Abstract
This study aimed to investigate the effect of Junduqing extractive on proliferation, apoptosis, migration and invasion of nasopharyngeal carcinoma (NPC) cells and the involved mechanism. Junduqing extractive was prepared. CCK-8 assay found that IC50 of Junduqing extractive in HNE-1 cells was 2.99 mg/ml, so its concentration of 1.0, 2.0 and 3.0 mg/ml was selected to perform the following experiments. HNE-1, HNE-2 and HONE1 cells were then divided into four groups: (1) Control (no treatment); (2) 1.0 mg/ml (1.0 mg/ml Junduqing); (3) 2.0 mg/ml (2.0 mg/ml Junduqing) and (4) 3.0 mg/ml (3.0 mg/ml Junduqing). Cell viability, apoptosis, migration and invasion were examined by CCK-8 assay, annexin V-FITC/PI staining, scratch wound assay and transwell assay, respectively. Compared with the control group, the viability, migration rates and invasive capacity of HNE-1, HNE-2 and HONE1 cells with Junduqing treatments decreased significantly. Higher concentration of Junduqing extractive caused lower viability, smaller migration rates and weaker invasive capacity. Compared with the control group, the apoptosis of HNE-1, HNE-2 and HONE1 cells after treatment with 2.0 and 3.0 mg/ml of Junduqing extractive increased remarkably. Levels of Bcl-xL, Mcl-1, Caspase-3, Caspase-8 and Caspase-9 were examined by western blotting. Compared with the control group, the expression of Bcl-xL and Mcl-1 and the expression of Caspase-3, Caspase-8 and Caspase-9 in HNE-1, HNE-2 and HONE1 cells were significantly down-regulated and up-regulated, respectively, after treatment with Junduqing extractive. In conclusion, Junduqing extractive could inhibit the proliferation, migration and invasion, and promote the apoptosis of human NPC cells through down-regulating Mcl-1 and Bcl-xL and up-regulating Caspase-3, Caspase-8 and Caspase-9.
Introduction
Nasopharyngeal carcinoma (NPC) is one of the most common malignant tumours in head and neck in southern China [Citation1]. Radiotherapy is often effective in early-stage patients while chemotherapy is the main treatment for middle and late-stage patients. However, the toxicity and side effects of antineoplastic chemotherapeutic drugs currently used in the clinic are great, so their clinical application is limited. How to reduce the toxicity and side effects of anti-cancer drugs has become a major problem that we need to solve urgently. In recent years, the research on the prevention and treatment of NPC by traditional Chinese medicine is popular year by year, and these traditional Chinese medicines have achieved satisfactory results [Citation2–4].
Junduqing granule is a traditional Chinese medicine of clearing away heat and detoxification, whose formulation is mainly composed of many Chinese medicines such as Rhizoma et Radix B. cusiae, Flos Lonicerae, Andrographis paniculata and Salvia miltiorrhiza [Citation5]. It has been used in our hospital for a long time [Citation5]. It is effective in clinical treatment of multiple diseases such as influenza, pharyngitis and upper respiratory tract infection [Citation5]. Our previous studies have found that Junduqing has obvious analgesic, anti-inflammatory and antipyretic effects, and meanwhile, it has extensive pharmacological effects such as anti-respiratory syncytial virus (RSV), anti-adenovirus (ADV) and anti-Coxsackievirus B3 (CoxB3) in vitro [Citation6–9]. During the clinical application of Junduqing, some clinicians report that the symptoms of early NPC patients and Epstein–Barr (EB) virus-positive patients improved obviously after long-term medication of Junduqing granules. Considering that most of the traditional Chinese medicine prescriptions have extensive antiviral effects, we speculate that Junduqing granules may also have some inhibitory effects on EB virus and even NPC. In vitro study reveals that Junduqing inhibits EB virus by suppressing the expression of BcLF and BARF1 genes [Citation10]. Interestingly, BcLF and BARF1 genes play an important role in the carcinogenesis of NPC cells [Citation11].
Therefore, we speculated that Junduqing may have potential anti-NPC effect. The purpose of this study was to investigate the effect of Junduqing extractive on proliferation, apoptosis, migration and invasion of NPC cells and the involved mechanism, so as to offer theoretical basis for the clinical treatment of NPC by Junduqing.
Materials and methods
Materials and cells
RPMI-1640 medium was purchased from KeyGen Biotech (Jiangsu, China). Foetal bovine serum (FBS) was bought from Biological Industries (Kibbutz Beit Haemek, Israel). Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis kit was obtained from Multi Sciences (Zhejiang, China). Polyvinylidene fluoride (PVDF) membrane was gotten from Millipore (Billerica, MA). SuperSignal® west pico chemiluminescent substrate was purchased from Thermo Fisher (Waltham, MA). Rabbit anti-Bcl-xL polyclonal antibody (ab32370), rabbit anti-Mcl-1 polyclonal antibody (ab32087), rabbit anti-Caspase-3 polyclonal antibody (ab184787) and rabbit anti-Caspase-9 polyclonal antibody (ab32539) were bought from Abcam (Cambridge, MA). Rabbit anti-Caspase-8 polyclonal antibody (bsm-33190M) was obtained from Bioss Antibodies (Beijing, China). Peroxidase-conjugated goat anti-rabbit IgG (H + L) (ZB-2301) was bought from ZSGB-Bio (Beijing, China).
Human NPC cell lines (HNE-1, HNE-2 and HONE1) were purchased from Stem Cell Bank of Chinese Academy of Sciences. Cells were cultured in RPMI-1640 medium containing FBS at 37 °C and 5% CO2.
Junduqing extractive preparation
A. paniculata was immersed in 85% ethanol and then percolated. The ethanol was recovered from the leachate, which was concentrated into a thick paste. After removing impurities, Rhizoma et Radix B. Cusiae, Flos Lonicerae, Radix Rubiae, S. ningpoensis and S. miltiorrhiza were washed, immersed in water, decocted twice for each 1.5 h. The decoction mixture was filtrated, centrifuged and concentrated into a thick paste which was mixed with the A. Paniculata thick paste. Cryodrying of the mixture was conducted to obtain the Junduqing extractive.
Exploration of Junduqing extractive IC50
HNE-1 cells were seeded in 96-well plate at 100 μl/well and cultured at 37 °C and 5% CO2 overnight. Cells were then cultured in the medium containing different concentrations of Junduqing extractive for 24 h. The CCK8 reagent was added at 10 μl/well and cells were cultured at 37 °C and 5% CO2 for 2 h. Absorbance (OD value) was measured at 450 nm using a microplate reader (RT-6100; Rayto, Houston, TX). IC50 was calculated accordingly.
Experimental grouping
Cells were divided into four groups: (1) Control (no treatment); (2) 1.0 mg/ml (cells were cultured in the medium containing 1.0 mg/ml Junduqing extractive for 24 h); (3) 2.0 mg/ml (cells were cultured in the medium containing 2.0 mg/ml Junduqing extractive for 24 h) and (4) 3.0 mg/ml (cells were cultured in the medium containing 3.0 mg/ml Junduqing extractive for 24 h). After treatment, cells were examined as follow.
Cell viability
Cells were cultured in the medium containing Junduqing extractive for 0, 12, 24, 48 and 72 h, and the cell viability was examined similarly as demonstrated in the above CCK-8 assay.
Cell apoptosis
Cells were cultured in the medium containing Junduqing extractive for 24 h and treated with Annexin V-FITC/PI apoptosis kit according to the manufacturer’s manual. Briefly, 1 × 106 – 3 × 106 cells were collected and resuspended in 300 μl precooled 1 × binding buffer. Cell suspension was then incubated with 3 μl Annexin V-FITC and 5 μl PI at room temperature for 10 min in the dark. Precooled 1 × binding buffer (200 μl) was added into every cell suspension. Eventually, cells were examined using a flow cytometer (NovoCyte 2060R; Acea Biosciences, Hangzhou, China).
Cell migration
A horizontal line across the well centre was drawn using a marker pen behind the 24-well plate. After cells were cultured in the medium containing Junduqing extractive for 24 h, they were seeded in the 24-well plate at about 5 × 105/well and cultured overnight. A scratch was made using a pipette tip along the back horizontal line. Cells were washed with phosphate buffer solution (PBS) three timed to remove the scratched cells. Cells were then cultured in serum-free medium at 37 °C and 5% CO2 for 48 h and examined microscopically (CKX41; Olympus, Japan).
Cell invasion
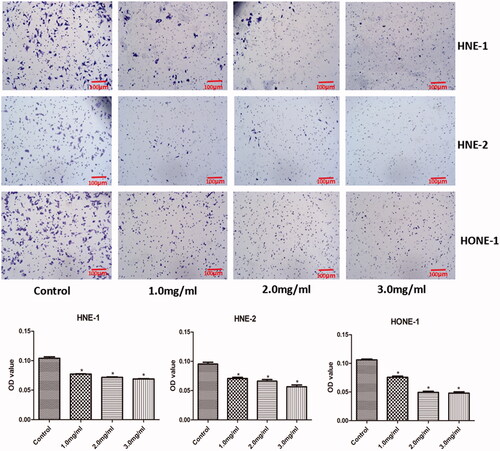
Cells were cultured in the medium containing Junduqing extractive for 24 h, collected, and resuspended in the medium containing 0.2% FBS at 5 × 105/ml. Matrigel was distributed uniformly onto the membranelle of transwell insert in advance and then the transwell insert containing Matrigel was placed in the well of 24-well plates. About 100 μl of cell suspension containing 5 × 104 cells were transferred into the upper chamber of transwell insert. About 500 μl of the medium containing 10% FBS was added into its lower chamber. Cells were cultured at 37 °C and 5% CO2 for 24 h and washed with PBS twice. Cells in the upper chamber were erased carefully using cotton swabs. Cells were fixed in 4% paraformaldehyde for 20–30 min, washed with PBS twice, stained in 0.1% crystal violet for 30 min and washed with PBS twice. Cells in the lower chamber were examined microscopically (CKX41; Olympus, Japan). The staining solution in the well was removed and 2 ml acetic acid (33%) was added into each well. After full mixing, the absorbance (OD value) was measured at 570 nm.
Western blotting
Cells were cultured in the medium containing Junduqing extractive for 24 h and incubated in radioimmunoprecipitation assay (RIPA) buffer for 30 min. The mixture was centrifuged at 4 °C and 10000 rpm for 10 min and the supernatant was collected carefully to obtain total protein. Protein concentration was measured using BCA kit. Subsequently, the protein was denatured, loaded to carry out sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) for 1–2 h and transferred for 30–50 min to PVDF membranes through a wet method. It was then incubated with primary antibodies at 4 °C overnight, washed and incubated with secondary antibody at room temperature for 1–2 h. After adding chemiluminescent substrate, the membrane was evaluated using a gel imaging system (ChemiDoc™ XRS+; Bio-Rad, Irvine, CA). A “Quantity one” software version 4.62 (Bio-Rad, Irvine, CA) was employed to analyse gray values of the blots. GAPDH served as internal control.
Statistical analysis
Data were statistically analysed with one-way analysis of variance (ANOVA) followed by Tukey post-hoc test using SPSS software version 19.0 (SPSS Inc., Chicago, IL). The significant difference was defined at p < .05.
Results
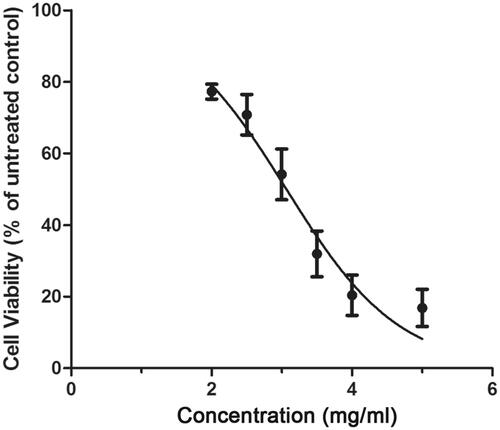
Junduqing extractive IC50
The survival curve of HNE-1 cells after treatment with different concentrations of Junduqing extractive was determined by CCK-8 assay and the results were shown in . Cell viability decreased sharply with increasing concentration of Junduqing extractive. The IC50 of Junduqing extractive in HNE-1 cells was 2.99 mg/ml, so its concentration of 1.0, 2.0 and 3.0 mg/ml was selected to perform the following experiments.
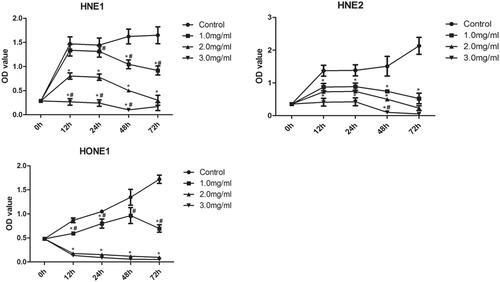
Cell viability
The viability of HNE-1, HNE-2 and HONE1 cells in various groups was also examined by CCK-8 assay and the results were shown in . Compared with the control group, the viability of all HNE-1, HNE-2 and HONE1 cells decreased significantly with the increase of the concentration of Junduqing extractive (p < .05). Higher concentration of Junduqing extractive caused lower viability of all HNE-1, HNE-2 and HONE1 cells (p < .05). Moreover, cell viability in the control group increased with time, while cell viability in the groups containing Junduqing extractive generally decreased with time.
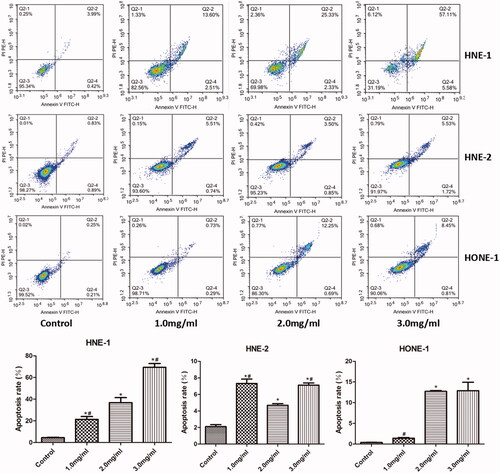
Cell apoptosis
The apoptosis of HNE-1, HNE-2 and HONE1 cells in various groups was assessed by Annexin V-FITC/PI staining and the results were shown in . Compared with the control group, the apoptosis of all HNE-1, HNE-2 and HONE1 cells after treatment with 2.0 and 3.0 mg/ml of Junduqing extractive increased remarkably (p < .05). Moreover, 3.0 mg/ml of Junduqing extractive induced most apoptosis of these three cells. As to HNE-2 cells, the apoptotic rates at various concentrations of Junduqing extractive were less than 10% and the apoptotic rate of 2.0 mg/ml group was not greater than that of 1.0 mg/ml group, which was different from other two cells. This might be due to the fact that HNE-2 cells might not be dose-dependent sensitive to Junduqing extractive. Moreover, these results suggested that the effects of Junduqing extractive dose on the apoptotic rate of NPC cells might be cell line-dependent.
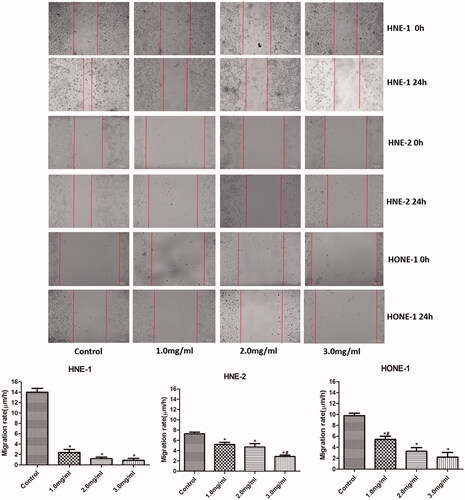
Cell migration
The migration of HNE-1, HNE-2 and HONE1 cells in various groups was evaluated by scratch wound assay and the results were shown in . Compared with the control group, the migration rates of all HNE-1, HNE-2 and HONE1 cells were reduced notably with the elevation of the concentration of Junduqing extractive (p < .05). Higher concentration of Junduqing extractive induced smaller migration rates of all HNE-1, HNE-2 and HONE1 cells.
Cell invasion
The invasion of HNE-1, HNE-2 and HONE1 cells in various groups was evaluated by transwell assay and the results were shown in . Compared with the control group, the density of all HNE-1, HNE-2 and HONE1 cells in the lower chamber were lowered significantly with the increasing concentration of Junduqing extractive (p < .05). Higher concentration of Junduqing extractive led to less HNE-1, HNE-2 and HONE1 cells in the lower chamber.
Levels of Bcl-xL, Mcl-1, Caspase-3, Caspase-8 and Caspase-9
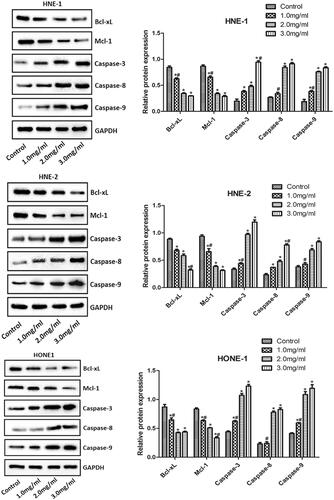
The levels of Bcl-xL, Mcl-1, Caspase-3, Caspase-8 and Caspase-9 in HNE-1, HNE-2 and HONE1 cells of various groups were examined by western blotting and the results were shown in . Compared with the control group, the expression of Bcl-xL and Mcl-1 and the expression of Caspase-3 in HNE-1, HNE-2 and HONE1 cells were significantly down-regulated and up-regulated, respectively, with the increasing concentration of Junduqing extractive (p < .05). Compared with the control group, the expression of Caspase-8 and Caspase-9 in HNE-1, HNE-2 and HONE1 cells after treatment with 2.0 and 3.0 mg/ml of Junduqing extractive was up-regulated remarkably (p < .05). Higher concentration of Junduqing extractive resulted in lower expression of Bcl-xL and Mcl-1 and higher expression of Caspase-3, Caspase-8 and Caspase-9 (p < .05).
Figure 6. The levels of Bcl-xL, Mcl-1, Caspase-3, Caspase-8 and Caspase-9 in HNE-1, HNE-2 and HONE1 cells of various groups which were examined by western blotting. Cells were cultured in the medium containing 1.0, 2.0 and 3.0 mg/ml of Junduqing extractive for 24 h. *p < .05 vs. Control; #p < .05 vs. 2.0 mg/ml.
Discussion
NPC is characterized by concealed development, hard early discovery, strong malignancy, easy metastasis and easy recurrence. Nowadays, high-dose radiotherapy combined with chemotherapy is its main treatment in clinic. This treatment strategy kills both cancer cells and normal cells without selectivity, leading to great toxicity and side effects. Therefore, it is urgent to research and develop new drugs with high efficiency and low toxicity.
In this study, we revealed that Junduqing extractive at all low, moderate and high doses was able to inhibit the proliferation, migration and invasion, and promote the apoptosis of all HNE-1, HNE-2 and HONE1 cells. These results suggested that Junduqing extractive could suppress the development and progression of NPC at cell level in vitro. Active ingredients in Rhizoma et Radix B. Cusiae such as indirubin, indigo, tryptanthrin and steroids have been proved to have extensive anti-tumour effects [Citation12]. Honeysuckle polysaccharides in Flos Lonicerae can inhibit S180 sarcoma in mice, which may be related to the regulation of Bcl-2/Bax apoptotic pathway and the promotion of TNF-α secretion [Citation13]. Andrographolide, as the main active ingredient of A. paniculata, has shown satisfactory anti-tumour activity in many in vivo and in vitro models, and its derivatives also have certain anti-tumour activity [Citation14]. Andrographolide exerts anti-cancer effect by induction of cell cycle arrest and apoptosis, immune regulation and anti-angiogenesis of tumour tissues [Citation15–19]. Tanshinone and salvianolic acid have been proved to be the main characteristic and active ingredients of S. miltiorrhiza and they have significant cardiovascular and cerebrovascular protective activities [Citation20–23]. Previous reports reveal that tanshinones and salvianolic acids are effective in the inhibition of NPC, gastric cancer, breast cancer and liver cancer in vitro and in vivo [Citation20–23]. These might be one of the reasons why Junduqing extractive could inhibit the proliferation, migration and invasion, and promote the apoptosis of human NPC cells.
Mcl-1 is a member of the anti-apoptotic Bcl-2 family. It can act as an apical regulator to inhibit cell apoptosis by blocking the release of cytochrome C [Citation24,Citation25]. Rapid degradation of Mcl-1 may directly lead to cell apoptosis [Citation25]. Down-regulation of Mcl-1 expression induced by exogenous small-molecular inhibitor can trigger the apoptosis of human renal carcinoma (Caki) cells [Citation26]. Our results also showed that the low, moderate and high doses of Junduqing extractive could reduce the expression of Mcl-1 and enhance the apoptosis of human NPC cells. This was consistent with the above reports [Citation24–26]. These results suggested that the decrease of Mcl-1 expression might be responsible for the increase of human NPC cell apoptosis.
Bcl-xL is also a member of the anti-apoptotic Bcl-2 family. In the endogenous apoptotic pathway, Bcl-xL plays an anti-apoptotic role by blocking the destruction of outer mitochondrial membrane by Bax [Citation27]. In the exogenous apoptotic pathway, Bcl-xL interferes with the assembly of death-inducing signal complex, thus inhibiting the activity of Caspase [Citation28]. Our results demonstrated that Junduqing extractive could suppress the expression of Bcl-xL and thus promote the apoptosis of human NPC cells.
Caspase is an aspartic acid-specific cysteine protease, which plays a crucial role in apoptotic process. Inhibition of Caspase-3 expression can effectively prevent cell apoptosis [Citation29]. Activated Caspase-9 can effectively incise and activate downstream Caspase-3 to induce cell apoptosis [Citation30]. Apoptotic signals stimulated by death receptor and its ligand system are transduced to regulatory Caspase-8 which then activates the effector Caspases to induce cell apoptosis [Citation31]. Our results revealed that the expression of Caspase-3, Caspase-8 and Caspase-9 in human NPC cells were significantly up-regulated by Junduqing extractive, which enhanced the apoptosis of human NPC cells. These results suggested that the apoptosis of human NPC cells induced by Junduqing extractive was directly associated with the activation of Caspases.
In conclusion, Junduqing extractive could inhibit the proliferation, migration and invasion, and promote the apoptosis of human NPC cells through down-regulating Mcl-1 and Bcl-xL and up-regulating Caspase-3, Caspase-8 and Caspase-9. These results proved that Junduqing extractive might be a potentially effective drug for NPC treatment. It should be stated that it was necessary to confirm this conclusion in the future animal and clinical trials.
Disclosure statement
The authors report no conflict of interest.
Data availability
The analysed data sets generated during this study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Wei KR, Zheng RS, Zhang SW, et al. Nasopharyngeal carcinoma incidence and mortality in China, 2013. Chin J Cancer. 2017;36(1):90.
- Cichello SA, Yao Q, Dowell A, et al. Proliferative and inhibitory activity of Siberian ginseng (Eleutherococcus senticosus) extract on cancer cell lines; A-549, XWLC-05, HCT-116, CNE and beas-2b. Asian Pac J Cancer Prev. 2015;16(11):4781–4786.
- Guo YQ, Sun HY, Chan CO, et al. Centipeda minima (Ebushicao) extract inhibits PI3K-Akt-mTOR signaling in nasopharyngeal carcinoma CNE-1 cells. Chin Med. 2015;10(1):26.
- Su M, Li Y, Chung HY, et al. 2beta-(Isobutyryloxy)florilenalin, a sesquiterpene lactone isolated from the medicinal plant Centipeda minima, induces apoptosis in human nasopharyngeal carcinoma CNE cells. Molecules. 2009;14(6):2135–2146.
- Li YJ, Peng XB, Zhao WG. Study on the analgesic and anti-inflammatory effects of Junduqing granule. Chin Pharm. 2011;14:1278–1280.
- Zhao WG, Li YJ, Yu YL, et al. Therapeutic effect of Junduqing granules on the treatment of anemopyretic cold. Chin J Modern Drug Appl. 2012;6:11–13.
- Zhao WG, Li YJ, Peng XB. Study on antipyretic effect of Junduqing Granule. Inform Tradit Chin Med. 2012;29:36–37.
- Peng XB, Zhao WG, Li YJ. Study on acute toxicity test of Junduqing granule. Chin J Modern Drug Appl. 2011;5:24–25.
- Han Y, Zhao WG, Li YJ. Pharmacodynamic study on in vitro antiviral effect of Junduqing granules. Chin Pharm. 2015;26:3070–3071.
- Guo SS, Bao L, Cui XL. Experimental study on antivirus effects of Junduqing granule in vitro. Chin J Pharm. 2015;12:325–329.
- Wang L, Tam JP, Liu DX. Biochemical and functional characterization of Epstein-Barrvirus-encoded BARF1 protein: interaction with human hTid1 protein facilitates its maturation and secretion. Oncogene. 2006;25(31):4320–4331.
- Zou P, Hong Y, Koh HL. Chemical fingerprinting of Isatis indigotica root by RP-HPLC and hierarchical clustering analysis. J Pharmaceut Biomed. 2005;38(3):514–520.
- Li JF, Zhao JY, Liao LM. D-optimal design for enzymatic extraction of honeysuckle polysaccharide research. Food Ind. 2017;38(4):144–147.
- Sukardiman H, Widyawaruyanti A, Sismindari , et al. Apoptosis inducing effect of Andrographolide on TD-47 human breast cancer cell line. Afr J Trad Compl Alt Med. 2007;4:345–351.
- Varma A, Padh H, Shrivastava N. Andrographolide: a new plant-derived antineoplastic entity on horizon. Evid Based Complement Alternat Med. 2011;2011:1.
- Rajagopal D, Kumar RA, Deevi DS, et al. Andrographolide, a potential cancer therapeutic agent isolated from Andrographis paniculata. J Exp Ther. 2003;3(3):147–158.
- Calabrese C, Berman SH, Babish JG, et al. A phase I trial of andrographolide in HIV positive patients and normal volunteers. Phytother Res. 2000;14(5):333–338.
- Hidalgo MA, Romero A, Figueroa J, et al. Andrographolide interferes with binding of nuclear factor-kappaB to DNA in HL-60-derived neutrophilic cells. Br J Pharmacol. 2005;144(5):680–686.
- Handa SS, Sharma A. Hepatoprotective activity of andrographolide from Andrographis paniculata against carbontetrachloride. Indian J Med Res. 1990;92:276–283.
- Zhou J, Zhang S, Ong CN, et al. Critical role of pro-apoptotic Bcl-2 family members in andrographolide-induced apoptosis in human cancer cells. Biochem Pharmacol. 2006;72(2):132–144.
- Chen J, Shi DY, Liu SL, et al. Tanshinone IIA induces growth inhibition and apoptosis in gastric cancer in vitro and in vivo. Oncol Rep. 2012;27(2):523–528.
- Guerram M, Jiang ZZ, Yousef BA, et al. The potential utility of acetyltanshinone IIA in the treatment of HER2-overexpressed breast cancer: induction of cancer cell death by targeting apoptotic and metabolic signaling pathways. Oncotarget. 2015;6(26):21865–21877.
- Han B, Zhang X, Zhang Q, et al. Protective effects of salvianolate on microvascular flow in a porcine model of myocardial ischaemia and reperfusion. Arch Cardiovasc Dis. 2011;104(5):313–324.
- Hiraki M, Suzuki Y, Alam M, et al. MUC1-C stabilizes MCL-1 in the oxidative stress response of triple-negative breast cancer cells to BCL-2 inhibitors. Sci Rep. 2016;6(1):26643.
- Woo SM, Kwon TK. Jaceosidin induces apoptosis through Bax activation and down-regulation of Mcl-1 and c-FLIP expression in human renal carcinoma Caki cells. Chem Biol Interact. 2016;260:168–175.
- Woo SM, Min K, Seo BR, et al. YM155 sensitizes TRAIL-induced apoptosis through cathepsin S-dependent down-regulation of Mcl-1 and NF-κB-mediated down-regulation of c-FLIP expression in human renal carcinoma Caki cells. Oncotarget. 2016;7(38):61520–61532.
- Renault TT, Dejean LM, Manon S. A brewing understanding of the regulation of Bax function by Bcl-xL and Bcl-2. Mech Ageing Dev. 2017;161(Pt B):201–210.
- Ono M, Ejima K, Higuchi T, et al. Equol enhances apoptosis-inducing activity of genistein by increasing Bax/Bcl-xL expression ratio in MCF-7 human breast cancer cells. Nutr Cancer. 2017;69(8):1300–1307.
- Freire R, d’Adda Di Fagagna F, Wu L, et al. Cleavage of the Bloom’s syndrome gene product during apoptosis by caspase-3 results in an impaired interaction with topoisomerase IIIα. Nucleic Acids Res. 2001;29(15):3172–3180.
- Mitupatum T, Aree K, Kittisenachai S, et al. mRNA expression of Bax, Bcl-2, p53, cathepsin B, caspase-3 and caspase-9 in the HepG2 cell line following induction by a novel monoclonal Ab Hep88 mAb: cross-talk for paraptosis and apoptosis. Asian Pac J Cancer Prev. 2016;17(2):703–712.
- Pu X, Storr SJ, Zhang Y, et al. Caspase-3 and caspase-8 expression in breast cancer: caspase-3 is associated with survival. Apoptosis. 2017;22(3):357–368.