Abstract
Nowadays, the synthesis and characterization of gold nanoparticles (AuNPs) from plant based extracts and effects of their anticancer have concerned an important interest. Marsdenia tenacissima (MT), a conventional Chinese herbal medicine, has long been used for thousands of years to treat tracheitis, asthma, rheumatism, etc. In this present study, we optimize the reaction of parameters to manage the nanoparticle size, which was categorized by high-resolution transmission electron microscopy (HR-TEM). A different characterization method, for example, UV-visible spectroscopy (UV-vis), fourier-transform infrared spectroscopy (FT-IR) and X-ray diffraction (XRD) were performed to consider the synthesized AuNPs getting from the MT leaf extract. The MT-AuNPs were analyzed for their cytotoxicity property against HepG2 cells by MTT analysis. The apoptosis was evaluated by using reactive oxygen species (ROS), migration assay, mitochondrial membrane potential (MMP) and apoptotic protein expression. Interestingly, the findings of our study observed the cytotoxicity effect of synthesized MT-AuNPs at a concentration of 59.62 ± 4.37 μg after 24 hrs treatment. Apoptosis was induced by the MT-AuNPs with enhanced ROS, changed MMP and inhibit the migration assay. Finally, the apoptosis was confirmed by the considerable up-regulation of Bax, caspase-9 and caspase-3, while the anti-apoptotic protein expressions of Bcl-2 and Bcl-XL were down-regulated. Although, in this studies, we evaluated the characterization, synthesis and anticancer action of gold nanoparticles from MT (MT-AuNPS) helpful for liver cancer therapeutics.
Introduction
Liver cancer is the third majority widespread cause of death and the fifth most commonly diagnosed cancer along with all types of cancer. In developing countries, the death rate is rising day by day and 85% of the people are suffering generally men rather than women [Citation1]. The liver cancer incidence rates are regarding three times more in men than in women [Citation2]. Several chemopreventive and therapeutic drugs were to remove the cancer cells during the process of apoptosis. Apoptosis is a system by which cells endure passing away to regulate cell proliferation and in response to DNA damage [Citation3]. The activation of caspases is a vital process in the stimulation of apoptosis. The caspase is formed as inactive zymogens and which is undergoing to proteolytic establish during apoptosis. The caspase-9 is an initiator caspase, whereas caspase-3 is one of the effector proteins that play an essential function in the start of apoptosis and to control the cancer formation [Citation4].
The metal nanoparticle synthesis using plant compartments is accepted for alleviating cancer because of the relatively simple to control the synthesis of monodispersed nanoparticles and its biocompatibility, eco-friendly and non-toxic properties [Citation5]. Nanoparticles are a lesser in size than several biological molecules such as nucleic acids, lipids and proteins. Gold nanoparticles (AuNPs) are a type of metal nanoparticle has become important in remedial research field due to their self-assembly, nontoxic nature and better drug release [Citation6]. Since molecular imaging essentially depends on the finding of particular biomarkers, the presence of AuNPs bound to particular ligands on tissue cells may be a means of finding diseased tissues resembling tumors [Citation7]. Several AuNPs complexes have been mostly used in the novel category of antitumor agents in line to alleviate cancer. Thus, the development of efficient AuNPs becomes one of the significant trends in the study of cancer treatment [Citation8].
Marsdenia tenacissima (MT) is a perennial climber that is widely distributed in tropical to subtropical areas in Asia, mainly in the Yunnan and Guizhou Provinces of China [Citation9]. MT is bitter in taste and slender cold, which has been a lot of pharmacological activities such as alleviate asthma and cough, clearing away toxic and heat material, analgesia and anti-inflammation [Citation10]. Recent pharmacological research has revealed that MT has considerable immunomodulatory, antitumor, diuretic and hepatoprotective effects [Citation11–13]. MT is having polysaccharides, steroidal glycosides, organic acids, alkaloids, resin and pigment and further chemical components [Citation14]. Previous studies have revealed that MT has capable anti-hepatoma property when used only or combined with a chemotherapeutic. One water-soluble polysaccharide of Marsdenia tenacissima polysaccharide can develop immune function in normal control mice and prevent the tumor formation in H22 hepatoma cells in tumor-induced mice by notably improved the activities of antioxidant substances such as SOD, GPx and CAT [Citation15]. Thus, in the present study, we designed to synthesize and characterization of AuNPs with the remedial plant of MT and to investigate the anticarcinogenic potential of the synthesized Marsdenia tenacissima-gold nanoparticles (MT-AuNPs) against liver cancer HepG2 cell lines.
Materials and methods
Chemicals and reagents
Gold(III) chloride trihydrate (99.9%) are acquired from Sigma Aldrich (St Louis, MO, USA). Dulbecco’s modified Eagles medium (DMEM), phosphate buffered saline (PBS), 3–(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), fetal bovine serum (FBS) and dimethyl sulfoxide (DMSO) were acquired from Himedia Lab Ltd., Mumbai, India. The primary antibodies for caspase-9, Bcl-2, Bcl-XL, Bax, caspase-3 and β-actin were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). All the additional chemicals were used of analytical grade.
Cell culture
HepG2 liver cancer cell line was purchased. The cells were seeded in DMEM medium supplement with 10% FBS, 1X penicillin and streptomycin. The cells were maintained at 37°C in a humidified atmosphere with 5% of CO2 and 95% of air incubation.
Preparation of MT extract
The collected MT leaves were authenticated, thoroughly washing by running tap water and finally rinsed with sterile distilled water. The eviscerated leaves were shadowed dried at room temperature for taking approximately 10–15 days. After the dried leaves were powdered by use an electric grinder. The 50 grams of fine powder were mixed up with 500 ml of distilled water and boiled for 30 min at 60°C temperature. The powdered samples were stored in a brown bottle and sheltered from revelation to sunlight until further use.
Synthesis of gold nanoparticles
The 5 ml of MT aqueous leaf extract was added with 95 ml of 1 mM gold(III) chloride trihydrate and kept at room temperature in favor of 30 min for the formation of MT-AuNPs. The color changes were observed from yellow to ruby red indicates the synthesis of MT-AuNPs and it was to conclude by characterization using various techniques.
Characterization of AuNPs
The synthesized AuNPs were distinguished by UV-visible spectrometer (UV-vis) for their fascination potential by the absorbance spectrum was got to use Shimadzu UV-1800 and scanned at a wavelength range of 300–700 nm. The morphological analysis containing size and shape was analyzed with HR-TEM by using the method of JEOL JEM-2000 EX microscope (Tokyo, Japan). The MT-AuNPs aqueous re-dispersed suspension of a drop was located on a carbon-coated copper grid and air-dried prior to the assessment of TEM. The crystallographic structure of the synthesized MT-AuNPs was investigated by X-ray diffraction (XRD) method by using Shimadzu XRD-6000/6100 model (Kyoto, Japan). The Fourier-transform infrared spectroscopy (FT-IR) spectra of MT-AuNPs were used to find out the presence of functional groups. The FTIR absorption spectra were carried out at room temperature at the level of 4000–600 cm−1 using the FT-IR (Shimadzu-8400) spectrometer.
Anticancer activity of AuNPs
Cell viability assay by MTT
The analysis of cytotoxicity of MT-AuNPs was carried out in HepG2 cells using MTT assay [Citation16]. The cells were suspended in a 96 well plate (5 × 103 cells/well) for 24 h. Consequently, cells were added with MT-AuNPs with various dosages (5, 10, 25, 50, 75 and 100 µg/ml) to the each well and the medium was changed with the new DMEM medium and cells were kept warm with MTT (5 mg/ml in PBS) at 37°C for 4 h. Following the 24 h incubation, removed the medium and added DMSO to each well to form the formazan crystals and it was read by the ELISA plate reader with an assessment of 570 nm wavelength.
Measurement of MMP
The MMP was assessed by Rhodamine-123 (Rh-123), lipophilic cationic dye [Citation17]. The cells were suspended in 6 wells plate on the concentration of 1 × 106 cells/well and the cells added with various dosages of MT-AuNPs (50 and 75 µg/ml) for 24 h treatment, the cells were developed with Rh-123 dye (30 min). The MMP was determined and measured at 485/530 nm under Spectrofluorometer.
Measurement of intracellular ROS
The intracellular ROS production was determined using fluorescence dye DCFH-DA that can enter into the intracellular matrix of cells where it is oxidized by ROS to fluorescent dichlorofluorescence (DCF) [Citation18]. The cells (5 × 105 cells/well) were prepared up to a final quantity of 2 ml in PBS. The 1 µl of DCFH-DA (1 mg/ml) was added to 1 ml aliquots of cells and keep warm at 37°C for 30 min underneath the dark condition and measured at 485 ± 10 and 530 ± 12.5 nm.
Scratch wound healing assay
The MT-AuNPs on cell migration was analyzed by a scratch wound healing assay of Luo et al. (2018 )[Citation19]. The wound healing analyze was used to find out the result of cell migration stimulated by the MT-AuNPs in the HepG2 cells. The cells were cultured in 12 well plates with concentration of 4 × 105 cells/well till it accomplished a confluence of 100. The medium was changed by PBS buffer to create a scratch by using a 1 ml tip. After that, the cells were washed and treated with 50 and 75 µg/ml of MT-AuNPs at 37°C in a 5% CO2 humidified environment for 0 h, 12 h and 24 h with control cells. The wound healing was identified and took pictures with an inverted microscope.
Western blotting analysis
The protein lysates from control and synthesized MT-AuNPs (50 and 75 µg/ml) treated HepG2 cells were isolated in cold lysis buffer (1% NP40, 1 mM DTT and protease inhibitors in PBS). Totally 50 µg of proteins were separated by SDS-PAGE tracked by immunoblotted with specific primary antibodies for caspase-3, Bax, caspase-9, Bcl-2 and Bcl-XL (Sigma Aldrich, USA) dilution of 1:1000 at 4°C overnight and identified using an ECL kit (Millipore, Billerica, MA, USA).
Statistical analysis
All the experimental data were examined as mean ± SD. The findings were evaluated by one-way ANOVA and Duncan examination using SPSS. A value of p < .05 was measured as statistically significant.
Results
Characterization of MT-AuNPs
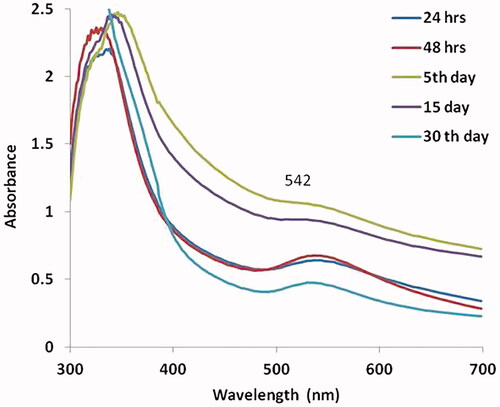
shows the synthesis of gold nanoparticles by UV-vis spectroscopy on HepG2 cells. The stability of synthesized MT-AuNPs was identified by the maximum absorbance range of peak and the color changes. The samples were determined at various time periods of 24 h, 48 h, 5 days, 15 days and 30 days. The highest peak was observed by the maximum peak range of 542, which indicates that the time raises the amount of conversion of gold nanoparticle as well, increases.
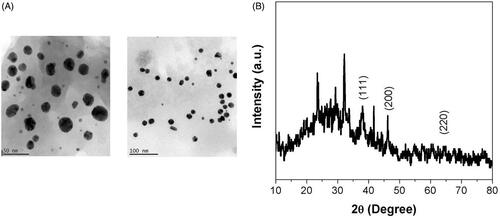
shows the HR-TEM analysis of MT-AuNPs on HepG2 cell lines. The MT-AuNPs treated images clearly exposed the shape and size of the nanoparticles as a role of the extract application, which evidently observed with a size of 30 to 50 nm.
shows the crystalline structure of the synthesized MT-AuNPs was observed by using XRD measurements. The XRD pattern demonstrated a number of Bragg reflections, which can be categorized according to the face-centered cubic (FCC) structure of gold. The diffraction peaks observed at 2θ = 111, 200 and 220 are found to this information for the standard gold metal. Therefore, the XRD pattern showed that the MT-AuNPs were actually crystalline.
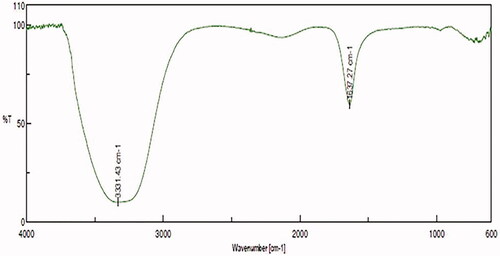
shows the FTIR spectra of the MT extracts and its binding efficient to the AuNPs. The FTIR spectra demonstrated in the presence of various functional groups. For example, the peaks showed at 3331.43 cm−1 and 1637.27 cm−1 for the MT-AuNPs, which were allocated to the free O-H and C = C stretching modes may be from the alcohols and alkenes.
Anticancer activity of MT-AuNPs
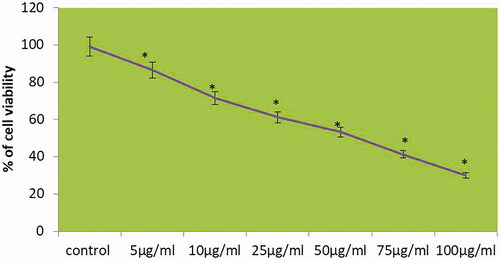
shows the cytotoxic effect of MT-AuNPs on HepG2 cells. The inhibitory concentration 50 (IC50) of MT-AuNPs were analyzed with the different concentration (5, 10, 25, 50, 75 and 100 μg/ml) and 24 h time incubation by MTT assay. The IC50 value of MT-AuNPs at a concentration of 59.62 ± 4.37 μg for HepG2 cells after 24 h treatment was determined from the growth inhibition curve and we have chosen for 50 and 75 μg doses of MT-AuNPs for the further work.
Figure 4. MT-AuNPs inhibit the viability of HepG2 liver carcinoma cells. This experiment was repeated thrice and the bars in the graph represent S.E. (*p<.05).
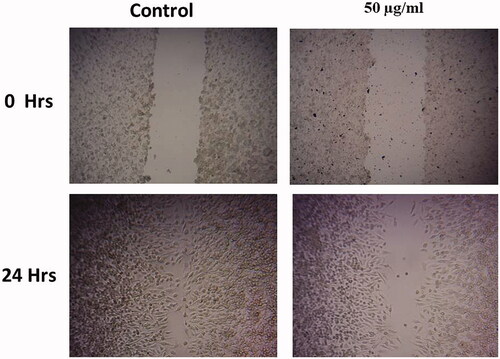
shows the cell migration assay of MT-AuNPs on HepG2 cells. The control cells explained augmented cell proliferation at 0–24 h incubations. The cell migration was very dynamic and the wound closure was almost complete. There was no indication of the wound after 24 h. While the treatment with MT-AuNPs (50 and 75 μg/ml) reduced cell migration in HepG2 cells in a dose-dependent behavior.
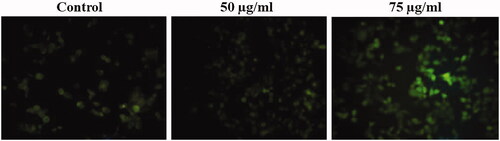
shows the HepG2 cells treated with MT-AuNPs in production of ROS. The high fluorescence intensity was noticed in cells incubated with MT-AuNPs evaluated with the control cells, which showed that the ROS content is elevated in the cells, were treated at different concentrations (50 and 75 μg/ml) of MT-AuNPs.
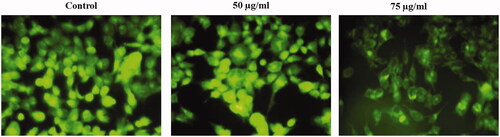
shows the HepG2 cells treated with MT-AuNPs in changes of MMP. We observed that there are noticeable increased green fluorescence and healthy mitochondria in the control of HepG2 cells, demonstrating the presence of Rh-123 dye aggregation. On the other hand, there is low green fluorescence in the HepG2 cells treated with MT-AuNPs (50 and 75 μg/ml), representing collapse of membrane occurred.
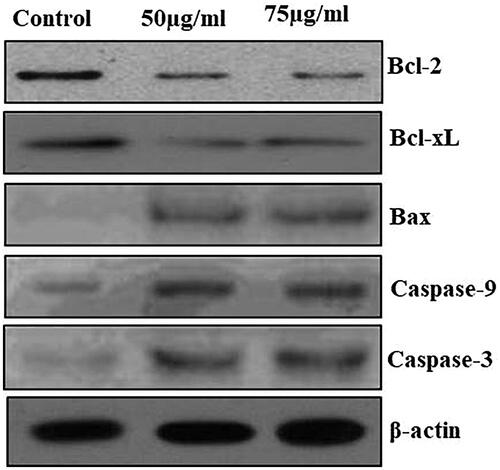
and show the apoptotic protein expressions of control and MT-AuNPs treated to HepG2 cells. The control cells showed enhanced expression of anti-apoptotic proteins (Bcl-XL and Bcl-2), whereas, diminished expression of pro-apoptotic proteins (Bax, caspase-3 and caspase-9) were observed. Treatment with MT-AuNPs at different concentrations (50 and 75 μg/ml) to HepG2 cells showed low expression of anti-apoptotic proteins and improved the pro-apoptotic protein expressions.
Figure 8. Effect of MT-AuNPs on colorimetric caspase activity in HepG2 liver cancer cells by ELISA method. This experiment was repeated thrice and the bars in the graph represent S.E. (*p<.05, #p<.01).
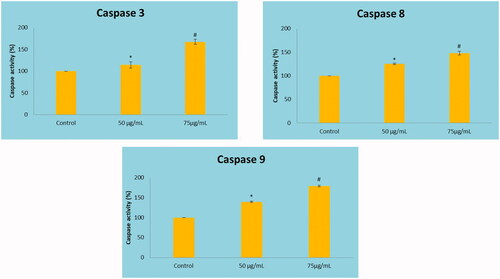
Figure 9. The anticancer effect of MT-AuNPs on apoptotic signaling proteins in HepG2 cell lines were examined by Western blotting technique. The cells were treated with MT-AuNPs (50 and 75 μg) for 24 h and the protein expressions of Bcl-2, Bax, Bcl-XL, caspase-3 and caspase-9 were determined. β-actin was used as a loading control.
Discussion
Nanoscience and nanotechnology have a graceful perspective across a wide spectrum of cancer study, including monitoring, diagnostic and therapeutic strategies and provide novel advances in these areas [Citation20]. Nanoparticles are frequently referred to as size particles with a maximum of 100 nm. Nanoparticles reveal a unique property, which is relatively different than those of larger particles. The novel properties of nanoparticles associated with variation in particular characteristics like shape, size and distribution have been established [Citation21].
UV-visible spectroscopy is the main method to determine the stability and development of metal nanoparticles [Citation22]. The size of the NPs may be lesser at a high dosage of extract due to the phytochemicals that can efficiently stabilize the NPs [Citation23]. The XRD dispersed study findings in a greater inset pattern of synthesized AuNPs, which agrees with the earlier findings of Philip (2009) [Citation24] that supports the crystalline nature of gold nanoparticles during circular rings analogous to the Bragg’s reflections. The results of the numerous researchers have confirmed the functional groups that perform in bio-reduction, capping and stabilization of NPs. The various functional groups with their related stretches were obtained in the current synthesized MT-AuNPs are in concordance with the earlier findings of Varshney et al. (2010) [Citation25]. In the present study, the results of UV-visible spectrum, a shift in the wavelength with λmax at 542 nm and visible color alteration to ruby red was noted, which point out the presence of the MT-AuNPs. The TEM results also explained the characteristics of the size and shape of nanoparticles. As well, XRD pattern analyses revealed the presence of nanoparticles with crystalline nature was confirmed. The FTIR examination of the MT-AuNPs samples has confirmed the presence of various functional groups such as alcohols and alkenes. Previous study also reported that the synthesized and characterization of Corchorus olitorius extract by using gold nanoparticles on HepG2 cell lines [Citation26].
The decreased level of IC50 values indicates the increased cytotoxic result of the sample was observed. These results exhibited significant cytotoxic and anticancer effects supported by the previous report [Citation27]. Previous studies showed that synthesized AuNPs and stated their immense effect to inhibit the cell viability of HepG2 cell lines [Citation26]. In the present study also informed that in agreement with this previous report. Plant-derived AuNPs are normally induced reactive oxygen species (ROS), which causes the cellular deaths. The ROS is disturbing the signal transduction pathways and also enhancing the cellular apoptosis [Citation28]. Previous reports showed that the formation of ROS either inside or outside of the cell may cause damage to the cell membrane [Citation29]. Recent research reported that MT induced cytotoxicity in erythrocytes due to the rising of ROS levels, elevating the level of erythrocyte shrinking and fragmentation [Citation30]. This present study also reported that MT-AuNPs enhanced ROS in HepG2 cells.
Apoptosis is cell essential machines that are helping to eliminate probable damaging cells, which is commonly controlled by Bcl-2 family members of proteins [Citation31,Citation32]. In this present study, we observed the protein expression of Bcl-2, Bcl-XL, Bax, caspase-3 and caspase-9 in response to MT-AuNPs shown in HepG2 cells for the reason that apoptotic cells are regulated during these pathways. The protein expression findings demonstrated that MT-AuNPs enhanced protein expression of pro-apoptotic Bax, caspase-9 and caspase-3. The expression of Bcl-2 and Bcl-XL was down-regulated in cells showing an MT-AuNPs dosage of 50 and 75 µg/ml. Likewise, Sellappa et al. (2015) [Citation33] found that AuNPs from Argemone Mexicana leaf extract against HepG2 cells, stimulating apoptosis by changing the expression of caspase-3. Our study also informed that AuNPs from MT inducing apoptosis agreeing with earlier reports [Citation33].
Conclusion
From our conclusion, the synthesized MT-AuNPs of the bioreduction were confirmed by UV-spectroscopy and morphological analysis was confirmed by spherical and oval shaped with a size range from 30 to 50 nm by TEM analysis. The crystalline nature of MT-AuNPs was recognized by XRD analysis. FT-IR analysis was confirmed by the occurrence of phytochemicals interaction in the stabilization and reduction of nanoparticles. Moreover, MT-AuNPs produces significant cytotoxicity on liver HepG2 cancer cells, which was confirmed by MTT cytotoxicity assay. Furthermore, MT-AuNPs induce apoptosis through inhibiting anti-apoptotic and enhancing pro-apoptotic pathway proteins. Overall, our findings, MT-based synthesis of AuNPs is faster, easier and eco-friendly, it has verified its powerful anticancer activity. The study extends additionally to the future for the purpose of the exact mechanism after its anticancer mediator in the molecular studies.
Disclosure statement
The author declares that there are no conflicts of interest.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30.
- Prasannaraj G, Sahi SV, Ravikumar S, et al. Enhanced cytotoxicity of biomolecules loaded metallic silver nanoparticles against human liver (HepG2) and prostate (PC3) cancer cell lines. J Nanosci Nanotechnol. 2016;16:4948–4959.
- Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257.
- Ajdari Z, Rahman H, Shameli K, et al. Novel gold nanoparticles reduced by Sargassum glaucescens: preparation, characterization and anticancer activity. Molecules. 2016;21:123.
- Khan MA, Jain DC, Bhakuni RS, et al. Occurrence of some antiviral sterols in Artemisia annua. Plant Sci. 1991;75:161–165.
- Yahia-Ammar A, Sierra D, Mérola F, et al. Self-assembled gold nanoclusters for bright fluorescence imaging and enhanced drug delivery. ACS Nano. 2016;10:2591–2599.
- Jain PK, El-Sayed IH, El-Sayed MA. Au nanoparticles target cancer. Nano Today. 2007;2:18–29.
- Sarkar S, Konar S, Prasad PN, et al. Micellear gold nanoparticles as delivery vehicles for dual tyrosine kinase inhibitor ZD6474 for metastatic breast cancer treatment. Langmuir. 2017;33:7649–7659.
- Zheng K, Zhang G, Jiang N, et al. Analysis of the transcriptome of Marsdenia tenacissima discovers putative polyoxypregnane glycoside biosynthetic genes and genetic markers. Genomics. 2014;104:186–193.
- Wang X, Yan Y, Chen X, et al. The antitumor activities of Marsdenia tenacissima. Front Oncol. 2018;8:473.
- Han SY, Zhao HY, Zhou N, et al. Marsdenia tenacissima extract inhibits gefitinib metabolism in vitro by interfering with human hepatic CYP3A4 and CYP2D6 enzymes. J Ethnopharmacol. 2014;151:210–217.
- Huang Z, Lin H, Wang Y, et al. Studies on the anti-angiogenic effect of Marsdenia tenacissima extract in vitro and in vivo. Oncol Lett. 2013;5:917–922.
- Zhu RJ, Shen XL, Dai LL, et al. Total aglycones from Marsdenia tenacissima increases antitumor efficacy of paclitaxel in nude mice. Molecules. 2014;19:13965–13975.
- Pang X, Kang LP, Yu HS, et al. New polyoxypregnane glycosides from the roots of Marsdenia tenacissima. Steroids. 2015;93:68–76.
- Jiang S, Qiu L, Li Y, et al. Effects of Marsdenia tenacissima polysaccharide on the immune regulation and tumor growth in H22 tumor-bearing mice. Carbohydr Polym. 2016;137:52–58.
- Leong CO, Suggitt M, Swaine DJ, et al. In vitro, in vivo, and in silico analyses of the antitumor activity of 2-(4-amino-3-methylphenyl)-5-fluorobenzothiazoles. Mol Cancer Ther. 2004;3:1565–1575.
- Johnson LV, Walsh ML, Chen LB. Localization of mitochondria in living cells with rhodamine 123. Proc Natl Acad Sci USA. 1980;77:990–994.
- Hafer K, Iwamoto KS, Schiestl RH. Refinement of the dichlorofluorescein assay for flow cytometric measurement of reactive oxygen species in irradiated and bystander cell populations. Radiat Res. 2008;169:460–468.
- Luo KW, Lung WY, Chun X, et al. EGCG inhibited bladder cancer T24 and 5637 cell proliferation and migration via PI3K/AKT pathway. Oncotarget. 2018;9:12261–12272.
- Maeng JH, Lee DH, Jung KH, et al. Multifunctional doxorubicin loaded superparamagnetic iron oxide nanoparticles for chemotherapy and magnetic resonance imaging in liver cancer. Biomaterials. 2010;31:4995–5006.
- Gurunathan S, Kalishwaralal K, Vaidyanathan R, et al. Biosynthesis, purification and characterization of silver nanoparticles using Escherichia coli. Colloids Surf B Biointerfaces. 2009;74:328–335.
- Karuppaiya P, Satheeshkumar E, Chao WT, et al. Anti-metastatic activity of biologically synthesized gold nanoparticles on human fibrosarcoma cell line HT-1080. Colloids Surf B Biointerfaces. 2013;110:163–170.
- Paul K, Bag BG, Samanta K. Green coconut (Cocos nucifera Linn) shell extract mediated size controlled green synthesis of polyshaped gold nanoparticles and its application in catalysis. Appl Nanosci. 2014;4:769–775.
- Philip D. Honey mediated green synthesis of gold nanoparticles. Spectrochim Acta A Mol Biomol Spectrosc. 2009;73:650–653.
- Varshney R, Bhadauria S, Gaur MS. Biogenic synthesis of silver nanocubes and nanorods using sundried Stevia rebaudiana leaves. AML. 2010;1:232–237.
- Ismail EH, Saqer AMA, Assirey E, et al. Successful green synthesis of gold nanoparticles using a Corchorus olitorius extract and their antiproliferative effect in cancer cells. IJMS 2018;19:2612.
- Kadir FA, Kassim NM, Abdulla MA, et al. PASS-predicted Vitex negundo activity: antioxidant and antiproliferative properties on human hepatoma cells-an in vitro study. BMC Complement Altern Med. 2013;13:343.
- Nayak D, Pradhan S, Ashe S, et al. Biologically synthesized silver nanoparticles from three diverse family of plant extracts and their anticancer activity against epidermoid A431 carcinoma. J Colloid Interface Sci. 2015;457:329–338.
- Hembram KC, Kumar R, Kandha L, et al. Therapeutic prospective of plant-induced silver nanoparticles: application as antimicrobial and anticancer agent. Artif Cells Nanomed Biotechnol. 2018;46:S38–S51.
- Hao K, Chen BY, Li KQ, et al. Cytotoxicity of anti-tumor herbal Marsdeniae tenacissimae extract on erythrocytes. J Zhejiang Univ Sci B. 2017;18:597–604.
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516.
- Ou L, Lin S, Song B, et al. The mechanisms of graphene-based materials-induced programmed cell death: a review of apoptosis, autophagy, and programmed necrosis. IJN. 2017;12:6633–6646.
- Sellappa S, RafiqKhan M, Selvaraj V, et al. Cytotoxic effect of green synthesized gold nanoparticles using Argemone mexicana leaf against HEPG2 cells. Indo Amer J Phar Res. 2015;5:3394–3398.