?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective
To investigate the potential mechanism of microRNA-34a (miR-34a) on proliferation, migration and invasion of paediatric neuroblastoma cells.
Methods
The expression of miR-34a and hepatocyte nuclear factor 4α (HNF4α) in paediatric neuroblastoma tissues were detected by RT-q PCR and Western blot, respectively. Cell proliferation, migration, invasion and the expression of matrix metalloproteinase 2 (MMP-2) and matrix metalloproteinase 14 (MMP-14) after transfection of miR-34a mimics or HNF4α siRNA into SH-SY5Y cells were detected by MTT assay, Transwell assay and Western blot assay, respectively. The target relationship between miR-34a and HNF4α was verified by TargetScan online prediction and dual-luciferase assay. Cell proliferation, migration and invasion of SH-SY5Y cells after overexpression of miR-34a and HNF4α were detected.
Results
The expression level of miR-34a was decreased (p < .05) while the expression level of HNF4α was increased (p < .05) in paediatric neuroblastoma tissues. Over- expression of Mi-34a or knockdown of HNF4α in SH-SY5Y cells could lead to a decreased of cell proliferation, migration, invasion and the expression of MMP-2 and MMP-14 (p < .05). The results of TargetScan online prediction and dual-luciferase assay indicted that HNF4α was a potential target gene for miR-34a. Overexpression of HNF4α could reverse the inhibition of miR-34a on proliferation, migration and invasion of SH-SY5Y cells.
Conclusion
The expression of miR-34a was down-regulated in paediatric neuroblastoma tissues, and overexpression of miR-34a could inhibit proliferation, migration and invasion of SH-SY5Y cells by targeting HNF4α.
Introduction
Neuroblastoma (NB), originating from sympathetic neurons, is a neoplasm caused by the deterioration of undifferentiated neuroblasts. It occurs mainly in the abdomen, mediastinum and neck of children and is the most common extracranial solid tumour in children [Citation1]. The incidence of neuroblastoma in children under 15 years old was 10.54/100,000, ranking the first in peripheral nervous system malignancies in children [Citation2]. Neuroblastoma is characterized by high degree of malignancy, rapid development, strong invasion and great differences in clinical manifestations. Although surgery, chemotherapy, radiotherapy, immunotherapy and other multidisciplinary comprehensive treatment can alleviate its clinical symptoms, the overall survival rate of NB in China is still difficult to improve. Immunotherapy and molecular targeted therapy are new directions in the clinical treatment of NB [Citation3,Citation4].
MicroRNA (miRNA) is a kind of endogenous non-coding RNA molecules that regulate gene expression by degrading target mRNA or inhibiting its translation. It has attracted much attention because of its important regulatory role in the development of the body and the development of diseases. At present, some miRNA molecules as tumour suppressors have shown a broad prospect in the clinical treatment of cancer [Citation5]. Previous studies have found that a variety of miRNAs are abnormally expressed in NB tissues and are associated with factors such as NB metastatic, tumour size and lymph node metastasis, including up-regulation of miR-181, miR-21, and miR-15a and down-regulation of miR-34a, miR-101 and miR-145 [Citation6–8]. In this study, the effect of miR-34a expression and overexpression of miR-34a in NB tissues on proliferation, migration and invasion of NB cells was examined to explore its mechanism in the development of NB.
Methods and materials
Clinical specimens
Thirty-five cases of paediatric neuroblastoma diagnosed by the department of pathology in the Henan Xinxiang Central hospital from July 2016 to April 2018 were selected as subjects. There were 19 males and 16 females aged from 3 months to 8 years, who underwent surgical resection of cancer tissues and were quickly put into liquid nitrogen. All the patients did not receive chemotherapy and radiotherapy before surgery. In addition, 15 cases of normal children's adrenal tissue preserved by the pathology department of our hospital were selected as the normal control. The tissues were collected and stored in the refrigerator at −80 °C. The neuroblastoma tissues and adrenal tissues were labelled as the non-tumour tissues group and the cancer tissues group, respectively. This study was approved by Henan Xinxiang Central Hospital, and prior written and informed consent was obtained from each patient.
Materials
Human neuroblastoma cell line SH-SY5Y was purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Foetal bovine serum, DMEM culture medium, trypsin and pcDNA 3.1 vector were purchased from Thermo Fisher Scientific (Waltham, MA). Trizol Plus RNA purification kit and Lipofectamine 2000 were purchased from Invitrogen (Carlsbad, CA). TaKaRa reverse transcription kit and real-time fluorescence quantitative kit were purchased from Bao Bioengineering Co., Ltd. (Dalian, China). PVDF membrane, ECL luminescent solution, RIPA lysate, crystal purple 4% and paraformaldehyde were purchased from Solarbio Company (Beijng, China). MTT, dimethyl sulphoxide were purchased from Sigma-Aldrich (St. Louis, MO). The double luciferase assay kit was purchased from Promega (Madison, WI). HNF4α 3’-UTR wild and mutant type plasmids were constructed by Han Heng biological Co., Ltd (Shanghai, China). Transwell was purchased from Corning Inc (Corning, NY). Control mimics and miR-34a mimics were designed and synthesized by Gemma Company (Shanghai, China). Rabbit anti-human hepatocyte nuclear factor 4a (HNF4α) polyclonal antibody, rabbit anti-human MMP-2 polyclonal antibody, rabbit anti-human MMP-14 polyclonal antibody and rabbit anti-human GAPDH polyclonal antibody were purchased from Emerald Technology Co., Ltd (Wuhan, China). Control siRNA and HNF4α siRNA were designed and synthesized by Wei Zhen Biotechnology Co., Ltd (Shandong, China). The primers were synthesized by Liuhe Huada Gene Company (Beijing, China). Cell incubator was purchased from Thermo Fisher Scientific (Waltham, MA). Enzyme labelling instrument of HBS-1096B was purchased from de tie Experimental Equipment Co., Ltd (Nanjing, China). Microscope was purchased from Bingyu Optical Instrument Co., Ltd (Shanghai, China).
Cell culture and grouping
To resuscitate these frozen cells, frozen SH-SY5Y cells were taken out and melted in 37 °C water bath. The cell suspension was transferred to a sterile centrifuge tube, and then washed with the serum-free DMEM medium. Resuscitated SH-SY5Y cells were recultured with DMEM containing 10% foetal bovine serum at 37 °C and 5% CO2 saturated humidity. The cells were subcultured at 80% confluence. SH-SY5Y cells were collected and suspended after trypsin digestion. The cell suspension concentration was adjusted to 1 × 105/mL and inoculated into 6-well plate. After the cells grew to about 45% confluence degree and cells transformation were performed showing as control mimics, miR-34a mimics, control siRNA, HNF4α siRNA, miR-34a mimics and pcDNA 3.1 empty vector, miR-34a mimics and HNF4α overexpression vectors were transfected into SH-SY5Y cells according to Lipofectamine 2000 transfection reagent instructions, generating the miR-NC group, miR-34a mimics group, si-NC group, si-HNF4α group, miR-34a mimics + pcDNA3.1 group and miR-34a mimics + pcDNA3.1-HNF4α group, respectively.
RT-qPCR
Total RNA was extracted from primary tissues and cell lines by the Trizol reagent according to the producer’s instructions. TaKaRa reverse transcription kit and fluorescence quantitative kit were used for reverse transcription and system preparation. GAPDH and U6 was used as internal reference for PCR amplification. Each RNA sample was repeated three times. The 2−△△Ct method was used to calculate the relative expression of genes. Primers and sequences are shown in .
Table 1. Primers and sequences of RT-qPCR.
Western blot
Western blot assays were performed to detect the protein expression of HNF4α, MMP-2 and MMP-14 in SH-SY5Y cells as well as neuroblastoma and adrenal tissues with various treatments. In brief, RIPA cell lysis solution containing protease inhibitor was used to lyse cells for 30 min. After 4 °C, 12,000 g centrifugation for 15 min, the supernatant was obtained. The protein sample was transferred to PVDF membrane after SDS-PAGE electrophoresis, and 5% skim milk powder was sealed at room temperature for 1 h. Rabbit anti-human HNF4α polyclonal antibody (1:1000), rabbit anti-human MMP-2 polyclonal antibody (1:1000), rabbit anti-human MMP-14 polyclonal antibody (1:1000) and rabbit anti-human GAPDH polyclonal antibody (1:1000) were added respectively and incubated at 4 °C overnight. After washed with TBST, horseradish peroxidase (HRP) labelled secondary antibody was added and incubated at room temperature for 2 h. TBST was used to wash the membrane three times, 10 min each time. Bands were detected by ECL Kit. Repeat the experiment three times.
MTT assay was used to detect cell proliferation
SH-SY5Y cells transfected with miR-NC, miR-34a mimics, si-NC, si-HNF4α, miR-34a mimics + pcDNA 3.1 and miR-34a mimics + pcDNA 3.1-HNF4α were seeded into 96-well plates with a density of 5000 cells/well and cultured at 24 h, 48 h and 72 h, then added 20 μL MTT (5 mg/mL) in each well, and continued to incubate for 4 h. The superfluous medium was discarded and 150 μL dimethylsulphoxide was added for 10 min oscillating reaction. The absorbance at 570 nm was measured as the indicator of cell viability by using enzyme labelling instrument.
Cell migration and invasion detection
The migration and invasion abilities of NB cells were detected by Transwell assay. Generally, SH-SY5Y cells with miR-NC, miR-34a mimics, si-NC, si-HNF4α, miR-34a mimics + pcDNA 3.1 and miR-34a mimics + pcDNA 3.1-HNF4α were digested by trypsin and resuspended with the serum free DMEM medium overnight. For migration assays, 3 × 104 cells were inoculated into the upper chamber of transwell uncoated matrix glue. For invasion assay, 3 × 104 cells were seeded into the upper chamber coated with matrix glue. Cells in the upper chamber were cultured in 600 μl DMEM medium and the medium containing 10% foetal bovine serum as chemoattractant was added into the lower compartment with six duplicated pores in each group. After 24 h of conventional culture, the cells in the upper chamber were gently wiped off with cotton swab. Cells attached to the lower surface were fixed with 4% paraformaldehyde for 30 min, stained with 0.1% crystal violet for 10 min, washed with double steaming water until the water was clear, followed by natural air-drying and then observed under microscope and photographed in five random fields. The number of penetrating cells was counted.
Double luciferase reporter gene experiment
HNF4α 3'-UTR wild-type plasmid vector (containing HNF4α 3'-UTR fragment) or mutant plasmid vector (excluding HNF4α 3'-UTR fragment) were co-transfected with mimics control and miR-34a mimics into NB cells, respectively, according to Lipofectamine 2000 transfection reagent instructions. At 48 h posttransfection, the luciferase activity was detected by dual luciferase reporter assay kit (Promega, Madison, WI) referring to protocols of manufacturer. Relative fluorescence intensity = firefly fluorescence intensity/hyphen fluorescence intensity.
Statistical analysis
The experiment was repeated for 3 times in each group. The obtained experimental data were statistically analyzed by SPSS17.0 (SPSS, Chicago, IL), and the results were expressed by mean ± standard deviation ( ± s). The T test was used for data comparison between two groups, and one-way ANOVA was used for data comparison among multiple groups. p < .05 was considered statistically significant.
Results
Expression of miR-34a and HNF4α in neuroblastoma tissue
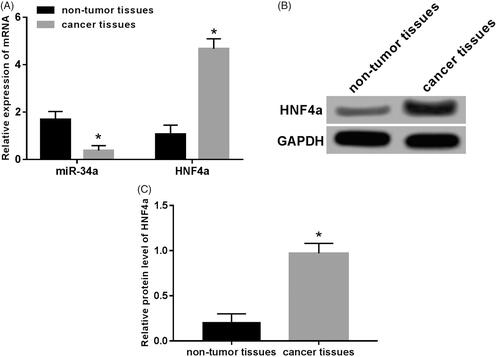
Expression of miR-34a and HNF4α were detected by RT-qPCR and Western blot. Results as shown in : compared with normal adjacent tissues, miR-34a expression was significantly down-regulated in human neuroblastoma tissues (p < .05), while the expression of HNF4α was significantly up-regulated in mRNA and protein levels (p < .05).
Figure 1. Expression of miR-34a and HNF4α in neuroblastoma tissue. (A) Expression of miR-34a and HNF4α mRNA in human neuroblastoma tissues and normal adjacent tissues; (B) gel electrophoresis of HNF4α protein expression; (C) the expression level of HNF4 proteins in human neuroblastoma and normal adjacent tissues. Note: Compared with the non-tumour tissue group, *p < .05.
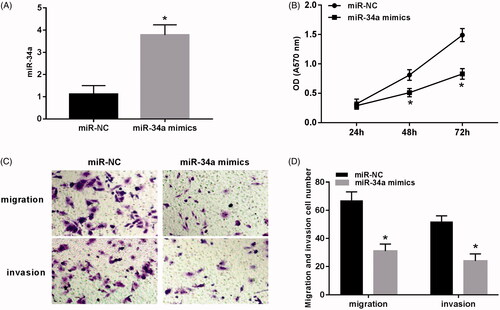
Overexpression of miR-34a inhibited the proliferation, migration and invasion of SH-SY5Y cells
The proliferation, migration and invasion were detected after transfection of miR-34a mimics to SH-SY5Y cells. The results () show: the proliferation capacity of the miR-34a mimics group was significantly lower than that of the miR-NC group at 48 h and 72 h (p < .05). The number of migration and invasion cells in the field of the miR-34a mimics group was significantly lower than that of the miR-NC group (p < .05).
Figure 2. Overexpression of miR-34a inhibited the proliferation, migration and invasion of SH-SY5Y cells. (A) Expression of miR-34a in SH-SY5Y cells; (B) overexpression of miR-34a inhibited proliferation of SH-SY5Y cells; (C) the detection of cell migration and invasion after overexpression of miR-34a; (D) overexpression of miR-34a inhibited migration and invasion of SH-SY5Y cells. Note: Compared with the miR-NC group, *p < .05.
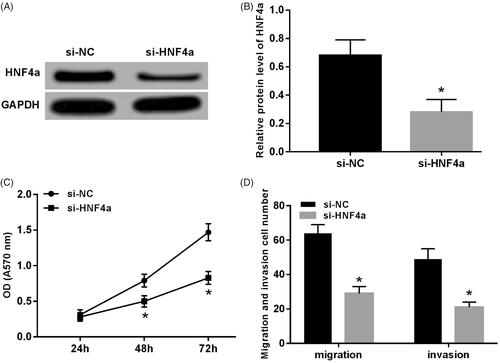
Knocking down HNF4α inhibited proliferation, migration and invasion of SH-SY5Y cells
After transfection of HNF4α to SH-SY5Y cells, the expression of the HNF4α was down-regulated (p < .05) (). The proliferation capacity of the si-HNF4α group cell was significantly lower than that of the si-NX group at 48 h and 72 h (p < .05) (). The number of migratory and invasive cells decreased in the visual field of si-HNF4α group (p < .05) ().
Figure 3. Knocking down HNF4α inhibited proliferation, migration and invasion of SH-SY5Y cells. (A) Gel electrophoresis of HNF4α protein expression; (B) expression level of HNF4α protein; (C) knocking down HNF4α inhibited SH-SY5Y cell proliferation; (D) knocking down HNF4α inhibited migration and invasion of SH-SY5Y cells. Note: Compared with the si-NC group, *p < .05.
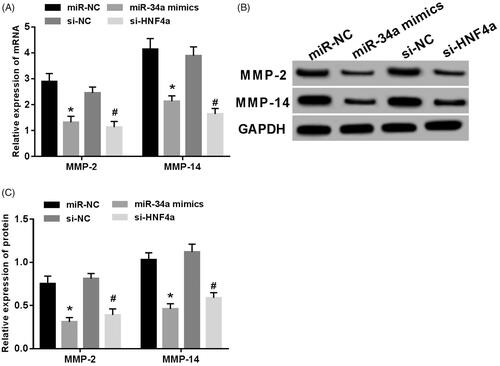
Effects of overexpression of miR-34a or knockdown of HNF4α on the expression of MMP-2 and MMP-14 in SH-SY5Y cells
MMP-2 and MMP-14 expression were detected after transfection of miR-34a mimics or HNF4α siRNA into SH-SY5Y cells. The result was shown in : mRNA and protein levels of MMP-2 and MMP-14 decreased significantly (p < .05), the difference was statistically significant.
Figure 4. Overexpression of miR-34a or knockdown of HNF4α inhibited MMP-2 and MMP-14 expression in SH-SY5Y cells. (A) mRNA expression of MMP-2 and MMP-14 in SH-SY5Y cells; (B) MMP-2 and MMP-14 protein electrophoresis in SH-SY5Y cells; (C) expression levels of MMP-2 and MMP-14 proteins in SH-SY5Y cells. Note: Compared with the miR-NC group,*p < .05; compared with si-NC group, #p < .05.
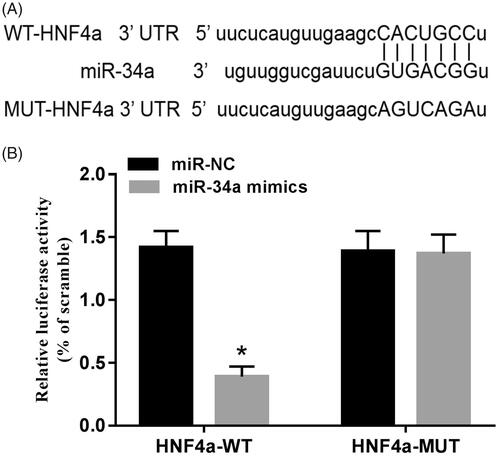
HNF4 is a potential target gene of miR-34a
By online analysis of TargetScan, it was found that HNF4α 3'-UTR contained miR-34a binding sites. Further detection of luciferase activity was found: The miR-34a mimics and HNF4α 3’-UTR wild plasmids were co-transfected into SH-SY5Y cells, the activity of cell luciferase was significantly reduced (p < .05), while the activity of cell luciferase was not significantly changed when co-transfected with HNF4α 3’-UTR mutant plasmids ().
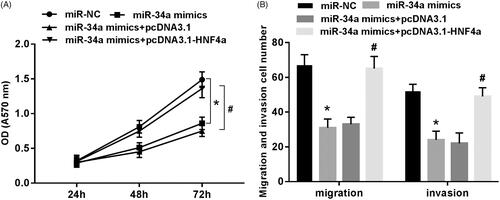
Overexpression of HNF4 can reverse the inhibitory effect of miR-34a on proliferation, migration and invasion of SH-SY5Y cells
As shown in : after the transfection of miR-34a mimics to SH-SY5Y cells, the cell proliferation capacity was significantly lower than that of the miR-NC group (p < .05), and the number of migration and invasion cells in the field was significantly reduced (p < .05). However, the proliferation, migration and invasion of SH-SY5Y cells were basically restored to normal level when the miR-34a mimics and pcDNA 3.1-HNF4α overexpression vectors were co-transfected into SH-SY5Y cells.
Figure 6. Overexpression of HNF4 can reverse the inhibitory effect of miR-34a on proliferation, migration and invasion of SH-SY5Y cells. (A) Proliferation detection of SH-SY5Y cells in each group; (B) migration and invasion detection of SH-SY5Y cells in each group. Note: Compared with the miR-NC group, *p < .05; compared with miR-34a mimics + pcDNA3.1 group, #p < .05.
Discussion
According to reports [Citation9,Citation10], the treatment based on NB risk grouping can improve the overall survival rate, and the survival rate of low-risk and medium-risk NB patients can be increased to more than 90% by surgical treatment, chemotherapy, immunotherapy, etc. However, high-risk NB patients are prone to complications after surgical treatment, and toxic side effects of chemotherapy and radiotherapy are likely to cause endocrine disorders and growth retardation, and the overall survival rate is only 40–50%. The abnormal expression of miRNA in various human tumour tissues can regulate the proliferation, differentiation, apoptosis, angiogenesis and other biological processes of cancer cells, thus play an inhibitory or promoting role in tumour development [Citation11,Citation12]. For example, miR-499 plays a role of tumour inhibition in liver cancer by inhibiting cell migration and inducing apoptosis [Citation13]. MiR-195 inhibits cervical cancer cell proliferation by blocking cell cycle [Citation14].
MiR-34 was first found in nematodes in 2001. It is widely distributed in mammals, nematodes and arthropods. It is highly conserved in evolution. The miR-34 gene family is formed by local and tandem repeats in animal development and evolution [Citation15]. Members of the miR-34 family, including miR-34a, miR-34b and miR-34c, play an important role in inhibiting the development of tumours such as liver cancer, breast cancer and lung cancer [Citation16,Citation17]. In addition, there is evidence that the miR-34 family is involved in neuronal differentiation. Overexpression of miR-34a can inhibit p53-induced apoptosis of neural stem cells and arrest cell cycle at G1 phase to promote differentiation of neural stem cells into neurons [Citation18]. Multiple studies showed that miR-34a can inhibit the proliferation of various tumour cells, such as osteosarcoma of prostate cancer, by blocking cell cycle and playing a role in tumour inhibition [Citation19,Citation20]. This study found that miR-34a was low expressed in paediatric neuroblastoma tissues, and overexpression of miR-34a could inhibit proliferation and invasion of SH-SY5Y cells. Moreover, it was found that HNF4α was the potential target of miR-34a through online prediction by TargetScan and biluciferase reporter gene experiments.
Hepatocyte nuclear factor 4α (HNF4α) belongs to the steroid hormone receptor superfamily, which can regulate cell differentiation and metabolism at the transcriptional level. Human HNF4α is located on 20q13.12 chromosome. It is a highly conserved zinc finger protein, which can be activated by various pathways such as acetylated phosphorylation. It binds with the target gene promoter sequence to form a dimer and regulates chromosomal structure, hepatocyte polarization and cholesterol metabolism [Citation21]. Some studies found that HNF4α can be used as a marker for the diagnosis of invasive mucinous adenocarcinoma, and has inhibitory effect on colon cancer. Knocking out HNF4α can promote the metastasis of colon cancer and contribute to the occurrence and development of colon cancer [Citation22,Citation23]. In neuroblastoma cells, HNF4α can promote cell invasion, metastasis and angiogenesis by targeting MMP-14. The lower differentiation of neuroblastoma and higher expression level of HNF4α, the shorter survival time of patients [Citation24]. In this study, HNF4αwas knocked down by RNA interference in SH-SY5Y cells, the ability of proliferation, migration and invasion was decreased (p < .05), and the expression of MMP-2 and MMP-14 was down-regulated at mRNA and protein levels (p < .05). Overexpression of HNF4α reversed the inhibitory effect of miR34a on proliferation, migration and invasion of SH-SY5Y cells.
In conclusion, in children with neuroblastoma, the expression of microRNA-34a was low while that of HNF4 alpha was high. Overexpression of miR-34a could inhibit the proliferation, migration and invasion of neuroblastoma cells by targeting HNF4α. This study may provide a new target for the clinical treatment of neuroblastoma in children.
Disclosure statement
The authors declare that they had no conflict of interest. The authors alone are responsible for the consent and writing of this article.
References
- Al-Shammari NF, Redha E, Al Hajeri MH. Cervical neonatal neuroblastoma with recurrent SVT. Gulf J Oncol. 2009;6:45–57.
- Davidoff AM. Neuroblastoma. Semin Pediatr Surg. 2012;21:2–14.
- Pinto NR, Applebaum MA, Volchenboum SL, et al. Advances in risk classification and treatment strategies for neuroblastoma. J Clin Oncol. 2015;33:3008–3017.
- Wang J. Therapeutic advancement of pediatric neuroblastoma. J Pharm. 2018;24:58–61.
- Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16:203–222.
- Xin C, Buhe B, Hongting L, et al. MicroRNA-15a promotes neuroblastoma migration by targeting reversion-inducing cysteine-rich protein with Kazal motifs (RECK) and regulating matrix metalloproteinase-9 expression. FEBS J. 2013;280:855–866.
- Liu G, Xu Z, Hao D. MicroRNA 451 inhibits neuroblastoma proliferation, invasion and migration by targeting macrophage migration inhibitory factor. Mol Med Rep. 2016;13:2253.
- Roth SA, Hald ØH, Fuchs S, et al. MicroRNA-193b-3p represses neuroblastoma cell growth via downregulation ofCyclin D1,MCL-1andMYCN. Oncotarget. 2018;9:18160–18179.
- Whittle SB, Smith V, Doherty E, et al. Overview and recent advances in the treatment of neuroblastoma. Expert Rev Anticancer Ther. 2017;17:369–386.
- Maris JM. Recent advances in neuroblastoma. N Engl J Med. 2010;362:2202–2211.
- Lin S, Gregory RI. MicroRNA biogenesis pathways in cancer. Nat Rev Cancer. 2015;15:321–333.
- Kong YW, Ferland-McCollough D, Jackson TJ, et al. microRNAs in cancer management. Lancet Oncol. 2012;13:e249–e258.
- Sandbothe M, Buurman R, Reich N, et al. The microRNA-449 family inhibits TGF-β-mediated liver cancer cell migration by targeting SOX4. J Hepatol. 2017;66:1012–1021.
- Ning W, Wei H, Yin D, et al. MicroRNA-195 inhibits proliferation of cervical cancer cells by targeting cyclin D1a. Tumor Biol. 2016;37:4711–4720.
- Lau NC, Lim LP, Weinstein EG, et al. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science. 2001;294:858–862.
- Lan V, Son HV, Trang VL, et al. Methylation profiles of miR34 gene family in Vietnamese patients suffering from breast and lung cancers. Mol Med Rep. 2018;18:2476–2484.
- Chamani F, Sadeghizadeh M, Masoumi M, et al. Evaluation of MiR-34 family and DNA methyltransferases 1, 3A, 3B gene expression levels in hepatocellular carcinoma following treatment with dendrosomal nanocurcumin. Asian Pac J Cancer Prev. 2016;17:219–224.
- Jauhari A, Singh T, Singh P, et al. Regulation of miR-34 family in neuronal development. Mol Neurobiol. 2018;55:936–945.
- Duan K, Yongchao GE, Zhang X, et al. miR-34a inhibits cell proliferation in prostate cancer by downregulation of SIRT1 expression. Oncol Lett. 2016;5:3223–3227.
- Xun C, Chen XG, Hu X, et al. MiR-34a and miR-203 inhibit survivin expression to control cell proliferation and survival in human osteosarcoma cells. J Cancer. 2016;7:1057–1065.
- Ji LS, Sun XH, Zhou ZH, et al. Research progress of the relationship between hepatocyte nuclear factor 4 alpha and hepatocellular carcinoma. China Med Herald. 2018;15:32–35.
- Sugano M, Nagasaka T, Sasaki E, et al. HNF4α as a marker for invasive mucinous adenocarcinoma of the lung. Am J Surg Pathol. 2013;37:211–218.
- Saandi T, Baraille F, Derbal-Wolfrom L, et al. Regulation of the tumor suppressor homeogene Cdx2 by HNF4α in intestinal cancer. Oncogene. 2013;32:3782–3788.
- Xiang X. Hepatocyte nuclear factor 4 alpha promotes the invasion, metastasis, and angiogenesis of neuroblastoma cells via targeting matrix metalloproteinase 14. Cancer Lett. 2015;359:187–197.