Abstract
Luteolin is a representative of natural flavonoid that has anti-tumour properties. This study designed to check its impact on breast cancer and the underlying mechanisms. MDA-MB-453 and MCF-7 cells were administrated with luteolin and the following techniques were carried out: CCK-8 assay, FITC-PI double-staining and Western blot. qRT-PCR analysis was utilized to see the effects of luteolin on miR-203 expression. Besides, miR-203 expression was silenced by transfection with specific inhibitor. Luteolin remarkably declined MDA-MB-453 and MCF-7 cells viability and accelerated apoptosis which accompanied by Bax up-regulation, Bcl-2 down-regulation and Caspase-3 cleavage. Also, luteolin impeded TGFβ1-induced EMT, as evidenced by the decreased levels of Vimentin, Zeb1 and N-cadherin, as well as the increased level of E-cadherin. miR-203 was highly expressed in 22 pair of breast cancer tissues than the matched paracancerous tissues. Luteolin could elevate miR-203 level. Besides, luteolin’s anti-tumour effects were partially eliminated by miR-203 silence. Further, luteolin inhibited Ras/Raf/MEK/ERK signalling, while the inhibitory effects were flattened by miR-203 silence. Luteolin significantly reduced breast cancer cells growth and EMT. Luteolin exerted its anti-tumour effects possibly involved the elevated expression of miR-203 and the inhibited Ras/Raf/MEK/ERK signalling.
Introduction
Breast cancer is the most frequently diagnosed cancer and the dominant cause of cancer death among women younger than 40 years [Citation1]. As established in 2017, there are 255,180 new cases and 41,070 deaths in situ of patients with breast cancer in United States [Citation1]. There is a noteworthy upward trend in incidence and mortality rates of this cancer [Citation2]. To date, therapeutic options for this solid tumour are limited to three fundamental modalities, i.e. surgical resection, radiation therapy and chemotherapy. These modalities are used in clinic alone or in combination with each other to treat breast cancer, but the outcome is still dissatisfied [Citation3,Citation4]. Thus, it is important to find new treatment for this cancer.
Luteolin (chemical construction is shown in ) is a representative of natural flavonoid existing in many natural herbs, vegetables and fruits. Overwhelming evidences suggest luteolin as a pharmacological agent for inflammation-related diseases and neurodegenerative diseases [Citation5,Citation6], which may be owing to its excellent anti-inflammatory and antioxidant properties. Besides that, luteolin was reported as a potential anti-tumour agent that could inhibit a wide range of human cancers, such as gastric cancer [Citation7], colorectal cancer [Citation8], melanoma [Citation9], ovarian cancer [Citation10] and so forth. Also, luteolin was capable of reducing breast cancer growth and metastasis [Citation11]. But, the regulation response of luteolin treatment in breast cancer cells is still unclear, which are required to be investigated to better explain how luteolin exerted its anti-tumour function.
The pathogenesis of breast cancer is complex involved with multi-factor induction, multi-gene participation and multi-pathway. microRNAs (miRNAs) are a sort of tiny non-coding RNAs that are participate in the initiation and progression of breast cancer [Citation12,Citation13]. Considering their functions in tumour cells growth and metastasis, some of them have been recognized as oncogenic miRNAs or anti-oncogenic miRNAs [Citation14]. For instance, miR-216a acted as an anti-oncogenic miRNAs in breast cancer as its overexpression induced apoptosis death of MCF-7 cells [Citation15]. Of contrast, miR-4513 was capable of accelerating breast cancer cells growth, migration and invasion, which suggested miR-4513 as an oncogenic miRNA [Citation16]. miR-203 is one of such oncogenic miRNA that locates at chromosome 14q32.33. The anti-tumour role of miR-203 has been found in various kinds of cancers, such as colorectal cancer [Citation17], hepatocellular carcinoma [Citation18], gastric cancer [Citation19], prostate cancer [Citation20] and so forth. However, miR-203 exerted contradictory roles in breast cancer. As reported by Muhammad et al., anti-miR-203 could suppress the growth and stemness of ER-positive breast cancer [Citation21], indicating tumour-promoting role of miR-203. Wang et al. illustrated that miR-203 functioned as an anti-tumour factor in inhibiting triple-negative breast cancer cells proliferation and migration [Citation22]. This contradiction attracts our interest to study the exact role of miR-203 in breast cancer.
In this, the impacts of luteolin in the growth and epithelial-mesenchymal transition (EMT) of breast cancer cells were checked. More importantly, the regulation of luteolin on miR-203 expression was studied to further explain luteolin’s anti-tumour function.
Materials and methods
Clinical specimens
Twenty-two pairs of breast cancer tissues and the matched paracancerous tissues were attained from Linyi Central Hospital (Linyi, China). Patients with breast cancer donated the tissues during tumour excision. None of them were treated with other therapies before that. Written informed consents were signed. The usage of tissue samples was approved by the ethics committee of Linyi Central Hospital (Linyi, China).
Cell treatment
Human breast cancer cell lines MDA-MB-453 and MCF-7 were both from ATCC (Manassas, VA). The two cells were respectively cultivated in Leibovitz’s L-15 and EMEM medium (both ATCC) completed with 10% FBS (Gibco, Grand Island, NY). Cells were raised at 37 °C in a dampen condition with 5% CO2.
Powder luteolin (≥98% (TLC)) from Sigma-Aldrich (St. Louis, MO) was dissolved in DMSO to form a storage solution with 0.5 M. The storage solution was thinned by culture medium to make working solution with 10 μM. Cells were administrated by 10 μM luteolin for 24 as elsewhere described [Citation23].
Transfection
miR-203 inhibitor and its respective control (NC) were brought from Genepharma (Shanghai, China). They were transfected into cell with the help of Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Transfection procedure was performed under non-serum condition and was lasted for 48 h.
qRT-PCR
Total miRNAs in cells and tissues were collected by miRNeasy Mini Kit (Qiagen, Shenzhen, China). Reverse transcription was carried out by Mir-X™ miRNA First Strand Synthesis Kit (Takara, Dalian, China) with specific primer for hsa-miR-203 (5′-GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGAC-3′). Quantification of miR-203 level was checked by Mir-X™ miRNA qRT-PCR TB Green™ Kit (Takara) with specific primers (Forward: 5′-GGGGTGAAATGTTTAGGAC-3′, Reverse: 5′-CAGTGCGTGTCGTGGAGT-3′). U6 snRNA was detected for RNA normalization and data was figured out by 2−ΔΔCt method.
Cell viability
Post-transfection, cells in 96-well plates (5 × 103 cells/well) were administrated with luteolin for 24 h. Then, 20 μL CCK-8 (Dojindo Molecular Technologies, Kyushu, Japan) was added. And 1 h later, the absorbance of samples was detected by a Microplate Reader (Bio-Rad, Hercules, CA).
Apoptosis assay
Post-transfection, cells in 6-well plates (5 × 105 cells/well) were administrated with luteolin for 24 h. Collected cells in epoxide tube and stained them with fluorochromes (FITC and PI) according to the instruction of Apoptosis Detection kit (Beyotime, Shanghai, China). Flow cytometry was carried out in FACS can (Beckman Coulter, Fullerton, CA). Data were figured out by FlowJo software (Treestar, San Carlos, CA).
Western blot
Total protein from cell was collected by RIPA buffer (Beyotime) and the purity of the extracts was verified by BCA method. After suffering from SDS-PAGE system and transmembrane, the proteins were probed by the primary antibodies against Bax (orb314609), Bcl-2 (orb135113), Caspase-3 (orb378617), cleaved Caspase-3 (orb106556), Vimentin (orb34040), Zeb1 (orb153501), E-cadherin (orb213706), N-cadherin (orb89454), Ras (orb379158), Raf (orb97430), MEK (orb129686), p-MEK (orb10463), ERK (orb95116), p-ERK (orb10606) and β-actin (orb378579, Biorbyt, San Francisco, CA). After incubating with secondary antibody, the bands were developed by ECL Plus Western Blotting Substrate (Pierce, Carlsbad, CA). Grey level of bands was measured by Image Lab™ Software (Bio-Rad).
Statistics
Data were shown as mean ± SD. Student t-test and ANOVA combined with Duncan post-hoc test in SPSS software (SPSS Inc., Chicago, IL) were utilized for statistical analyses. p Values lower than .05 was set as statistical difference and presented as asterisks.
Results
Luteolin suppressed breast cancer cells growth and EMT progress
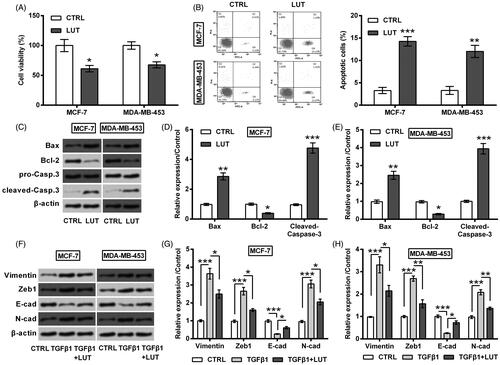
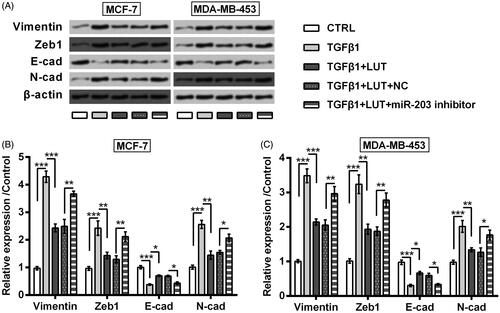
MCF-7 and MDA-MB-453 cells were administrated with 10 μM luteolin for 24, after which cell viability was checked. As seen in , the viability of MCF-7 and MDA-MB-453 cells was repressed by luteolin (p < .05). On the contrary, luteolin raised apoptosis rate (p < .05, ), along with Bax up-regulation (p < .01), Bcl-2 down-regulation (p < .05) and Caspase-3 cleavage (p < .001, ). Also, the effects of luteolin on EMT process of these two cell lines were checked. To this end, MCF-7 and MDA-MB-453 cells were treated by 5 ng/mL TGFβ1 for 24 h to make an experimental EMT model. Data in displayed that, accumulation of Vimentin, Zeb1 and N-cadherin was clearly increased while E-cadherin was lowered by addition with TGFβ1 (p < .001). Luteolin treatment remarkably ameliorated these alterations evoked by TGFβ1 (p < .05 or p < .01).
Figure 2. Luteolin suppressed breast cancer cells growth and EMT progress. MCF-7 and MDA-MB-453 cells were administrated with 10 μM luteolin for 24. (A) Cell viability, (B) apoptosis rate, (C–E) accumulation of apoptosis-related proteins, and (F–H) expression of EMT-markers were checked by CCK-8 assay, FITC-PI double-staining and Western blot. 5 ng/mL TGFβ1 was utilized to treat cells for 24 h to make an experimental EMT model. *, ** and *** stand for p values <.05, .01, and .001.
Luteolin increased miR-203 expression
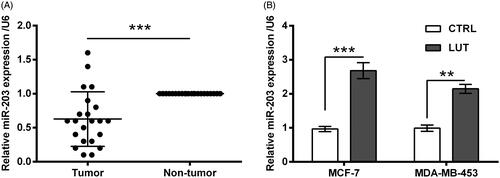
miR-203 expression in 22 pair of breast cancer tissues and the matched paracancerous tissues was monitored. qRT-PCR result shown that miR-203 was lower expressed in tumour tissues than non-tumour tissues (p < .001, ). Besides, in vitro data indicated that miR-203 expression was raised by luteolin treatment (p < .01 or p < .001, ).
Figure 3. Luteolin increased miR-203 expression. (A) miR-203 expression in 22 pair of breast cancer tissues and the matched paracancerous tissues was monitored by qRT-PCR. (B) miR-203 expression in MCF-7 and MDA-MB-453 cells was monitored by qRT-PCR after treating with luteolin. **and ***stand for p values <.01 and .001.
Luteolin suppressed breast cancer cells growth and EMT progress via miR-203
Next, miR-203 inhibitor was transfected into MCF-7 and MDA-MB-453 cells to check the involvement of miR-203 in luteolin’s effects. As seen in , miR-203 expression was dropped steeply by miR-203 inhibitor (p < .001). Importantly, the suppressive impacts of luteolin on MCF-7 and MDA-MB-453 cells were impeded by miR-203 silence. In deed luteolin did not that significantly impact cell viability (p < .05, ), apoptosis rate (p < .05, ), expression of apoptosis-related proteins (p < .05 or p < .01, ), as well as expression of EMT-related biomarkers (p < .05 or p < .01, ) when miR-203 was silenced.
Figure 4. Luteolin suppressed breast cancer cells growth via miR-203. (A) miR-203 expression in MCF-7 and MDA-MB-453 cells was monitored by qRT-PCR after transfection with miR-203 inhibitor and NC. The transfected cells were treated by luteolin. (B) Cell viability, (C) apoptosis rate, and (D–F) accumulation of apoptosis-related proteins were checked by CCK-8 assay, FITC-PI double-staining and Western blot. *, ** and *** stand for p values <.05, .01 and .001.
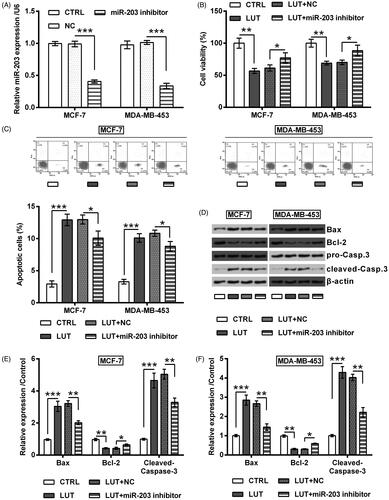
Figure 5. Luteolin suppressed breast cancer cells EMT progress via miR-203. MCF-7 and MDA-MB-453 cells were transfected with miR-203 inhibitor and NC. The transfected cells were treated by luteolin. (A–C) Expression of EMT-markers was checked by Western blot. 5 ng/mL TGFβ1 was utilized to treat cells for 24 h to make an experimental EMT model. *, ** and *** stand for p values <.05, .01 and .001.
Luteolin inhibited Ras/Raf/MEK/ERK signalling via miR-203
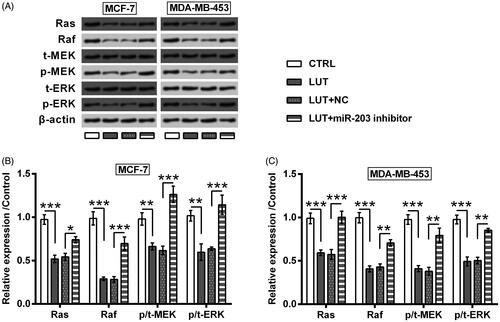
Lastly, the downstream signalling which is responsible for luteolin’s anti-tumour property was explored. Ras/Raf/MEK/ERK has long been known as critical signalling in driving the initiation of cancers, including breast cancer [Citation24]. Herein, the expression of Ras and Raf, as well as the phosphorylation of MEK and ERK were repressed by luteolin (p < .01 or p < .001, ). Nonetheless, these alterations made by luteolin treatment were attenuated or reversed by miR-203 suppression (p < .05, p < .01 or p < .001).
Figure 6. Luteolin inhibited Ras/Raf/MEK/ERK signalling via miR-203. MCF-7 and MDA-MB-453 cells were transfected with miR-203 inhibitor and NC. The transfected cells were treated by luteolin. The expression of Ras and Raf, as well as the phosphorylation of MEK and ERK were checked by Western blot. *, ** and *** stand for p values <.05, .01 and .001.
Discussion
With the improvement of aetiology and pathogenesis research, treatment and therapy for breast cancer is significantly improved. However, the overcome of this cancer is still not optimistic since the high tumour metastasis, recurrence and treatment resistance, which are principal causes of death in patients with cancer [Citation25–27]. Recent decades, scholars around the world noticed that natural herbs are beneficial in the management of breast cancer [Citation28,Citation29]. Luteolin is one of the medicine herbs that has been considered as an anti-tumour flavonoid. Herein, in vitro data suggested luteolin as an effective anti-growth and anti-EMT agent in breast cancer cells. Luteolin elevated miR-203 expression and luteolin conferred its anti-tumour properties possibly through up-regulating miR-203 and inhibiting Ras/Raf/MEK/ERK signalling.
Excessive proliferation is one main character of human cancers. The anti-proliferating and pro-death functions of luteolin in breast cancer cells have been previously revealed [Citation23,Citation30]. This was also observed in this study that MCF-7 and MDA-MB-453 cells viability was repressed while apoptosis was induced by luteolin. The apoptosis death made by luteolin treatment possibly via a mitochondria-dependent way, as the balance between Bax and Bcl-2 was tilted and Caspase-3 was clearly cleaved. Besides that, the effects of luteolin on MCF-7 and MDA-MB-453 cells EMT process were studied. Morbidity and mortality in patients with solid tumours always result from the disruption of normal biological function by disseminating tumour cells, and EMT process has been intensely investigated as the underlying causes of cancer metastasis. During EMT process, E-cadherin shifting to N-cadherin is considered as a hallmark event [Citation31]. Besides, increased expression of Vimentin and Zeb1 is another characteristic that reinforcing EMT process [Citation31]. This work demonstrated that luteolin suppressed EMT progress as N-cadherin, Vimentin and Zeb1 were low expressed while E-cadherin was highly expressed. This finding confirmed the anti-metastatic effects of luteolin in breast cancer which has been reported elsewhere [Citation11,Citation32].
Despite recent studies have shown the anti-breast cancer properties of luteolin, the underlying mechanisms underlined its function are still unclear. A small fraction of literatures focused on the regulation of luteolin on miRNA expression in order to explain luteolin’s function via a novel angle [Citation33]. In respect of human cancer, miR-34a-5p [Citation34], miR-301 [Citation35] and miR-630 [Citation36] have been reported as the downstream effectors of luteolin. Herein, we for the first time illustrated miR-203 as another anti-tumour executor of luteolin. miR-203 has been widely identified as an anti-tumour miRNA [Citation17–20], but its exact function in breast cancer is confusing. Two of the previous studies reported miR-203 as an oncogene, as silence of its expression inhibited breast cancer cells colony formation, transformation, stemness and migration [Citation21,Citation37]. Others suggested miR-203 as an anti-oncogene through performing in vitro experiments [Citation22,Citation38,Citation39]. This contradiction may due to the different cell lines used and the difference in testing parameters. Our study was confirmed with the findings which suggested miR-203 as an anti-oncogene. More importantly, present work provided a viewpoint that luteolin resisted breast cancer owing to its promoting effects on miR-203 expression.
Ras/Raf/MEK/ERK signalling pathway exists in all types of eukaryotic cells and plays a critical role in tumour cells proliferation, apoptosis and transformation [Citation40]. In terms of breast cancer, Ras/Raf/MEK/ERK signalling plays dominating physiological effects in driving breast cancer [Citation24]. Ras is a proto-oncogene that produces Ras protein to function as a molecular switch in controlling intracellular signalling networks [Citation40]. Raf is one of the downstream genes of Ras. Ras strongly activates Raf and the following MEK/ERK kinase cascade. The present data found that luteolin could inhibit Ras/Raf/MEK/ERK signalling significantly and the inhibitory effects of luteolin on this signalling possibly via up-regulating miR-203.
To sum up, this work demonstrated the anti-breast cancer properties of luteolin, as it significantly reduced breast cancer cells growth and EMT progress. miR-203 was found to be a downstream effector of luteolin. Further, luteolin exerted its anti-tumour effects might be associated the inhibited Ras/Raf/MEK/ERK signalling. The findings of this study further suggested luteolin as a potential nature cure for breast cancer, and provided one of the explanations of luteolin’s effect.
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The research was encouraged by the Medical Ethics Committee of Linyi Central Hospital.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The datasets used and/or analysed during this study are available from the corresponding author on reasonable request.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30.
- Ghoncheh M, Pournamdar Z, Salehiniya H. Incidence and mortality and epidemiology of breast cancer in the world. Asian Pac J Cancer Prev. 2016;17:43–46.
- Shamsi M, Pirayesh Islamian J. Breast cancer: early diagnosis and effective treatment by drug delivery tracing. Nucl Med Rev. 2017;20:45–48.
- Board PDQATE. Breast cancer treatment (PDQ(R)): patient version. PDQ cancer information summaries. Bethesda (MD): National Cancer Institute; 2002.
- Aziz N, Kim MY, Cho JY. Anti-inflammatory effects of luteolin: a review of in vitro, in vivo, and in silico studies. J Ethnopharmacol. 2018;225:342–358.
- Nabavi SF, Braidy N, Gortzi O, et al. Luteolin as an anti-inflammatory and neuroprotective agent: a brief review. Brain Res Bull. 2015;119:1–11.
- Zang MD, Hu L, Fan ZY, et al. Luteolin suppresses gastric cancer progression by reversing epithelial-mesenchymal transition via suppression of the Notch signaling pathway. J Transl Med. 2017;15:52.
- Liu Y, Lang T, Jin B, et al. Luteolin inhibits colorectal cancer cell epithelial-to-mesenchymal transition by suppressing CREB1 expression revealed by comparative proteomics study. J Proteomics. 2017;161:1–10.
- Yao X, Jiang W, Yu D, et al. Luteolin inhibits proliferation and induces apoptosis of human melanoma cells in vivo and in vitro by suppressing MMP-2 and MMP-9 through the PI3K/AKT pathway. Food Funct. 2019;10:703–712.
- Wang H, Luo Y, Qiao T, et al. Luteolin sensitizes the antitumor effect of cisplatin in drug-resistant ovarian cancer via induction of apoptosis and inhibition of cell migration and invasion. J Ovarian Res. 2018;11:93.
- Cook MT. Mechanism of metastasis suppression by luteolin in breast cancer. Breast Cancer (Dove Med Press). 2018;10:89–100.
- Bahiraee A, Ebrahimi R, Halabian R. The role of inflammation and its related microRNAs in breast cancer: a narrative review. J Cell Physiol. 2019. [Epub ahead of print]. doi: 10.1002/jcp.28742
- Uhr K, Prager-van der Smissen WJC, Heine AAJ, et al. MicroRNAs as possible indicators of drug sensitivity in breast cancer cell lines. PLoS One. 2019;14:e0216400.
- O’Bryan S, Dong S, Mathis JM, et al. The roles of oncogenic miRNAs and their therapeutic importance in breast cancer. Eur J Cancer. 2017;72:1–11.
- Cui Y, Wang J, Liu S, et al. MiR-216a promotes breast cancer cell apoptosis by targeting PKCalpha. Fundam Clin Pharmacol. 2019;33:397–404.
- Li Y, Zhu H, Wang J, et al. miR-4513 promotes breast cancer progression through targeting TRIM3. Am J Trans Res. 2019;11:2431–2438.
- Okugawa Y, Toiyama Y, Hur K, et al. Circulating miR-203 derived from metastatic tissues promotes myopenia in colorectal cancer patients. J Cachexia Sarcopeni. 2019;10:536–548.
- Simile MM, Peitta G, Tomasi ML, et al. MicroRNA-203 impacts on the growth, aggressiveness and prognosis of hepatocellular carcinoma by targeting MAT2A and MAT2B genes. Oncotarget. 2019;10:2835–2854.
- Li J, Zhang B, Cui J, et al. miR-203 inhibits the invasion and EMT of gastric cancer cells by directly targeting Annexin A4. Oncol Res. 2019;27:789–799.
- Chen LZ, Ding Z, Zhang Y, et al. MiR-203 over-expression promotes prostate cancer cell apoptosis and reduces ADM resistance. Eur Rev Med Pharmacol Sci. 2018;22:3734–3741.
- Muhammad N, Bhattacharya S, Steele R, et al. Anti-miR-203 suppresses ER-positive breast cancer growth and stemness by targeting SOCS3. Oncotarget. 2016;7:58595–58605.
- Wang C, Zheng X, Shen C, et al. MicroRNA-203 suppresses cell proliferation and migration by targeting BIRC5 and LASP1 in human triple-negative breast cancer cells. J Exp Clin Cancer Res. 2012;31:58.
- Huang L, Jin K, Lan H. Luteolin inhibits cell cycle progression and induces apoptosis of breast cancer cells through downregulation of human telomerase reverse transcriptase. Oncol Lett. 2019;17:3842–3850.
- Saini KS, Loi S, de Azambuja E, et al. Targeting the PI3K/AKT/mTOR and Raf/MEK/ERK pathways in the treatment of breast cancer. Cancer Treat Rev. 2013;39:935–946.
- Dittmer J. Mechanisms governing metastatic dormancy in breast cancer. Semin Cancer Biol. 2017;44:72–82.
- Kabat GC, Ginsberg M, Sparano JA, et al. Risk of recurrence and mortality in a multi-ethnic breast cancer population. J Racial Ethn Health Disparities. 2017;4:1181–1188.
- Tang Y, Wang Y, Kiani MF, et al. Classification, treatment strategy, and associated drug resistance in breast cancer. Clin Breast Cancer. 2016;16:335–343.
- Li Y, Li S, Meng X, et al. Dietary natural products for prevention and treatment of breast cancer. Nutrients. 2017;9:728.
- Shareef M, Ashraf MA, Sarfraz M. Natural cures for breast cancer treatment. Saudi Pharm J. 2016;24:233–240.
- Park SH, Ham S, Kwon TH, et al. Luteolin induces cell cycle arrest and apoptosis through extrinsic and intrinsic signaling pathways in MCF-7 breast cancer cells. J Environ Pathol Toxicol Oncol. 2014;33:219–231.
- Thiery JP, Acloque H, Huang RY, et al. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890.
- Lin D, Kuang G, Wan J, et al. Luteolin suppresses the metastasis of triple-negative breast cancer by reversing epithelial-to-mesenchymal transition via downregulation of β-catenin expression. Oncol Rep. 2017;37:895–902.
- Zhang Z, Xu P, Yu H, et al. Luteolin protects PC-12 cells from H2O2-induced injury by up-regulation of microRNA-21. Biomed Pharmacother. 2019;112:108698.
- Jiang ZQ, Li MH, Qin YM, et al. Luteolin inhibits tumorigenesis and induces apoptosis of non-small cell lung cancer cells via regulation of microRNA-34a-5p. Int J Mol Sci. 2018;19:pii: E447.
- Han K, Meng W, Zhang JJ, et al. Luteolin inhibited proliferation and induced apoptosis of prostate cancer cells through miR-301. OncoTargets Ther. 2016;9:3085–3094.
- Sakurai MA, Ozaki Y, Okuzaki D, et al. Gefitinib and luteolin cause growth arrest of human prostate cancer PC-3 cells via inhibition of cyclin G-associated kinase and induction of miR-630. PLoS One. 2014;9:e100124.
- He S, Zhang G, Dong H, et al. miR-203 facilitates tumor growth and metastasis by targeting fibroblast growth factor 2 in breast cancer. OncoTargets Ther. 2016;9:6203–6210.
- Lin J, Wang L, Gao J, et al. MiR-203 inhibits estrogen-induced viability, migration and invasion of estrogen receptor α-positive breast cancer cells. Exp Ther Med. 2017;14:2702–2708.
- Zhang Z, Zhang B, Li W, et al. Epigenetic silencing of miR-203 upregulates SNAI2 and contributes to the invasiveness of malignant breast cancer cells. Genes Cancer. 2011;2:782–791.
- McCubrey JA, Steelman LS, Chappell WH, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773:1263–1284.