Abstract
Background
Cyclooxygenase-2 (Cox-2) is critical for tumor invasion, angiogenesis, and poor prognosis in many human cancers. It was reported to be an abnormal expression in many human malignancies, including basal cell carcinoma (BCC). However, the prognostic significance of cox-2 in BCC was still unclear. The aim of this study was to investigate the prognostic roles of cox-2 for patients with BCC.
Methods
We detected the expression of cox-2 both at mRNA and protein level in tumor tissue and adjacent normal tissues from 180 patients with BCC by quantitative real-time polymerase chain reaction (qRT-PCR) and western blot analysis, respectively.
Results
Cox-2 expression was significantly increased in BCC tissues compared with the adjacent normal cohorts (p < .001). Its expression was significantly associated with angiogenesis (p < .001) and depth of invasion (p < .001). Kaplan–Meier analysis suggested patients with high expression of cox-2 had a shorter overall survival rate than those with low expression (log rank test, p < .001).
Conclusions
The expression of cox-2 was up-regulated in BCC and it could be used as a bio-marker for the prognosis of BCC patients with a high risk of recurrence.
Introduction
Basal cell carcinoma (BCC) is one of the most common type of cutaneous malignancy, which accounts for ∼75% of all skin cancers and 80–90% of non-melanoma skin cancer (NMSC) [Citation1–3]. It affects predominantly the head and neck regions in fair skin types and its incidence is increasing [Citation4]. The risk factors of BCC include sunlight exposure, fair skin, nevi, smoking, industrial chemical agents, and so on [Citation5–7]. In addition, the progression of BCC is significantly influenced by the host immune response and the inflammatory cells in the microenvironment [Citation8,Citation9]. Even though BCC usually grows slowly and rarely metastasizes, sometimes it can cause significant morbidity by local tissue destruction and invasion which could lead to disfigurement [Citation10]. The main treatment for BCC is surgery depending on the histologic subtype, location, and patient comorbidities [Citation2,Citation11]. However, patients with an aggressive form of BCC will probably undergo functional and esthetic deformities, even at an increased mortality rate [Citation12,Citation13].
Cyclooxygenase-2 (cox-2) is one isoform of cox which is a key factor in the synthesis of prostaglandins (PG) from arachidonic acid [Citation14]. It is usually induced by several extracellular or intracellular stimuli, such as growth factors, proinflammatory cytokines, infectious agents, mitogens and hormones [Citation15–18]. It is not expressed in normal tissues, but up-regulated in malignant tumors which may be closely associated with tumorigenesis and metastasis [Citation19,Citation20]. Cox-2 was considered to be over-expressed and could predict the recurrence of BCC in previous studies as well as related to cell apoptosis, angiogenesis, and tumorigenesis [Citation21,Citation22]. However, its role in the prognosis of BCC was rarely reported.
In the present study, we detected the cox-2 expression in BCC tissues and the adjacent normal tissues. Then, the association between its expression and clinicopathologic characteristics was analyzed, too. In order to determine the prognostic value of cox-2 in BCC patients, Kaplan–Meier and cox regression analysis were conducted.
Materials and methods
Patients and specimens
A total of 180 patients with BCC (mostly located on face and neck) who received surgical excision without preoperative treatment (chemotherapy or radiotherapy) at Chinese PLA General Hospital were selected for this study. All patients with BCC were based on pathological identification. The study was approved by the Ethics Committee of the hospital. Written informed consent was obtained from each patient in advance, and all specimens were made anonymous according to the ethical and legal standards.
Tumor tissues and paired adjacent normal tissues were severally taken at the time of surgery from patients with BCC and immediately frozen by liquid nitrogen, immediately. Then, the frozen tissues specimens were stored at –80 °C until use. The clinicopathologic characteristics of the patients with BCC were recorded in a database. A 5 years’ follow up was conducted with all patients by telephone calls or questionnaire. Patients who died from unexpected events or other diseases were excluded from our study.
RNA extraction and quantitative real-time reverse transcriptase-polymerase chain reaction (qRT-PCR) analysis
Total RNA was extracted from all samples using TRIzol reagent (Invitrogen, San Diego, CA, USA). Only those samples of RNA with an OD A260/A280 ratio close to a value of 2.0 were available, which was measured by Agilent 2100 Bioanalyzer (Agilent Technologies). Then, the cDNA was synthesized using RevertAid First Strand cDNA Synthesis Kits (Fermentas, Germany). RT-PCR reaction was performed using the SYBR Premix Ex Taq™ II (TaKaRa Dalian, China) in an Applied Biosystems 7500 real-time PCR system (Applied Biosystems, Foster City, CA, USA). The glyceraldehyde phosphate dehydrogenase (GAPDH) served as an endogenous control. The primers of cox-2 and GAPDH were as follows: cox-2, forward-5′-ACATTAACT ATTTACAGGGTAACTGCTTAGG-3′ and reverse-5′-CCCCCTCCTTGTTTCTTGG A-3′; GAPDH, forward-5′-CATCTTCTTTTGCGTCGCC-3′ and reverse-5'-AAA AGC AGCCCTGGTGAC-3′. Relative quantification of cox-2 expression at mRNA level was calculated using the comparative CT (2−ΔΔCT) method. Each sample was used in triplicate.
Detection for angiogenesis
Staining of vessel was performed according to the previous study [Citation23]. Angiogenesis was evaluated by microvessel density under the light field of micoscope according to the method performed by Weidner et al. [Citation24]. The staining range was scored as follows: <5%, 0 point; 5–25%, 1 point; 26–50%, 2 points; 51–75%, 3 points; >75%, 4 points. Scores 0 and 1 were considered as negative, scores 2 and 3 were considered as positive.
Western blotting analysis
Total protein were extracted from all tissue samples using total protein lysis buffer, and protein concentration was determined by the BCA Protein Assay Kit, respectively. Briefly, protein was separated on 10% sodium dodecyl sulfate-polyacrylamide gels electrophoresis (SDS-PAGE). After electrophoresis separation, the protein was transferred to a polyvinylidene fluoride membrane (PVDF; Millipore, USA). After blocked with 5% blocking buffer, the membranes were incubated with primary antibodies overnight at 4 °C with primary cox-2 antibody (1:1000 dilution). After washing with 1 × PBST (PBS 0.1% Tween-20), membranes were incubated with HRP-conjugated secondary antibody (1: 2000 dilution) for 1 h at room temperature. Expressly, the membranes were adequately washed with TBST after each treatment with antibody. At last, the protein was visualized with ECL chemiluminescence reagents (Beyotime, China). The relative protein density was normalized to GAPDH protein.
Statistical analysis
The statistical analysis were performed with SPSS 18.0 software (SPSS, Chicago, IL, USA). All data were expressed as mean ± SD. The differences between two groups was compared using Student’s t-test, and chi-square test was used to analyze the associations between cox-2 expression and the clinicopathological features. Kaplan–Meier analysis with log–rank test was used for overall survival analysis. Univarite and multivariate analysis with cox regression analysis were used for estimating the prognostic value of cox-2 based on overall survival and disease-free survival (DFS). The difference was considered to be statistically significant when p < .05.
Results
The expression of cox-2 in BCC tissues and adjacent normal tissues
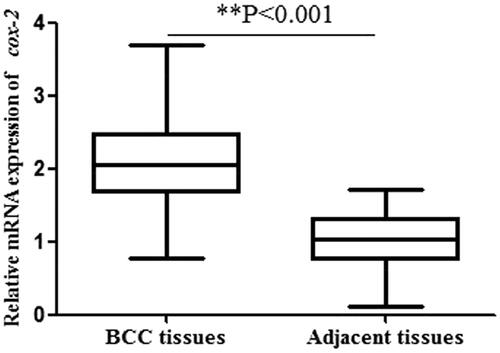
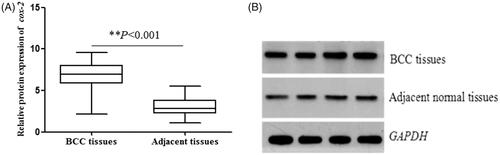
The relative mRNA expression of cox-2 (normalized to GAPDH) in 180 BCC tissues and matched adjacent normal samples were determined by qRT-PCR analysis. As shown in , the results suggested a significantly higher cox-2 expression in BCC tissues compared with adjacent tissues (2.10 ± 0.61 vs 0.99 ± 0.39, p < .001). Then, we investigated BCC protein expression by western blot. The results also showed the protein expression of cox-2 in BCC tissues was significantly higher than that in adjacent normal specimens (6.79 ± 1.57 vs 3.02 ± 1.00, p < .001, ) (Supplementary Figure 1). These results demonstrated that cox-2 could act as an oncogene in BCC.
Relationship between cox-2 expression and clinicopathological characteristics in patients with BCC
Subsequently, we analyzed the relationship of cox-2 expression with various clinicopathological features. Primarily, we divided the patients with BCC into two groups with a median expression level of cox-2 protein expression with 6.79. Tissues with a expression level more than 6.79 belonged to the high group and the others were of the low group. As shown in , cox-2 protein expression in BCC was significantly associated with the depth of invasion (p < .001). The positive angiogenesis in BCC tissues with cox-2 high expression was as high as 72.94% (62/85). But, the proportion of the positive angiogenesis in BCC tissues with low cox-2 expression was 28.42% (27/95). The difference was significant between BCC tissues with cox-2 high- and low-expression groups (p < .001). Concretely, high cox-2 expression was more frequently detected in BCC with advanced depth of tumor invasion and positive angiogenesis. According to the result, cox-2 expression might be useful for identifying the degree of BCC. However, there was no association of cox-2 expression with and age, gender, tumor size, location and nevi.
Table 1. The association of cox-2 expression with clinicopathological parameters in patients with BCC.
Association between cox-2 expression and overall survival of BCC
To explore the prognostic role of cox-2 in BCC, Kaplan–Meier and cox regression analysis with the data of follow-up were carried out, respectively. Kaplan–Meier analysis showed patients with high cox-2 expression had significantly shorter overall survival than those with low expression of cox-2 (log rank test, p < .001, , ). The overall survival rate of BCC patients in five years was 83.3% and the DFS rate was 78.3% in this study. Univariate and multivariate analysis adjusted for clinical factors indicated that cox-2 expression (HR = 5.320, 95% CI = 1.861–15.204, p = .002) and angiogenesis (HR = 2.437, 95% CI = 1.096–5.415, p = .029) were related to the prognosis of BCC and they might be independent prognostic indicators. Additionally, cox-2 and angiogenesis were also found to be associated with DFS of BCC patients (HR = 2.457, 95% CI = 1.227–4.919, p = .011; HR = 2.476, 95% CI = 1.215–5.045, p = .013) ().
Figure 3. Kaplan–Meier analysis for estimating the overall survival of patients with BCC. Patients with high expression of cox-2 had shorter overall survival than those with low cox-2 expression (log rank test, p < .001).
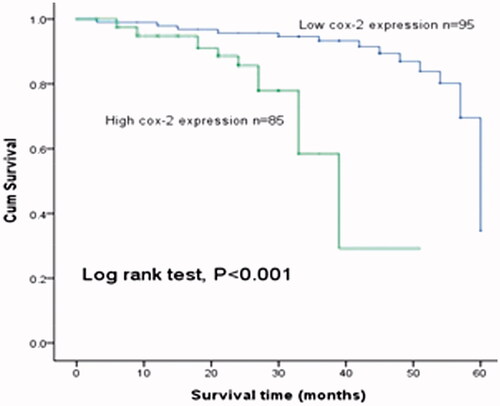
Table 2. Univariate and multivariate analysis of clinical factors and cox-2 in overall survival of BCC.
Table 3. Univariate and multivariate analysis of clinical factors and cox-2 in disease-free survival of BCC.
Discussion
As the most common form of skin cancer, the mortality of BCC is low due to the low rates of metastatic disease [Citation25]. Despite the theoretical advantage of more extensive histological assessment of excision margins for BCC, the 5 years recurrence rates for BCC after complete excision are 3.2–10% in pBCC and >17% in rBCC [Citation26]. Therefore, it is critical to determine effective markers for predicting the prognosis of BCC after surgical resection.
In previous studies, some markers were found to predict the recurrence of BCC. Actin was detected and found to be restricted in aggressive tumors, which could be a marker of aggressiveness in BCCs and used in clinical practice for surgical therapeutic decisions [Citation27]. Janisson et al. considered the aneuploidy was a risk factor for recurrences of BCC [Citation28]. Various researches suggested that cox-2 expression was connected with tumorigenesis of cancers by promoting invasion, stimulating proliferation, promoting angiogenesis and inhibiting apoptosis [Citation29,Citation30]. Recently, overexpression of cox-2 was found in BCC by increasing the expression of p53 protein [Citation31,Citation32]. Moreover, it acts as a risk factor for the recurrence of BCC [Citation21]. However, the role of cox-2 in the prognosis of BCC remains incompletely known.
In the present study, we detected the expression of cox-2 in the tissues of BCC patients and matched adjacent normal tissues, and the results demonstrated that cox-2 expression was significantly higher in BCC compared to normal cohorts. These might suggest that cox-2 served as an oncogene in BCC. Then, we investigated the relationship between cox-2 expression and clinical factors to explore whether it was involved in the development of BCC. The outcome proved its expression was influenced by the depth of invasion and angiogenesis significantly, which showed cox-2 participate in the progression of BCC. This was consistent with the previous studies [Citation21,Citation22,Citation33].
It was reported that the overexpression of cox-2 associated with a poor prognosis in several malignancies, such as colorectal cancer, colon cancer and breast cancer [Citation34–36]. So, we thought cox-2 might be related to the prognosis of BCC, too. Kaplan–Meier analysis revealed that patients with high cox-2 expression had a shorter overall survival compared to those with low cox-2 expression. Then, univariate and multivariate analysis were performed and the results indicated that cox-2 was an independent predictor of the prognosis of patients with BCC after curative excision.
In summary, cox-2 expression is up-regulated in tumor tissues compared to adjacent normal tissues. Its expression is related to the depth of invasion and angiogenesis. Besides, this is the first report to reveal that cox-2 expression is a reliable predictor of the prognosis for patients with BCC. However, more larger and deeper studies are necessary to confirm and predict the value of cox-2 in the prognosis of BCC.
Supplementary_Figure_Legends.doc
Download ()Supplementary_Figure_1.tif
Download ()Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Sobjanek M, Zablotna M, Michajlowski I, et al. 308 G/A TNF-alpha gene polymorphism influences the course of basal cell carcinoma in a Polish population. Arch Med Sci. 2015;11:599–604.
- Ballester-Sanchez R, Pons-Llanas O, Candela-Juan C, et al. Efficacy and safety of electronic brachytherapy for superficial and nodular basal cell carcinoma. J Contemp Brachytherapy. 2015;7:231–238.
- Bobadilla F, Wortsman X, Munoz C, et al. Pre-surgical high resolution ultrasound of facial basal cell carcinoma: correlation with histology. Cancer Imaging. 2008;8:163–172.
- Al-Niaimi F, Sheth N, Kurwa HA, et al. Photodynamic therapy followed by Mohs micrographic surgery compared to Mohs micrographic surgery alone for the treatment of basal cell carcinoma: results of a pilot single-blinded randomised controlled trial. J Cutan Aesthet Surg. 2015;8:88–91.
- Han J, Colditz GA, Hunter DJ. Risk factors for skin cancers: a nested case-control study within the Nurses' Health Study. Int J Epidemiol. 2006;35:1514–1521.
- Lock-Andersen J, Drzewiecki KT, Wulf HC. Naevi as a risk factor for basal cell carcinoma in Caucasians: a Danish case-control study. Acta Derm Venereol. 1999; 79:314–319.
- Lock-Andersen J, KT and Wulf D. HC. Eye and hair colour, skin type and constitutive skin pigmentation as risk factors for basal cell carcinoma and cutaneous malignant melanoma. A Danish Case-Control Study. Acta Derm Venereol. 1999;79:74–80.
- Kaur P, Mulvaney M, Carlson JA. Basal cell carcinoma progression correlates with host immune response and stromal alterations: a histologic analysis. Am J Dermatopathol. 2006;28:293–307.
- Kaporis HG, Guttman-Yassky E, Lowes MA, et al. Human basal cell carcinoma is associated with Foxp3+ T cells in a Th2 dominant microenvironment. J Invest Dermatol. 2007;127:2391–2398.
- Wong CS, Strange RC, Lear JT. Basal cell carcinoma. BMJ. 2003;327:794–798.
- Bath-Hextall FJ, Perkins W, Bong J, et al. Interventions for basal cell carcinoma of the skin. Cochrane Database Syst Rev. 2007;CD003412.
- Dixon AY, Lee SH, McGregor DH. Histologic evolution of basal cell carcinoma recurrence. Am J Dermatopathol. 1991;13:241–247.
- de Rosa G, Vetrani A, Zeppa P, et al. Comparative morphometric analysis of aggressive and ordinary basal cell carcinoma of the skin. Cancer. 1990;65:544–549.
- Wang JL, Wang X, Yang D, et al. Association between 8473T > C polymorphism in the cyclooxygenase-2 gene and the risk of nasopharyngeal carcinoma. Int J Clin Exp Pathol. 2015;8:7441–7445.
- Mitsudomi T, Suda K, Yatabe Y. Surgery for NSCLC in the era of personalized medicine. Nat Rev Clin Oncol. 2013;10:235–244.
- Allaj V, Guo C, Nie D. Non-steroid anti-inflammatory drugs, prostaglandins, and cancer. Cell Biosci. 2013; 3: 8
- Chow LW, Yip AY, Loo WT, et al. Celecoxib anti-aromatase neoadjuvant (CAAN) trial for locally advanced breast cancer. J Steroid Biochem Mol Biol. 2008;111:13–17.
- Jana D, Sarkar DK, Ganguly S, et al. Role of cyclooxygenase 2 (COX-2) in prognosis of breast cancer. Indian J Surg Oncol. 2014;5:59–65.
- Chell S, Kaidi A, Williams AC, et al. Mediators of PGE2 synthesis and signalling downstream of COX-2 represent potential targets for the prevention/treatment of colorectal cancer. Biochim Biophys Acta. 2006;1766:104–119.
- Chu AJ, Chou TH, Chen BD. Prevention of colorectal cancer using COX-2 inhibitors: basic science and clinical applications. Front Biosci. 2004;9:2697–2713.
- El-Khalawany MA, Abou-Bakr AA. Role of cyclooxygenase-2, ezrin and matrix metalloproteinase-9 as predictive markers for recurrence of basal cell carcinoma. J Can Res Ther. 2013;9:613–617.
- Tjiu JW, Liao YH, Lin SJ, et al. Cyclooxygenase-2 overexpression in human basal cell carcinoma cell line increases antiapoptosis, angiogenesis, and tumorigenesis. J Invest Dermatol. 2006;126:1143–1151.
- Park MK, Ko EJ, Jeon KY, et al. Induction of angiogenesis by malarial infection through hypoxia dependent manner. Korean J Parasitol. 2019;57:117–125.
- Weidner N, Semple JP, Welch WR, et al. Tumor angiogenesis and metastasis–correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8.
- Ting PT, Kasper R, Arlette JP. Metastatic basal cell carcinoma: report of two cases and literature review. J Cutan Med Surg. 2005;9:10–15.
- Mosterd K, Krekels GA, Nieman FH, et al. Surgical excision versus Mohs' micrographic surgery for primary and recurrent basal-cell carcinoma of the face: a prospective randomised controlled trial with 5-years' follow-up. Lancet Oncol. 2008;9:1149–1156.
- Adegboyega PA, Rodriguez S, McLarty J. Stromal expression of actin is a marker of aggressiveness in basal cell carcinoma. Hum Pathol. 2010;41:1128–1137.
- Janisson-Dargaud D, Durlach A, Lorenzato M, et al. Aneuploidy, but not Ki-67 or EGFR expression, is associated with recurrences in basal cell carcinoma. J Cutan Pathol. 2008;35:916–921.
- Lau MT, Wong AS, Leung PC. Gonadotropins induce tumor cell migration and invasion by increasing cyclooxygenases expression and prostaglandin E(2) production in human ovarian cancer cells. Endocrinology. 2010;151:2985–2993.
- Gangwar R, Mandhani A, Mittal RD. Functional polymorphisms of cyclooxygenase-2 (COX-2) gene and risk for urinary bladder cancer in North India. Surgery. 2011;149:126–134.
- Chen Z, Yang J, Huang Q. Correlation and expression of COX-2 and P53 protein in basal cell carcinoma of eyelid. J Huazhong Univ Sci Technol Med Sci. 2009;29:383–386.
- Karagece Yalcin U, Seckin S. The expression of p53 and COX-2 in basal cell carcinoma, squamous cell carcinoma and actinic keratosis cases. Turk Patoloji Derg. 2012;28:119–127.
- Tjiu JW, Chen JS, Shun CT, et al. Tumor-associated macrophage-induced invasion and angiogenesis of human basal cell carcinoma cells by cyclooxygenase-2 induction. J Invest Dermatol. 2009;129:1016–1025.
- Wang D, Dubois RN. The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene. 2010;29:781–788.
- Ogino S, Kirkner GJ, Nosho K, et al. Cyclooxygenase-2 expression is an independent predictor of poor prognosis in colon cancer. Clin Cancer Res. 2008;14:8221–8227.
- Ristimaki A, Sivula A, Lundin J, et al. Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res. 2002;62:632–635.