Abstract
Background
CircRNA circ_0026344 was previously revealed as a tumour-suppressive gene in colorectal cancer (CRC) progression. The purpose of this research was to investigate the role of circ_0026344 in CRC cells metastasis induced by chemokines.
Methods
Two human CRC cell lines SW480 and Caco-2 were treated by CCL20 and CXCL8. Cell proliferation, migration/invasion, expression of epithelial-mesenchymal transition (EMT) inducers and the expression of circ_0026344 were measured using sulforhodamine B assay, Transwell chamber, western blot and qRT-PCR, respectively. The effects of circ_0026344 on CRC cells migration/invasion and the expression of EMT inducers were evaluated. Moreover, the downstream miRNA and signalling pathways of circ_0026344 were studied.
Results
CCL20 and CXCL8 synergized to facilitate the proliferation, migration and invasion of CRC cells. At the meantime, E-cadherin was downregulated, whereas N-cadherin, Vimentin and Snail were up-regulated by CCL20 and CXCL8 co-stimulation, which was accompanied by the mobilization of PI3K/AKT/ERK signalling. More interestingly, the expression of circ_0026344 was down-regulated by CCL20 and CXCL8 co-stimulation. Silence of circ_0026344 increased the migratory and invasive capacities of CRC cells and increased EMT process as well. Overexpression of circ_0026344 led to a contrary impact. miR-183 was negatively regulated by circ_0026344, and the inhibitory effects of circ_0026344 overexpression on Wnt/β-catenin pathway were reversed when miR-183 was overexpressed.
Conclusion
Overexpression of circ_0026344 restrained CRC metastasis and EMT induced by CCL20 and CXCL8 synergistical treatment. miR-183 was a downstream effector of circ_0026344, and the anti-tumour function of circ_0026344 might be involved in the repressed Wnt/β-catenin signalling.
CCL20 and CXCL8 synergize to decrease the expression of circ_0026344;
Silence of circ_0026344 promotes CRC cells migration, invasion and EMT process;
miR-183 is a downstream effector of circ_0026344.
Highlights
Introduction
Colorectal cancer (CRC) refers to the epithelial cells of colorectal mucosa transforming into malignant cells under multiple pathogenic factors, such as diet, environment or genes. It is the third most prevailing malignancy in both male and female and is the third leading cause of cancer-associated death in the United States [Citation1]. The incidence of CRC is increased rapidly worldwide, accompanied by age increasing and the adverse effects of many lifestyle-related factors. For decades, surgery, chemotherapy and radiotherapy are the main treatments for CRC. However, single surgical treatment can not cure CRC, even if combined with chemotherapy and radiotherapy, high recurrence and metastasis still remain.
Chemokines are a family of chemoattractant cytokines that result in the directed migration of leukocytes, as well as the migration of other type of cells. Chemokines can be divided into four subfamilies, i.e., CC, CXC, C and CX3C, based on the order of two conserved cysteine residues. An accumulating number of evidence shows that chemokines are frequently expressed in various tumours to regulate tumour cells function in both physiological and pathological conditions [Citation2,Citation3]. In terms of CRC, several chemokines are highly expressed in tumour tissue and cell line, like CXCL1 [Citation4], CXCL8 [Citation5], CXCL10 [Citation6] and CCL20 [Citation7]. Functional assays demonstrated that CXCL8 and CCL20 co-stimulation led to the increase of CRC cells proliferation and migration [Citation8,Citation9]. Stimulation of CRC cells by CXCL8 and CCL20 led to the mobilization of PI3K/AKT/ERK pathway, which is a key driver of tumour growth and metastasis [Citation9,Citation10]. Moreover, a later study reported that CXCL8 and CCL20 synergize to facilitate CRC metastatic progress by coordinated induction of epithelial-mesenchymal transition (EMT) via PI3K/AKT/ERK pathway. Thus, targeting CXCL8 and CCL20 is of particular interest in CRC as a method that restricts tumour development and to considerably restrain its metastatic spreading.
Circular RNAs (circRNAs) are an emerging kind of non-coding RNAs, form a covalently closed continuous loop, which is misinterpreted as by-products of splicing errors. CircRNAs are widely expressed in human non-cancerous and cancerous tissues, as well as human serum and plasma. It is believed that circRNAs serve as miRNA sponges by absorbing and sequestering miRNA molecules and act through the repression of downstream tumour-suppressive genes. Nowadays, certain circRNAs have been considered as promising biomarkers and therapeutic targets since they are aberrantly expressed in tumours and involved in the onset and progression of tumours [Citation11,Citation12]. A previous study has revealed that circ_0026344 was down-regulated in CRC tissues and circ_0026344 overexpression led to CRC cell lose both in vitro and in vivo [Citation13], indicating circ_0026344 worked as a tumour-suppressive gene. The present work attempted to study whether circ_0026344 also play a role in CRC metastasis induced by CXCL8 combined with CCL20. The findings will help us to further understand the function of circ_0026344 in the onset and progression of CRC.
MicroRNAs (miRNAs) are a series of translated single-stranded RNA molecules that negatively control target genes via translational inhibition or degradation of complementary mRNAs [Citation14]. Abnormal expression of miRNA in exact tissues may be involved in the process of malignant transformation and the development of tumours [Citation15]. Additionally, studies have indicated that particular miRNA expression profiles in tumour tissues may conduce to the diagnosis and prognosis of malignant tumours [Citation16]. The miR-183 family is highly conservative, including three members, miR-96, miR-182 and miR-183 [Citation17]. These miRNAs have potential carcinogenic effects during tumourgenesis and have been exhibited to regulate cancer development and progression [Citation18,Citation19]. Previous reports suggested miR-183 family expressed higher in CRC tissues than normal colon tissues, and miR-183 is the member with the highest expression in this family [Citation20,Citation21]. In addition, previous studies have been shown that miR-183 overexpression in tumours is significantly associated with advanced clinical stage, lymph node and distant metastasis, and poor prognosis of CRC [Citation22]. Thus, we also explore the function of miR-183 in CRC.
Methods
Cell lines and treatment
Human CRC cell lines SW480 (ATCC® CCL-228) and Caco-2 (ATCC® HTB-37) obtained from ATCC (Manassas, VA) were authenticated using STR DNA typing method. SW480 cells were hatched in Leibovitz's L-15 medium (ATCC), whereas Caco-2 cells were cultured in eagle's minimum essential medium (ATCC). To make the complete growth medium, fetal bovine serum (Gibco, Grand Island, NY) was added into the medium to final concentrations of 10% and 20%, respectively. The cells were subcultured in 75 cm2 flask at 37 °C in an atmosphere with 5% CO2.
Cells were treated by 100 ng/mL of CCL20 and CXCL8 for 48 h [Citation10]. CCL20 and CXCL8 were purchased from R&D Systems (Minneapolis, MN).
Transfection
For circ_0026344 overexpression, circRNA sequence was cloned into PLCDH-cir vector (Ribobio, Guangzhou, China) for producing lentivirus. si-circ_0026344 (5′-GTTAAATCCTGAGTCCTCTCA-3′), miR-183 mimics (5′-UAUGGCACUGGUAGAAUUCACU-3′) and corresponding control were procured from GenePharma (Shanghai, China). Transfections were carried out by using Lipofectamine™2000 (Invitrogen, Carlsbad, CA) according to the standard protocol.
Sulforhodamine B (SRB) assay
Cells seeded in 96-well plates were treated by CCL20 and CXCL8 as described above, and then cell proliferation was measured by SRB assay. In brief, 50 μL pre-cooling trichloroacetic acid (TCA, Sangon Biotech, Shanghai, China) solution (30%, w/v) was added to fix cell for 1 h at 4 °C. Following five washes with deionized water, 70 μL SRB solution (Sigma-Aldrich, St. Louis, MO) was used to stain cells with a final concentration of 0.4% (w/v) for 30 min. The cells were rinsed four times with 1% acetic acid and incubated with 100 μL Tris-base solution (10 mM, pH10.5) for 20 min. The absorption value of each sample was calculated by GloMax®-Multi Detection System (Promega, Madison, WI) at 540 nm.
Migration and invasion assay
Cell migration was evaluated by utilizing a modified two-chamber transwell system (Costar-Corning, New York). The pre-treated cells were collected and suspended in serum-free medium at a final concentration of 1 × 105 cells/mL. 200 μL cell suspension was added into the upper side of the 24-well chamber, and the lower side of the chamber was filled with 600 μL the complete medium. After 24 h of incubation at 37 °C, the cells in the lower side were dyed by crystal violet (Sangon Biotech) and counted microscopically.
Cell invasion was tested similar to the measurement of cell migration, except the chamber was pre-coated with 2 mg/mL Matrigel (Millipore, Bedford, MA) containing 8 mm diameter pores overnight.
qRT-PCR
Total RNA was extracted from cells by mixing the cells with Trizol reagent (Invitrogen). For the assessment of circ_0026344, the extracted RNA was reversely transcribed by using a PrimeScript RT reagent Kit with gDNA Eraser (TaKaRa, Dalian, China). qPCR was carried out using SYBR Green PCR kit (TaKaRa) with three replication each. For the test of miR-183, Mir-X™ miRNA First Strand Synthesis Kit and Mir-X™ miRNA qRT-PCR SYBR® Kit both from Takara were utilized for the processes of reverse transcription and qPCR. Data were calculated according to 2−ΔΔCt method and normalized to GAPDH and U6 snRNA.
Western blot
The primary antibodies applied in this process were listed as follows. Anti-PI3K (ab86714), anti-p-PI3K (ab182651), anti-AKT (ab8805), anti-p-AKT (ab38449), anti-ERK (ab32537), anti-p-ERK (ab194776), anti-E-cadherin (ab15148), anti-N-cadherin (ab76057), anti-Vimentin (ab8978), anti-Snail (ab53519), anti-Wnt3a (ab28472), anti-β-catenin (ab32572) and anti-β-actin (ab8226) were all purchased from Abcam (Cambridge, MA).
After the indicated treatment, total proteins were extracted from cell by utilizing RIPA buffer (Beyotime, Shanghai, China) containing protease inhibitor PMSF (Beyotime). The lysate was centrifuged at 14,000 g for 15 min at 4 °C and the supernatant was implied or immediately stored at 80 °C. The protein concentration was detected by BCA Protein Assay Kit (Pierce, Rockford, IL). Protein (0.1 mg) from each sample was resolved over SDS-PAGE and transferred to a polyvinylidene fluoride membrane. The blot was blocked in blocking buffer (Beyotime) for 1 h at room temperature, incubated with appropriate primary antibodies in blocking buffer at 4 °C overnight, followed by incubation with anti-mouse or anti-rabbit secondary antibody horseradish peroxidase conjugate and distinguished by chemiluminescence and autoradiography utilizing X-ray film (Applygen Technologies Inc., Beijing, China). Densitometric measurements of the bands in Western blot analysis were performed using Image Lab™ Software (Bio-Rad, Hercules, CA).
Statistical analysis
All experiments were repeated three times in triplicate. Results were presented as mean ± SD. Statistical analyses were performed using SPSS 19.0 software (Chicago, IL, USA) with Student t-test or ANOVA. A p value < .05 was considered to indicate a significant result.
Results
CCL20 and CXCL8 synergize to facilitate the proliferation and metastasis of cultured CRC cells
To start with, two human CRC cell lines SW480 and Caco-2 were treated by CCL20 and CXCL8. As a result, cell proliferation was significantly increased by CCL20 and CXCL8 as compared to control group (p < .05, ). At the meantime, relative migration and invasion were both promoted by CCL20 and CXCL8 when compared to control group (p < .05, ). The results were consistent with previous findings, suggesting chemokines, in particular CCL20 and CXCL8, are critical regulators in the proliferating and metastatic processes of tumour cancer [Citation10].
Figure 1. CCL20 and CXCL8 synergize to facilitate the proliferation and metastasis of cultured CRC cells. SW480 and Caco-2 cells were treated by CCL20 and CXCL8 at concentration of 100 ng/mL for 48 h. Non-treated cells served as control. (A) Cell proliferation measured by SRB assay. (B) Relative migration and (C) invasion tested by transwell chamber. (D) Expression of core proteins in PI3K/AKT/ERK pathway quantified by western blot analysis. (E and F) Semi-quantitative results based on the data from western blot analysis. *p < .05 compared to control group.
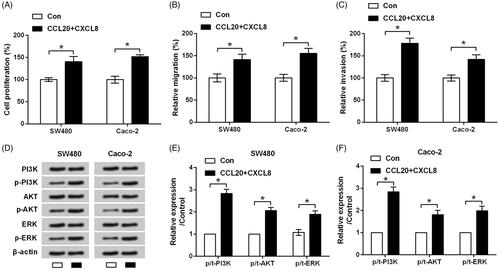
Further, the results from western blotting showed that CCL20 and CXCL8 significantly increased the phosphorylation levels of PI3K, AKT and ERK (p < .05) while did not impact the total levels of these proteins (). It seems that CCL20 and CXCL8 synergize to facilitate the proliferation and metastasis of cultured CRC cells through activation of PI3K/AKT/ERK signalling.
CCL20 and CXCL8 synergize to facilitate the EMT process
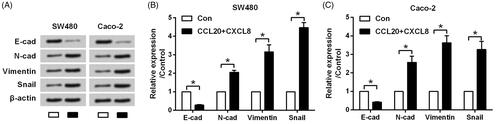
Next, the expression changes of EMT inducers were assessed to evaluate if CCL20 and CXCL8 synergize to facilitate the metastasis of cultured CRC cells via regulating EMT. Results in displayed that protein levels of E-cadherin were repressed, whereas protein levels of N-cadherin, Vimentin and Snail were upsurge in response to CCL20 and CXCL8 treatment (all p < .05). This phenomenon suggested that CCL20 and CXCL8 synergized to facilitate the metastasis of SW480 and Caco-2 cells through promoting the process of EMT.
Figure 2. CCL20 and CXCL8 synergize to facilitate the EMT process. SW480 and Caco-2 cells were treated by 100 ng/mL CCL20 and CXCL8 for 48 h. Non-treated cells served as control. (A) Expression of EMT inducers detected by western blot analysis. (B and C) Semi-quantitative results based on the data from western blot analysis. *p < .05 compared to control group.
CCL20 and CXCL8 synergize to decrease the expression of circ_0026344
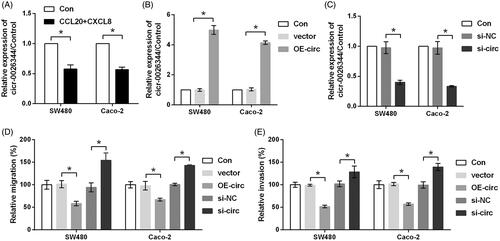
Then, qRT-PCR was carried out to test whether CCL20 and CXCL8 treatment could alter the expression of circ_0026344. Data in showed that the expression of circ_0026344 was prominently down-regulated by CCL20 and CXCL8 as compared to control group (p < .05), indicating circ_0026344 might be a key factor involved in CCL20 and CXCL8 co-stimulation. Subsequently, the expression of circ_0026344 in both SW480 and Caco-2 cells was overexpressed or suppressed to measure the function of circ_0026344 on CRC cells metastasis. Transfection efficiency tested by qRT-PCR was shown in , in which the up-regulation and down-regulation of circ_0026344 were observed (p < .05). Further study indicated that relative migration and invasion were both significantly repressed by circ_0026344 overexpression while were enhanced by circ_0026344 suppression (p < .05, ), confirming our abovementioned hypothesis.
Figure 3. CCL20 and CXCL8 synergize to decrease the expression of circ_0026344. (A) SW480 and Caco-2 cells were treated by 100 ng/mL CCL20 and CXCL8 for 48 h. Non-treated cells served as control. Expression of circ_0026344 was measured by qRT-PCR. (B) Expression of circ_0026344 was evaluated by qRT-PCR after the cells were transfected with circ_0026344 overexpression vector (OE-circ) or an empty vector. (C) Expression of circ_0026344 was measured by qRT-PCR after the cells were transfected with circ_0026344 siRNA (si-circ) or the non-targeting negative control (si-NC). Following transfection, relative (D) migration and (E) invasion were estimated by using transwell chamber. *p < .05 compared to the indicated group.
Silence of circ_0026344 promotes EMT process
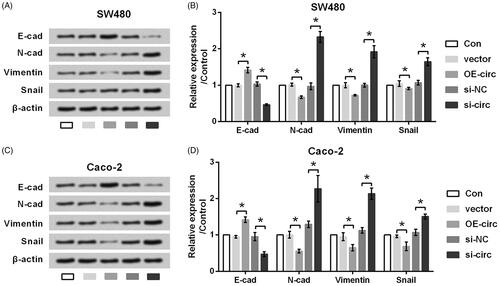
Also, the effects of circ_0026344 dysregulation on the expression of EMT inducers were calibrated. showed that protein levels of E-cadherin were up-regulated by circ_0026344 overexpression while down-regulated by circ_0026344 suppression (p < .05). Of contrast, protein levels of N-cadherin, Vimentin and Snail were down-regulated by circ_0026344 overexpression while up-regulated by circ_0026344 suppression (p < .05). Based on these results, we found that circ_0026344 silence exhibited similar impacts to CCL20 and CXCL8 co-stimulation.
Figure 4. Silence of circ_0026344 promotes EMT process. (A) SW480 and (C) Caco-2 cells were transfected with circ_0026344 overexpression vector (OE-circ) or circ_0026344 siRNA (si-circ). An empty vector and a non-targeting sequence (si-NC) were transfected as negative controls. Expression of EMT inducers was detected by western blot analysis. (B and D) Semi-quantitative results based on the data from western blot analysis. *p < .05 compared to the indicated group.
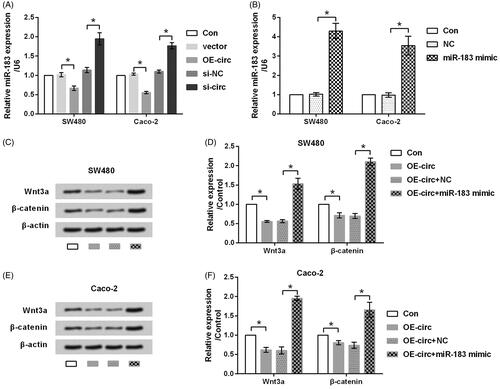
miR-183 is a downstream effector of circ_0026344
It is well-established that circRNAs exerts functions though sponging miRNAs in cancer [Citation23]. miR-183 has been recognized as an oncogene and was found to facilitate the proliferation and metastasis of CRC cells [Citation24,Citation25]. Thus, we are interested to study whether circ_0026344 impacted EMT process via regulating miR-183. qRT-PCR data in exhibited that the expression of miR-183 was down-regulated by circ_0026344 overexpression while up-regulated by circ_0026344 suppression (p < .05), indicating miR-183 was negatively regulated by circ_0026344. Afterwards, the effects of miR-183 on circ_0026344’s function were tested. To this end, the expression of miR-183 in SW480 and Caco-2 cells was overexpressed by transfection with miR-183 mimic. Transfection efficiency presented in indicated that miR-183 mimic dramatically increased miR-183 expression (p < .05). Further study revealed that circ_0026344 overexpression significantly down-regulated the expression levels of core proteins in Wnt/β-catenin pathway (p < .05, ). However, the impacts of circ_0026344 overexpression on Wnt/β-catenin pathway were reversed by miR-183 mimic (p < .05). Thus, we preliminary conclude that circ_0026344 restrains metastasis of CRC cells possibly via down-regulating miR-183.
Figure 5. miR-183 is a downstream effector of circ_0026344. (A) SW480 and Caco-2 cells were transfected with circ_0026344 overexpression vector (OE-circ) or circ_0026344 siRNA (si-circ). An empty vector and a non-targeting sequence (si-NC) were transfected as negative controls. Expression of miR-183 was estimated by qRT-PCR. (B) Expression of miR-183 was estimated by qRT-PCR after the cells were transfected with miR-183 mimic or the scrambled negative control (NC). (C) SW480 and (E) Caco-2 cells were transfected with OE-circ alone or in combination with miR-183, after which the expression of core proteins in Wnt/β-catenin pathway was evaluated by western blot analysis. (D and F) Semi-quantitative results based on the data from western blot analysis. *p < .05 compared to the indicated group.
Discussion
Tumour metastasis is an important biological feature of malignant tumours. Metastasis, including malignant invasion, intravascular perfusion, circulatory system survival, extravasation and secondary site colonization, is the most lethal attribute of cancer. Once the tumour has metastasized, the prognosis is often poor. Specifically in CRC, the 5-year survival rate of patients with distant metastasis is less than 10% [Citation26]. Thus, inhibition of CRC metastasis has been considered as an effective aspect for improving patients’ prognosis. In this assay, we explored the potential of circ_0026344 in regulating CRC cells metastasis. As a result of the study, the expression of circ_0026344 was down-regulated by CCL20 and CXCL8 co-stimulation, which are key chemokines in mediating EMT. Besides, silence of circ_0026344 increased the migratory and invasive capacities of cultured CRC cells (SW480 and Caco-2). At the meantime, silence of circ_0026344 repressed the EMT process. Further study revealed that the function of circ_0026344 might be through regulating miR-183 and Wnt/β-catenin pathway.
CircRNAs were first discovered in RNA viruses as early as 1976 [Citation27]. Initially, due to their low richness in cells, circRNAs were barely getting any attention. However, with the development of the second-generation sequencing technique and bioinformatics, the richness of some circRNAs was found far greater than their originated mRNAs. Nowadays, circRNAs are widely known as a novel subset of competing endogenous RNAs, regulating target genes via affecting the function of miRNA and RNA-binding proteins. Also, the role of circRNAs in various kinds of cancers has been studied. A previous study demonstrated circ_0026344 as a tumour suppressive gene in CRC progression, as overexpression of circ_0026344 suppressed CRC cells growth and invasion while promoted apoptosis [Citation13]. However, great effort is still required to fully appreciate the complexity of circ_0026344.
It is well-known that chemokines are closely associated with tumour pathogenesis and metastasis. In CRC, stimulation of tumour cells with CCL20 and CXCL8 is sufficient to induce complete EMT [Citation10]. This phenomenon was further confirmed in this study as the proliferation, migration, invasion and the expression of epithelial marker E-cadherin were decreased by co-stimulation with CCL20 and CXCL8, which was accompanied by the up-regulated expression of mesenchymal biomarkers N-cadherin, Vimentin and Snail. Besides, PI3K/AKT/ERK pathway was demonstrated to be activated by CCL20 and CXCL8 co-stimulation, indicating this pathway was responsible for the induced EMT. More interestingly, we found that the expression of circ_0026344 was down-regulated in SW480 and Caco-2 cells after co-stimulating with CCL20 and CXCL8. The result suggested that circ_0026344 might be one of the effectors of CCL20 and CXCL8. Additionally, silence of circ_0026344 exhibited inhibitory effects on cultured CRC cells migration, invasion and EMT, further evidenced the tumour suppressive roles of circ_0026344. This is the first study revealed the anti-EMT effects of circ_0026344 on human cancers.
It is widely-accepted that circRNAs exert functions via sponging miRNA, functionally releasing mRNA transcripts that are normally targeted by miRNA. For the selected examples, circRNA_33287 worked as a key regulator in osteogenic differentiation via sponging miR-214-3p [Citation28]. Likewise, circ_0055625 plays a significant role in promoting CRC cells growth and metastasis by functioning as a miRNA sponge for miR-106b-5p [Citation29]. In the current study, miR-183, a recognized oncogene [Citation24], was found to be a downstream gene of circ_0026344 since it was negatively regulated by circ_0026344. This result was analogous with a preceding study, in which miR-21 and miR-31 were revealed as two downstream genes of circ_0026344 [Citation13]. Another study implied that miR-183 facilitated proliferation and suppressed apoptosis in CRC via targeting ABCA1 [Citation24]. Similar function was reported in diverse cancers. For instance, overexpressing miR-183 enhanced radioresistance via down-regulation of LRIG1. Moreover, miR-183 inhibitor repressed tumour growth, on the contrary, miR-183 mimic improved tumour growth in vivo experiments [Citation30]. Another research indicated that miR-183 exerted its oncogenic effect on papillary thyroid carcinoma. miR-183 facilitated proliferation, migration and invasion, as well as restrained apoptosis of papillary thyroid carcinoma cells [Citation31].
EMT process is driven by various pathways, containing TGF-β1/Smad, Wnt/β-catenin, Notch, Hedgehog and other pathways [Citation32]. Considering Wnt/β-catenin signalling was suggested as a downstream signalling of miR-183 [Citation33,Citation34,Citation35], we further studied whether circ_0026344 could impact this signalling. Data presented in this study demonstrated that circ_0026344 was capable of repressing Wnt/β-catenin signalling via a miR-183-dependent fashion. This finding implied the anti-EMT functions of circ_0026344 were via suppressing miR-183-dependent Wnt/β-catenin signalling.
Conclusions
On the whole, our findings illustrated that overexpressed circ_0026344 restrained CRC metastasis and EMT induced by CCL20 and CXCL8 synergistical treatment. Further experiments suggested miR-183 as a downstream effector of circ_0026344, and the anti-tumour function of circ_0026344 might be involved in the repressed Wnt/β-catenin signalling.
Author contributions
Conceives and designed the experiments: Tao Shen, Xianshuo Cheng and Yunfeng Li. Performed the experiments: Tao Shen, Xianshuo Cheng, Xin Liu and Cuifeng Xia. Analyzed the data: Tao Shen, Xianshuo Cheng and Hongtao Zhang. Contributed reagents/materials/analysis tools: Dingguo Pan and Xuan Zhang. Wrote the paper: Yunfeng Li.
| Abbreviations | ||
| CRC | = | Colorectal cancer |
| EMT | = | Epithelial-mesenchymal transition |
| circRNAs | = | Circular RNAs |
| TCA | = | Trichloroacetic acid |
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA A Cancer J Clinicians. 2014;64(2):104–117.
- Acharyya S, Oskarsson T, Vanharanta S, et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell. 2012;150(1):165–178.
- Agostini M, Janssen KP, Kim IJ, et al. An integrative approach for the identification of prognostic and predictive biomarkers in rectal cancer. Oncotarget. 2015;6(32):32561–32574.
- Zhuo C, Wu X, Li J, et al. Chemokine (C-X-C motif) ligand 1 is associated with tumor progression and poor prognosis in patients with colorectal cancer. Biosci Rep. 2018;38(4):BSR20180580.
- Xiao YC, Yang ZB, Cheng XS, et al. CXCL8, overexpressed in colorectal cancer, enhances the resistance of colorectal cancer cells to anoikis. Cancer Lett. 2015;361(1):22–32.
- Bai M, Chen X, Ba YI. CXCL10/CXCR3 overexpression as a biomarker of poor prognosis in patients with stage II colorectal cancer. Mol Clin Oncol. 2016;4(1):23–30.
- Vicinus B, Rubie C, Stegmaier N, et al. miR-21 and its target gene CCL20 are both highly overexpressed in the microenvironment of colorectal tumors: significance of their regulation. Oncol Rep. 2013;30(3):1285–1292.
- Ghadjar P, Rubie C, Aebersold DM, et al. The chemokine CCL20 and its receptor CCR6 in human malignancy with focus on colorectal cancer. Int J Cancer. 2009;125(4):741–745.
- Shen T, Yang Z, Cheng X, et al. CXCL8 induces epithelial-mesenchymal transition in colon cancer cells via the PI3K/Akt/NF-kappaB signaling pathway. Oncol Rep. 2017;37(4):2095–2100.
- Cheng XS, Li YF, Tan J, et al. CCL20 and CXCL8 synergize to promote progression and poor survival outcome in patients with colorectal cancer by collaborative induction of the epithelial-mesenchymal transition. Cancer Lett. 2014;348(1–2):77–87.
- Arnaiz E, Sole C, Manterola L, et al. CircRNAs and cancer: biomarkers and master regulators. Semin Cancer Biol. 2018;58:90–99.
- Fang S, Pan J, Zhou C, et al. Circular RNAs serve as novel biomarkers and therapeutic targets in cancers. CGT. 2019;19(2):125–133.
- Yuan Y, Liu W, Zhang Y, et al. CircRNA circ_0026344 as a prognostic biomarker suppresses colorectal cancer progression via microRNA-21 and microRNA-31. Biochem Biophys Res Comm. 2018;503(2):870–875.
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297.
- Yuan D, Li K, Zhu K, et al. Plasma miR-183 predicts recurrence and prognosis in patients with colorectal cancer. Cancer Biol Ther. 2015;16(2):268–275.
- Yanaihara N, Caplen N, Bowman E, et al. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 2006;9(3):189–198.
- Pierce ML, Weston MD, Fritzsch B, et al. MicroRNA-183 family conservation and ciliated neurosensory organ expression. Evol Dev. 2008;10(1):106–113.
- Abraham D, Jackson N, Gundara JS, et al. MicroRNA profiling of sporadic and hereditary medullary thyroid cancer identifies predictors of nodal metastasis, prognosis, and potential therapeutic targets. Clin Cancer Res. 2011;17(14):4772–4781.
- Xu X, Dong Z, Li Y, et al. The upregulation of signal transducer and activator of transcription 5-dependent microRNA-182 and microRNA-96 promotes ovarian cancer cell proliferation by targeting forkhead box O3 upon leptin stimulation. Int J Biochem Cell Biol. 2013;45(3):536–545.
- Earle JS, Luthra R, Romans A, et al. Association of microRNA expression with microsatellite instability status in colorectal adenocarcinoma. JMD. 2010;12(4):433–440.
- Zhang QH, Sun HM, Zheng RZ, et al. Meta-analysis of microRNA-183 family expression in human cancer studies comparing cancer tissues with noncancerous tissues. Gene. 2013;527(1):26–32.
- Zhou T, Zhang GJ, Zhou H, et al. Overexpression of microRNA-183 in human colorectal cancer and its clinical significance. Eur J Gastroenterol Hepatol. 2014;26(2):229–233.
- Rong D, Sun H, Li Z, et al. An emerging function of circRNA-miRNAs-mRNA axis in human diseases. Oncotarget. 2017;8(42):73271–73281.
- Bi DP, Yin CH, Zhang XY, et al. MiR-183 functions as an oncogene by targeting ABCA1 in colon cancer. Oncol Rep. 2016;35(5):2873–2879.
- Zhang Q, Ren W, Huang B, et al. MicroRNA-183/182/96 cooperatively regulates the proliferation of colon cancer cells. Mol Med Rep. 2015;12(1):668–674.
- Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet (London, England). 2014;383(9927):1490–1502.
- Sanger HL, Klotz G, Riesner D, et al. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc Natl Acad Sci USA. 1976;73(11):3852–3856.
- Peng W, Zhu S, Chen J, et al. Hsa_circRNA_33287 promotes the osteogenic differentiation of maxillary sinus membrane stem cells via miR-214-3p/Runx3. Biomed Pharmacother. 2019;109:1709–1717.
- Zhang J, Liu H, Zhao P, et al. Has_circ_0055625 from circRNA profile increases colon cancer cell growth by sponging miR-106b-5p. J Cell Biochem. 2019;120(3):3027–3037.
- Fan H, Yuan R, Cheng S, et al. Overexpressed miR-183 promoted glioblastoma radioresistance via down-regulating LRIG1. Biomed Pharmacother. 2018;97:1554–1563.
- Wei C, Song H, Sun X, et al. miR-183 regulates biological behavior in papillary thyroid carcinoma by targeting the programmed cell death 4. Oncol Rep. 2015;34(1):211–220.
- Gonzalez DM, Medici D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci Signal. 2014;7(344):re8.
- Yang X, Wang L, Wang Q, et al. MiR-183 inhibits osteosarcoma cell growth and invasion by regulating LRP6-Wnt/beta-catenin signaling pathway. Biochem Biophys Res Comm. 2018;496(4):1197–1203.
- Chen Y, Song W. Wnt/catenin beta1/microRNA 183 predicts recurrence and prognosis of patients with colorectal cancer. Oncol Lett. 2018;15(4):4451–4456.
- Chen C, Xiang H, Peng YL, et al. Mature miR-183, negatively regulated by transcription factor GATA3, promotes 3T3-L1 adipogenesis through inhibition of the canonical Wnt/beta-catenin signaling pathway by targeting LRP6. Cell Signal. 2014;26(6):1155–1165.
