Abstract
The long-term prognosis of patients with lung cancer remains poor and thus it is imminent to further elucidate the molecular mechanism for the oncogenesis of lung cancer. In this study, we observed that surfactant protein C (SFTPC) expression was downregulated in human lung adenocarcinoma tissues and cell lines, and low SFTPC expression correlated with poor overall survival of lung adenocarcinoma patients. Moreover, we found that overexpression of SFTPC could inhibit lung cancer cell proliferation in vitro and in vivo, but downregulation of SFTPC showed the opposite results. Besides, it was observed that miR-629-3p expression was upregulated in human lung adenocarcinoma tissues and cell lines. More importantly, we found that miR-629-3p could downregulate SFTPC expression by directly binding to the SFTPC 3'-UTR and inhibit the regulatory effect of SFTPC on lung adenocarcinoma cell proliferation. In conclusion, these data suggested that miR-629-3p-meditated downregulation of SFTPC may promote lung adenocarcinoma progression.
Introduction
Lung carcinoma is the major cause of cancer-associated deaths globally [Citation1–3]. In China, it is the most common cancer and the major cause of cancer-related deaths as well [Citation4]. Non-small-cell lung cancer (NSCLC) takes up more than 85% of lung cancer, of which 60% are lung adenocarcinoma (LUAD), being the most frequently diagnosed subtype of NSCLC [Citation5]. Of note, approximately 75% of lung cancer patients are diagnosed in the advanced stages of the disease [Citation3]. Up to date, several strategies are available for treating NSCLC patients, including surgical resection, chemotherapy, radiation therapy, targeted therapy, and the combination of these strategies [Citation5], but the long-term prognosis of NSCLC patients remains unsatisfying, with a 5-year overall survival rate less than 15% [Citation6]. Therefore, the further elucidation of the molecular mechanism for NSCLC is urgently needed, so as to identify novel therapeutic targets and then improve the long-term survival of patients.
Pulmonary surfactant is a protein–lipid complex covering alveolar surfaces, which helps to prevent the collapse of alveoli [Citation7]. Four key proteins participate in the formation of the protein–lipid complex, including surfactant protein A, B, C and D (SFTP-A, B, C, and D). These proteins are mainly yielded by alveolar epithelial type II cells, with abundance in pulmonary alveoli [Citation8]. SFTPA and SFTPD are hydrophilic proteins that play an essential role in the regulation of innate immune systems in the lung [Citation8,Citation9]. For instance, SFTPD can deter the dissemination of infectious microbes by suppressing the agglutination and growth inhibition of the microbes [Citation10], as well as through enhancing phagocytosis in macrophages [Citation11]. Inversely, SFTPB and SFTPC are hydrophobic proteins with the capacity of mitigating surface tension in the lung [Citation12]. Strikingly, it has been proposed that SFTPD expression was associated with tumour progression and survival of patients with lung cancer [Citation13–15]. Furthermore, recent evidence also showed that SFTPD could suppress the progression of pancreatic cancer by inducing epithelial-mesenchymal-transition (EMT) and apoptosis of pancreatic cancer cells [Citation16,Citation17]. In addition, it has also been suggested that SFTPA and SFTPB might function as tumour suppressor genes and their dysregulation were closely related to poor prognosis of lung cancer patients [Citation18–21]. As for SFTPC, one previous study reported that SFTPC deletion was observed in NSCLC tissues, implying that SFTPC downregulation might be involved in the progression of lung cancer [Citation22]. However, it remains unclear whether SFTPC also has an inhibitory effect on lung cancer progression as the other surfactant proteins do.
MicroRNAs (miRNAs) are a type of endogenous small molecule non-coding single-stranded RNAs composed of 21–24 nucleotides and display a repressive effect on target gene expression through post-transcriptional suppression and mRNA degradation [Citation23,Citation24]. A large number of studies have shown that miRNAs play a crucial part in regulating tumour progression, including malignant cell apoptosis, proliferation, invasion, growth, and metastasis [Citation23–27]. Therefore, by combining bioinformatics analysis and experimental validation of molecular biology we made a further exploration of whether SFTPC expression was regulated by microRNAs in lung cancer.
In this study, we observed that surfactant protein C (SFTPC) expression was downregulated in human lung adenocarcinoma tissues and cell lines, and low SFTPC expression correlated with poor overall survival of lung adenocarcinoma patients. Moreover, we found that overexpression of SFTPC could inhibit lung cancer cell proliferation in vitro and in vivo, but downregulation of SFTPC showed the opposite results. Besides, it was observed that miR-629-3p expression was upregulated in human lung adenocarcinoma tissues and cell lines. More importantly, we found that miR-629-3p could downregulate SFTPC expression by directly binding to the SFTPC 3'-UTR, and inhibit the regulatory effect of SFTPC on lung adenocarcinoma cell proliferation. Thus, it could be hypothesized that miR-629-3p-mediated downregulation of SFTPC could contribute to lung adenocarcinoma progression.
Materials and methods
Bioinformatics analysis
All available published SAGE data were applied to analyze SFTPC gene expression in human normal and malignant tissues. The analysis of Digital SFTPC gene expression profiles was analyzed and displayed with the SAGE Anatomic Viewer [Citation28]. The Oncomine database, a publicly accessible online cancer microarray database containing 715 datasets and 86,733 samples, was searched to investigate the transcription level of the SFTPC gene in lung cancer [Citation28]. The data on the SFTPC mRNA expression (log2-transformed) in lung carcinoma and normal tissues were obtained from the Oncomine database and then were subjected to statistical comparison. The TIMER database containing 10,897 samples across 32 types of cancer from The Cancer Genome Atlas (TCGA) was also used to estimate SFTPC mRNA expression levels in different types of cancer [Citation29]. Additionally, LUNG CANCER EXPLORER was employed to further identify the expression of SFTPC mRNA between in lung cancer tissues and their matched normal tissues and the prognostic significance of SFTPC expression in lung cancer [Citation30]. Furthermore, the HPA database was used to estimate SFTPC protein expression level in lung cancer tissues and normal tissues by analyzing immunohistochemistry (IHC) staining images [Citation31–33]. Two online tools, including Target scan [Citation34] and TACCO [Citation35], were used to screen for candidate microRNAs probably targeting SFTPC mRNA to suppress its protein expression. StarBase was utilized to predict the association between miR-629-3p expression and SFTPC expression in LUAD [Citation36].
Human tissue samples and ethics statement
Lung adenocarcinoma (n = 5) and adjacent normal tissues (n = 5) were obtained from the patients who underwent surgery in the Department of Thoracic surgery, Lanzhou University Second Hospital, Lanzhou, China. This study was approved by the Ethics Committee of Lanzhou University Second Hospital.
Cell culture and transfection
Lung adenocarcinoma cell lines (A549, H1650, and H441) and human bronchial epithelial cells (BEAS-2B) were obtained from the Chinese Academy of Sciences Cell Bank of Type Culture Collection (CBTCCCAS, Shanghai, China). Under the condition of a humidified atmosphere of 5% CO2 and a temperature of 37 °C, cells were cultured in Dulbecco’s modified Eagle’s medium or Roswell Park Memorial Institute (RPMI)-1640 medium supplemented with 10% fetal bovine serum (FBS) and 100 mg/ml streptomycin/penicillin.
MiR-629-3p mimics, miR-629-3p inhibitor, and human SFTPC/control small interfering RNA (siRNAs) (RiboBio, Guangzhou, China) were transfected in LUAD cells using Lipofectamine 2000 transfection reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol.
Cell proliferation assay
Cell proliferative capacity was assessed using the CCK-8 kit (Promega). After H1650 and A549 cells (3000 cells/well) were transfected with si-SFTPC and lenti-SFTPC for 24 h, respectively, these transfected cells were subsequently seeded into 96-well plates, where they were cultured at 37 °C with 5% CO2. The relative growth rate of cancer cells was detected using CCK-8 test every 24 h according to the manufacturer’s protocol.
Xenograft tumour model
All animal experimental procedures were approved by the Institutional Animal Care and Use Committee of Lanzhou University Second Hospital. The SFTPC expression constructing lenti-SFTPC and pCDH-CMV-MCS-EF1-copGFP control vector were purchased from System Biosciences (Mountain View, CA). The lentivirus was produced following the manufacturer’s instructions. A549 cells (5 × 106) infected with lenti-SFTPC or pCDH-CMV-MCS-EF1-copGFP control vector were injected subcutaneously into the left dorsal flank of BALB/c-nude mice (5–6 weeks, 18–20 g, n = 5). Every 3 days, measurements were performed to evaluate tumour volume and calculate tumour volume by the formula (length × width2)/2. On day 45, the mice were first euthanized and imaged, and then were sacrificed for the excision of the tumours.
RNA extraction and miRNA/mRNA detection
Total RNA was separated from tissue samples and cultured cells using TRIzol reagent (Invitrogen, Carlsbad, CA) in accordance with the manufacturer’s protocol provided by the manufacturer. To analyze gene expression, the real-time PCR system with a mixture of primers, cDNA templates and SYBR Green qPCR Master Mix was reacted using real-time PCR based on standard methods. The primers used in the current study included the following:
MiR-629-3p, Forward: GTTCTCCCAACGTAAGCCCAGC,
Reverse: CAGTGCGTGTCGTGGAGT;
U6, Forward: CGCTTCGGCAGCACATATAC,
Reverse: CAGGGGCCATGCTAATCTT;
SFTPC, forward: TTGGTCCTTCACCTCTGTCC,
Reverse: CTCCCACAATCACCACGAC;
GAPDH, forward: CTCACCGGATGCACCAATGTT,
Reverse: CGCGTTGCTCACAATGTTCAT;
GAPDH and U6 were regarded as internal controls for SFTPC and miR-629-3p respectively. Fold changes in gene expression were determined using 2−ΔΔCt method.
Luciferase assays
To determine whether miR-629-3p targeted SFTPC directly by interacting with its predicted 3'-untranslated region (UTR) binding sites, luciferase reporter assays were carried out. Mutant (MUT) and wild-type (WT) sequences of SFTPC 3'-UTR were amplified by PCR and cloned into control vector. 293T cells were co-transfected with SFTPC 3'-UTR wild-type plasmid or mutant plasmid and miR-629-3p mimics or miR-NC referring to the Lipofectamine 2000 transfection reagent instruction. The cells were maintained at 37 °C and 5% CO2 for 48 h. The luciferase activity was then detected by using a Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA).
Western blot analysis
Proteins were extracted from all cell-lysates, isolated with sodium dodecyl sulphate-polyacrylamide gel electrophoresis, and then transferred onto a polyvinylidene fluoride (PVDF) membranes. Subsequently, the membranes loaded with proteins were incubated with specific primary antibodies (SFTPC: 1:1500, Abcam; GAPDH: 1:8000, Abcam) overnight at 4 °C. After that, we further incubated the membranes using secondary anti-bodies (1:3000; Abcam, Cambridge, MA) for 1 h at room temperature. At last, the membranes was treated with chemiluminescence reagents (Santa Cruz, Dallas, TX) according to the manufacturer’s protocols and then visualized by the ChemiDoc XRS system (Bio-Rad, Hercules, CA, USA).
Statistical analysis
All statistical analysis was performed by utilizing the SPSS 18.0 software (SPSS Inc., Chicago, IL). All values were displayed as mean ± standard deviation (SD), which was calculated using data obtained from at least three repeated individual experiments for each group. The differences among groups were analyzed using by Student’s t-test or one-way analysis of variance (ANOVA). Differences were regarded as significant when p-value was less than < .05.
Results
SFTPC was downregulated and correlated with poor overall survival in lung adenocarcinoma (LUAD) patients
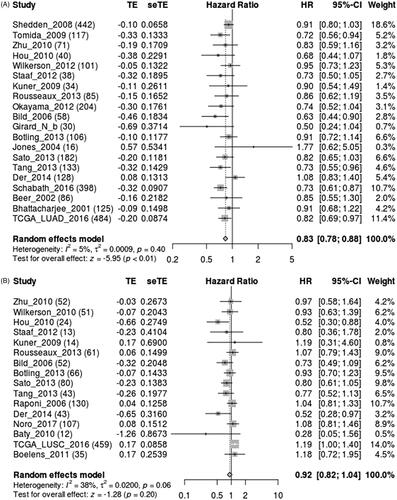
The expression of SFTPC in human lung cancer was first analyzed by using the SAGE Digital Gene Expression Display. SFTPC expression was downregulated specifically in lung cancer compared with that of the matched normal tissues (). Oncomine and TCGA data analysis also verified that SFTPC was obviously downregulated in lung cancer tissues (). Additionally, a meta-analysis based on lung cancer explorer further confirmed that SFTPC mRNA expression was significantly lower in lung cancer tissues than that of their matched normal tissues (). Consistently, the downregulation of SFTPC was confirmed in lung cancer tissues, including LUAD and LUSC (). Moreover, IHC staining images obtained from the HPA database observed that SFTPC protein had stronger staining in LUAD and lung squamous carcinoma (LUSC) than those in normal lung tissues (). A meta-analysis based on lung cancer explorer unveiled that lower SFTPC expression was closely associated with worse overall survival in LUAD patients, but not in LUSC (). Therefore, we further explored the potential roles of SFTPC in LUAD.
Figure 1. The expression of SFTPC in human cancers. (A) Expression profile for surfactant protein C (SFTPC) in human cancer using the SAGE digital gene expression displayer (https://cgap.nci.nih.gov/SAGE/Viewer). (B) Expression of SFTPC in different types of cancers (Oncomine: https://www.oncomine.org). (C) Expression of SFTPC across all TCGA tumours (TIMER: https://cistrome.shinyapps.io/timer/).
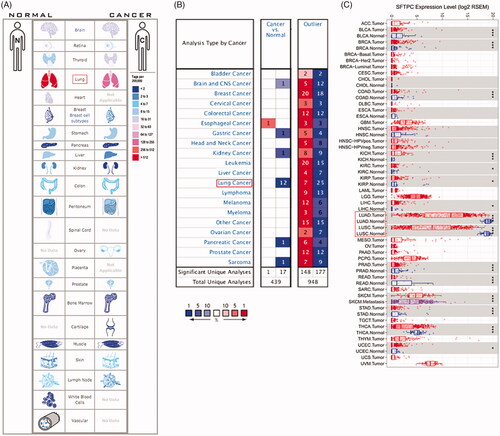
Figure 2. SFTPC expression is significantly downregulated in lung cancer. (A) A meta-analysis of SFTPC expression in lung cancers using Lung Cancer Explore (http://lce.biohpc.swmed.edu/lungcancer/index.php#about); (B) Expression of SFTPC in five pairs of LUSC tissues and the adjacent tissues. (C) Expression of SFTPC in five pairs of LUAD tissues and the adjacent tissues. **p < .01.
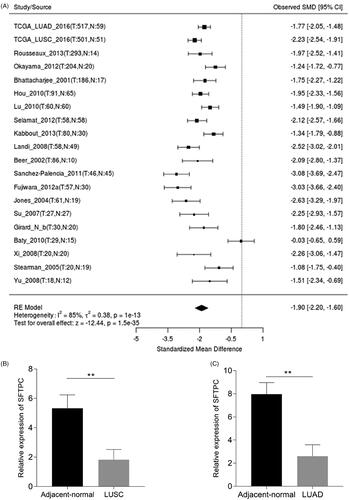
Figure 3. SFTPC protein expression was significantly lower in both LUAD and lung squamous carcinoma (LUSC) tissues in comparison with normal lung tissues. SFTPC immunohistochemistry (IHC) staining images: in normal lung tissues (left), in LUSC tissues (middle), and in LUAD tissues (right). Images were downloaded from the Human Protein Atlas (HPA) (http://www.proteinatlas.org/).
SFTPC regulated LUAD cell proliferation in vitro and tumour growth in vivo
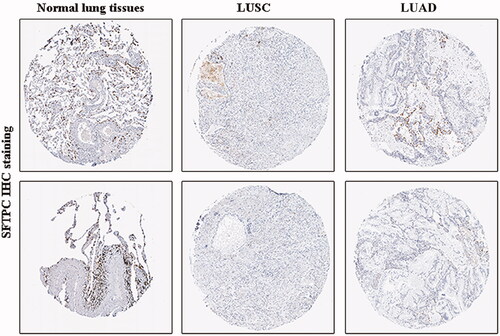
Both RT-qPCR and western blot showed that LUAD cell lines also had lower SFTPC expression than normal cell lines (). To gain insight into the biological effect of SFTPC on in vitro proliferation of LUAD cells, A549 cells were used to establish cell model stably overexpressing SFTPC using lentivirus infection, since A549 cells had a relatively low SFTPC expression among all the LUAD cell lines (). Additionally, we chose H1650 cells as the subject to establish cell model with SFTPC downregulation using SFTPC siRNA, considering that H1650 cells had a relatively high SFTPC expression among all the LUAD cell lines (). Western blot assay showed that SFTPC protein was significantly overexpressed in A549 cells after SFTPC transfection, but was downregulated in H1650 cells after SFTPC siRNA transfection (). CCK-8 assays showed that overexpression of SFTPC evidently inhibited the viability of A549 cells (), whereas downregulation of SFTPC enhances the viability of H1650 cells (). More importantly, In Xenograft model in vivo, the tumours formed by A549 cells with stable SFTPC overexpression were substantially smaller, in both size and weight compared with the control tumours (). Taken together, our results implied that SFTPC regulated LUAD cell proliferation in vitro and tumour growth in vivo.
Figure 5. SFTPC inhibits cell proliferation in LUAD cell lines. (A) Quantitative reverse-transcription polymerase chain reaction (qRT-PCR) analyzed SFTPC mRNA expression in LUAD cell lines. (B) Western blot analysis of SFTPC protein expression in LUAD cell lines. (C) Western blot analysis was used to detect the expression of SFTPC protein in A549 after lenti-APAF1 infection and in H1650 after si-SFTPC transfection. (D) Overexpression of SFTPC inhibited cell proliferation in A549 cell lines. (E) Inhibition of SFTPC promoted cell proliferation in H1650 cell lines. (F) SFTPC inhibited tumour growth in mice xenograft tumour model by subcutaneous inoculation with lenti-SFTPC or control vector A549 cells (five mice per group); (G) The weights of xenograft tumours are detected at the day 45. *p < .05; **p < .01; ***p < .001; ****p < .0001.
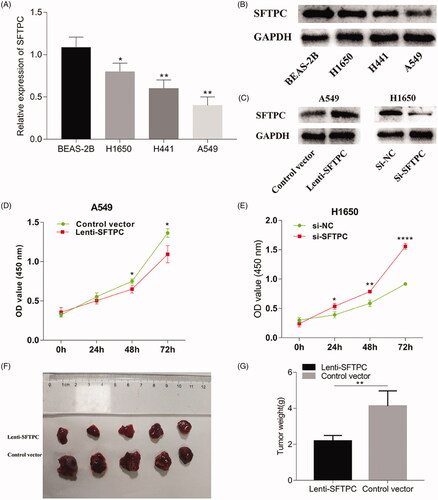
MiR-629-3p was upregulated in LUAD and a potential negative regulator to SFTPC
MicroRNAs, a type of endogenous small molecule non-coding single-stranded RNAs, display a repressive effect on target gene expression or mRNA degradation [Citation23,Citation24]. Mountains of evidence show that microRNAs play crucial parts in regulating malignant cell apoptosis, proliferation, invasion, growth and metastasis [Citation23–27]. Herein, we explored whether SFTPC expression was modulated by microRNAs in lung cancer. Two online tools, including Target scan and TACCO, were used to screen for candidate microRNAs probably targeting SFTPC mRNA. As shown in , two candidate microRNAs (miR-629-3p and miR-409-3p) were identified. RT-qPCR showed that miR-409-3p expression in lung cancer tissues was not significantly dysregulated (), but miR-629-3p was obviously upregulated in lung cancer tissues (). Consistently, we also found that miR-629-3p expression was upregulated in LUAD cell lines (). More importantly, data analysis by using Starbase 3.0 suggested that there was an inverse association between miR-629-3p expression and SFTPC expression in LUA (). Therefore, we selected miR-629-3p as the research subject in our next experiments.
Figure 6. miR-629-3p was upregulated in LUAD and a potential negative regulator to SFTPC. (A) miR-629-3p and miR-409-3p were potential regulatory factors to SFTPC mRNA. (B) RT-qPCR analyzed miR-409-3p expression in five pairs of LUAD tissues and the adjacent tissues. (C) qRT-PCR analyzed miR-629-3p expression in five pairs of LUAD tissues and the adjacent tissues. (D) qRT-PCR analyzed miR-629-3p expression in LUAD cell lines. (E) The level of miR-629-3p was negatively correlated with SFTPC in LUAD (Starbase 3.0). *p < .05; **p < .01; ***p < .001.
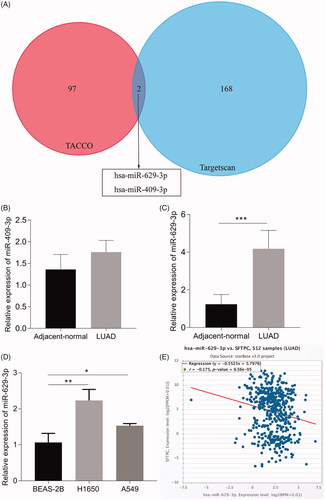
MiR-629-3p inhibited SFTPC expression by directly binding to the SFTPC 3'-UTR
Target scan also indicated that there were potential binding sites between miR-629-3p and 3'-UTR of SFTPC mRNA (). Furthermore, we utilized the luciferase reporter gene vector assays to explore whether miR-629-3p could bind to the 3'-UTR of SFTPC to suppress the SFTPC protein expression. A wild-type SFTPC 3'-UTR (named WT-SFTPC 3'-UTR) and a mutant-type SFTPC 3'-UTR (named MUT-SFTPC 3'-UTR) luciferase reporter gene vector in the predicted miR-629-3p binding site were constructed. The indicated vectors were co-transfected with miR-629-3p mimics into 293T cells, and then the luciferase activity was determined. Results showed that the luciferase activity of WT-SFTPC 3'-UTR vector was substantially mitigated by miR-629-3p mimics, but that of MUT-SFTPC 3'-UTR vector was not affected (). The results of luciferase reporter gene vector assays suggested that SFTPC may be a direct target of miR-629-3p. Thus, we made a further investigation on the effect of miR-629-3p on SFTPC expression and SFTPC-dependent regulation of LUAD cell proliferation. Results showed that miR-629-3p inhibitor substantially increased SFTPC expression in H1650 cells (), while miR-629-3p mimics significantly decreased SFTPC expression in A549 cells (). Additionally, we also observed that miR-629-3p inhibitor could suppress the proliferation of H1650 cells (), whereas miR-629-3p mimics could promote the proliferation of A549 cells (). These data indicated that miR-629-3p could inhibit SFTPC expression by directly binding to the SFTPC 3'-UTR.
Figure 7. MiR-629-3p inhibited SFTPC expression by directly binding to the SFTPC 3'-UTR. (A) There existed a putative miR-629-3p binding site in the 3'-UTR of SFTPC mRNA(Tagetscan). (B) Wild-type (WT) and mutant (MUT) sequences of SFTPC 3'-UTR, miR-629-3p mimics, and mimics’ negative control (NC) were cotransfected into 293T cells. (C) A549 or H1650 cells were transfected with miR-629-3p mimics or inhibitor and its corresponding control and then the expression of SFTPC was detected by Western blot assays (C) and qRT-PCR (D, E). Cell proliferation of lung cancer cell lines was detected after transfected with miR-629-3p inhibitor and SFTPC siRNA (H1650) or transfected with miR-629-3p mimics and infected lenti-SFTPC (A549) (F, G).
Discussion
Surfactant protein A, B, C and D (SFTP-A, B, C, and D) are the key elements of pulmonary surfactant that helps to prevent the collapse of alveoli [Citation7]. In addition to the essential physiological functions, these surfactant proteins were considered to play crucial roles in tumour progression and correlate with the clinical outcome of cancer patients. For instance, Hasegawa et al. [Citation13] demonstrated that SFTPD suppressed the proliferation, migration and invasion of A549 cells by inhibiting the epidermal growth factor signaling. Umeda et al. [Citation15] reported that high serum SFTPD levels were associated with a lower number of distant metastases and better prognosis. Besides, recent studies also suggested that SFTPD could suppress the progression of pancreatic cancer by inducing epithelial-mesenchymal-transition (EMT) and apoptosis of pancreatic cancer cells [Citation16,Citation17]. Furthermore, SFTPA and SFTPB have been proposed to function as tumour suppressor genes as well, and their downregulation was closely related to poor prognosis of patients with lung cancer [Citation18–21]. With respect to SFTPC, a previous study reported that SFTPC deletion was observed in NSCLC tissues, implying that SFTPC downregulation might be involved in NSCLC progression [Citation22]. Consistently, in the current study, we found that SFTPC was downregulated in lung adenocarcinoma tissues and cell lines (H1650, H441 and A549) and low SFTPC expression correlated with poor prognosis. Of note, our data showed that the expression level of SFTPC in H441 cells was lower than that of H1650 cells but higher than that of A549 cells. As far as we know, if a kind of functional protein has a low level in some cells, overexpression of the protein may exert more substantial effects on these cells than the downregulation of the protein. Inversely, when functional proteins are highly expressed in some cells, the downregulation of these proteins has a larger effect on these cells compared with overexpression of these proteins. Therefore, to sensitively determine the function of SFTPC and the molecular mechanism underlying the dysregulation of SFTPC expression, we chose A549 cells for subsequent SFTPC-overexpression experiments and utilized H1650 cells to conduct SFTPC-silencing experiments. Our function experiments showed that overexpression of SFTPC suppressed the A549 cell proliferation in vitro and tumour growth in vivo. Conversely, we observed that silence of SFTPC significantly promoted the H1650 cell proliferation in vitro. Thus, our study unveiled that SFTPC may act as a tumour suppressor gene and could be exploited as a prognostic biomarker and therapeutic target.
MicroRNAs, a type of endogenous small molecule non-coding single-stranded RNAs composed of 21–24 nucleotides, have a repressive effect on target gene expression through post-transcriptional inhibition or mRNA degradation [Citation23,Citation24]. Numerous studies have shown that microRNAs play a crucial part in regulating cancer cell apoptosis, proliferation, invasion, growth, and metastasis [Citation23–27]. Therefore, we made a further exploration of whether SFTPC downregulation in lung cancer was mediated by microRNAs. In this study, by bioinformatics analysis, we first found that miR-629-3p was upregulated in lung cancer and identified it as a microRNA candidate that may target SFTPC. Subsequently, we further tried to explore whether miR-629-3p directly targeted SFTPC to suppress its expression by experimental validation of molecular biology. Our results showed that miR-629-3p was upregulated in lung cancer tissues and cell lines, and miR-629-3p drastically inhibited SFTPC expression by directly binding to the SFTPC 3'-UTR. Of note, a study by Wang et al. [Citation37] showed that miR-629-3p was significantly upregulated in human breast cancer and inhibition of miR-629-3p drastically suppressed the viability and migration of breast cancer cells, and as well as the lung metastasis in vivo. Combing the findings of the previous study and the results of our study, we may speculate that miR-629-3p-mediated SFTPC downregulation promoted tumour proliferation and invasion of lung adenocarcinoma cells.
In conclusion, our results demonstrated that SFTPC was downregulated in lung adenocarcinoma clinical samples and cell lines, and overexpression of SFTPC could inhibit lung adenocarcinoma cell proliferation and invasion. In addition, our study also unveiled that miR-629-3p could inhibit SFTPC expression by directly binding to the SFTPC 3'-UTR. These findings suggested that miR-629-3p/SFTPC pathway may be a new target for the treatment of lung adenocarcinoma.
Ethical approval and consent to participate
This study was approved by the Ethics Committee of Lanzhou University Second Hospital. Informed consents were obtained from patients.
Disclosure statement
The authors declare that they have no competing interests.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108.
- Bray F, Ferlay J, Soerjomataram I, et al. Global Cancer Statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34.
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132.
- Wang J, Zou K, Feng X, et al. Downregulation of NMI promotes tumour growth and predicts poor prognosis in human lung adenocarcinomas. Mol Cancer. 2017;16:158.
- Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553:446–454.
- Whitsett JA, Weaver TE. Hydrophobic surfactant proteins in lung function and disease. N Engl J Med. 2002;347:2141–2148.
- Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol. 2005;5:58–68.
- Sano H, Kuroki Y. The lung collectins, SP-A and SP-D, modulate pulmonary innate immunity. Mol Immunol. 2005;42:279–287.
- Wu H, Kuzmenko A, Wan S, et al. Surfactant proteins A and D inhibit the growth of Gram-negative bacteria by increasing membrane permeability. J Clin Invest. 2003;111:1589–1602.
- Kudo K, Sano H, Takahashi H, et al. Pulmonary collectins enhance phagocytosis of Mycobacterium avium through increased activity of mannose receptor. J Immunol. 2004;172:7592–7602.
- Schurch D, Ospina OL, Cruz A, et al. Combined and independent action of proteins SP-B and SP-C in the surface behavior and mechanical stability of pulmonary surfactant films. Biophys J. 2010;99:3290–3299.
- Hasegawa Y, Takahashi M, Ariki S, et al. Surfactant protein D suppresses lung cancer progression by downregulation of epidermal growth factor signaling. Oncogene. 2015;34:4285–4286.
- Nakamura K, Kato M, Shukuya T, et al. Surfactant protein-D predicts prognosis of interstitial lung disease induced by anticancer agents in advanced lung cancer: a case control study. BMC Cancer. 2017;17:302.
- Umeda Y, Hasegawa Y, Otsuka M, et al. Surfactant protein D inhibits activation of non-small cell lung cancer-associated mutant EGFR and affects clinical outcomes of patients. Oncogene. 2017;36:6432–6445.
- Kaur A, Riaz MS, Murugaiah V, et al. A recombinant fragment of human surfactant protein D induces apoptosis in pancreatic cancer cell lines via Fas-mediated pathway. Front Immunol. 2018;9:1126.
- Kaur A, Riaz MS, Singh SK, et al. Human surfactant protein D suppresses epithelial-to-mesenchymal transition in pancreatic cancer cells by downregulating TGF-beta. Front Immunol. 2018;9:1844.
- Jiang F, Caraway NP, Nebiyou Bekele B, et al. Surfactant protein A gene deletion and prognostics for patients with stage I non-small cell lung cancer. Clin Cancer Res. 2005;11:5417–5424.
- Kim JG, Kim MJ, Choi WJ, et al. Wnt3A induces GSK-3β phosphorylation and β-Catenin accumulation through RhoA/ROCK. J Cell Physiol. 2017;232:1104–1113.
- Mitsuhashi A, Goto H, Kuramoto T, et al. Surfactant protein A suppresses lung cancer progression by regulating the polarization of tumour-associated macrophages. Am J Pathol. 2013;182:1843–1853.
- Sin DD, Tammemagi CM, Lam S, et al. Pro-surfactant protein B as a biomarker for lung cancer prediction. J Clin Oncol. 2013;31:4536–4543.
- Li R, Todd NW, Qiu Q, et al. Genetic deletions in sputum as diagnostic markers for early detection of stage I non-small cell lung cancer. Clin Cancer Res. 2007;13:482–487.
- Shirjang S, Mansoori B, Asghari S, et al. MicroRNAs in cancer cell death pathways: apoptosis and necroptosis. Free Radic Biol Med. 2019;139:1–15.
- Pinweha P, Rattanapornsompong K, Charoensawan V, et al. MicroRNAs and oncogenic transcriptional regulatory networks controlling metabolic reprogramming in cancers. Comput Struct Biotechnol J. 2016;14:223–233.
- Lv Y, Chen S, Wu J, et al. Upregulation of long non-coding RNA OGFRP1 facilitates endometrial cancer by regulating miR-124-3p/SIRT1 axis and by activating PI3K/AKT/GSK-3beta pathway. Artif Cells Nanomed Biotechnol. 2019;47:2083–2090.
- Sheedy P, Medarova Z. The fundamental role of miR-10b in metastatic cancer. Am J Cancer Res. 2018;8:1674–1688.
- Xue M, Shi D, Xu G, et al. The long noncoding RNA linc00858 promotes progress of lung cancer through miR-3182/MMP2 axis. Artif Cells Nanomed Biotechnol. 2019;47:2091–2097.
- Cui X, Yi Q, Jing X, et al. Mining prognostic significance of MEG3 in human breast cancer using bioinformatics analysis. Cell Physiol Biochem. 2018;50:41–51.
- Pan JH, Zhou H, Cooper L, et al. LAYN is a prognostic biomarker and correlated with immune infiltrates in gastric and colon cancers. Front Immunol. 2019;10:6.
- Cai L, Lin S, Girard L, et al. LCE: an open web portal to explore gene expression and clinical associations in lung cancer. Oncogene. 2019;38:2551–2564.
- Sun H, Ni SJ, Ye M, et al. Hedgehog interacting protein 1 is a prognostic marker and suppresses cell metastasis in gastric cancer. J Cancer. 2018;9:4642–4649.
- Thapa S, Chetry M, Huang K, et al. Significance of aquaporins’ expression in the prognosis of gastric cancer. Biosci Rep. 2018;38:BSR20171687
- Yan Y, Xu Z, Hu X, et al. SNCA is a functionally low-expressed gene in lung adenocarcinoma. Genes (Basel). 2018;9:16.
- Wu G, Yu W, Zhang M, et al. MicroRNA-145-3p suppresses proliferation and promotes apotosis and autophagy of osteosarcoma cell by targeting HDAC4. Artif Cells Nanomed Biotechnol. 2018;46:579–586.
- Chou PH, Liao WC, Tsai KW, et al. TACCO, a database connecting transcriptome alterations, pathway alterations and clinical outcomes in cancers. Sci Rep. 2019;9:3877.
- Zhang J, Zhang H, Qin Y, et al. MicroRNA-200c-3p/ZEB2 loop plays a crucial role in the tumour progression of prostate carcinoma. Ann Transl Med. 2019;7:141.
- Wang J, Song C, Tang H, et al. miR-629-3p may serve as a novel biomarker and potential therapeutic target for lung metastases of triple-negative breast cancer. Breast Cancer Res. 2017;19:72.