oral exposure to silver nanoparticles increases oxidative stress markers in the liver of male rats and deregulates the insulin signalling pathway and p53 and cleaved caspase 3 protein expression. Food Chem Toxicol. 2018;115:398–404.
- Kawamoto Y, Ayaki T, Urushitani M, et al. Activated caspase-9 immunoreactivity in glial and neuronal cytoplasmic inclusions in multiple system atrophy. Neurosci Lett. 2016;628:207–212.
- Shrimali D, Shanmugam MK, Kumar AP, et al. Targeted abrogation of diverse signal transduction cascades by emodin for the treatment of inflammatory disorders and cancer. Cancer Lett. 2013;341:139–149.
- Hu B, Zhang H, Meng X, et al. Aloe-emodin from rhubarb (Rheum rhabarbarum) inhibits lipopolysaccharide-induced inflammatory responses in RAW264.7 macrophages. J Ethnopharmacol. 2014;153:846–853.
- Meng G, Liu Y, Lou C, et al. Emodin suppresses lipopolysaccharide-induced pro-inflammatory responses and NF-kappaB activation by disrupting lipid rafts in CD14-negative endothelial cells. Br J Pharmacol. 2010;161:1628–1644.
- Xiao M, Zhu T, Zhang W, et al. Emodin ameliorates LPS-induced acute lung injury, involving the inactivation of NF-kappaB in mice. IJMS. 2014;15:19355–19368.
- Wang CG, Zhong L, Liu YL, et al. Emodin exerts an antiapoptotic effect on human chronic myelocytic leukemia K562 cell lines by targeting the PTEN/PI3K-AKT signaling pathway and deleting BCR-ABL. Integr Cancer Ther. 2017;16:526–539.
- Lu TX, Munitz A, Rothenberg ME. MicroRNA-21 is up-regulated in allergic airway inflammation and regulates IL-12p35 expression. J Immunol (Baltimore, MD: 1950). 2009;182:4994–5002.
- Han Z, Chen F, Ge X, et al. miR-21 alleviated apoptosis of cortical neurons through promoting PTEN-Akt signaling pathway in vitro after experimental traumatic brain injury. Brain Res. 2014;1582:12–20.
- Wei C, Li L, Kim IK, et al. NF-kappaB mediated miR-21 regulation in cardiomyocytes apoptosis under oxidative stress. Free Radic Res. 2014;48:282–291.
- Wu YR, Qi HJ, Deng DF, et al. MicroRNA-21 promotes cell proliferation, migration, and resistance to apoptosis through PTEN/PI3K/AKT signaling pathway in esophageal cancer. Tumor Biol. 2016;37:12061–12070.
- Hua JY, He YZ, Xu Y, et al. Emodin prevents intima thickness via Wnt4/Dvl-1/beta-catenin signaling pathway mediated by miR-126 in balloon-injured carotid artery rats. Exp Mol Med. 2015;47:e170.
- Xiang H, Tao X, Xia S, et al. Emodin alleviates sodium taurocholate-induced pancreatic acinar cell injury via MicroRNA-30a-5p-mediated inhibition of high-temperature requirement A/transforming growth factor beta 1 inflammatory signaling. Front Immunol. 2017;8:1488.
- Lin SZ, Xu JB, Ji X, et al. Emodin inhibits angiogenesis in pancreatic cancer by regulating the transforming growth factor-beta/drosophila mothers against decapentaplegic pathway and angiogenesis-associated microRNAs. Mol Med Rep. 2015;12:5865–5871.