Abstract
Zinc finger and BTB domain containing 20 (ZBTB20), a sequence-specific transcriptional repressor, has been found to be involved in tumorigenesis. However, its role(s) in gastric cancer and the molecular mechanisms involved are poorly investigated. Here, our data demonstrated that ZBTB20 expression was markedly upregulated in gastric cancer cell lines infected with Helicobacter pylori (H. pylori) and in gastric cancer tumor samples. Loss- and gain-of-function studies showed that ZBTB20 promoted cell proliferation, invasion and migration of gastric cancer cell lines. Mechanistically, the phosphorylation of NF-κBp65 and expression and activity of MMP-2 and MMP-9 were increased, while IκBα expression was decreased by ZBTB20 in gastric cancer cells. We further revealed that IκBα overexpression significantly inhibited NF-κB signaling as well as cell migration, invasion and proliferation in gastric cancer cell lines induced by ZBTB20 overexpression. Therefore, our findings emphasize an important role for ZBTB20 in controlling gastric cancer development, which is helpful to identify potential therapeutic targets for its treatment.
Keywords:
Introduction
Gastric cancer is one of the most common malignancies worldwide with an overall 5-year survival rate lower than 25% [Citation1]. Despite efforts to improve diagnostic technology and patient managements, the prognosis of gastric cancer patients has not been improved significantly [Citation2]. The formation of gastric cancer is a complex process, which eventually develops into gastric cancer from precancerous lesions such as superficial and atrophic gastritis, intestinal metaplasia, and dysplasia. Helicobacter pylori (H. pylori) infection is a common chronic infection that is one of the most important risk factors for gastric cancer compared with others such as genetic factors, high-salt diets, smoked foods, smoking, and alcohol abuse [Citation3]. H. pylori infection is necessary but not sufficient for the development of gastric adenocarcinoma. Globally, the infection rate of H. pylori is about 40%–80%, but only 1% of H. pylori infections progress to gastric cancer [Citation4]. It is suggested that individuals with different genetic backgrounds have different susceptibility to gastric cancer when exposed to the same environment. Given that different DNA, mRNA and protein expressions modulated by H. pylori infection could regulate gastric cancer cell proliferation, apoptosis, migration and invasion, they could serve as therapeutic targets in gastric cancer [Citation5,Citation6].
Zinc finger and BTB domain containing 20 (ZBTB20) has been implicated in developmental neurogenesis [Citation7], glucose and lipid homoeostasis, and the tumorigenesis of glioblastoma [Citation8], liver [Citation9] and lung cancer [Citation10]. Polymorphisms of ZBTB20 appeared to be associated with gastric cancer susceptibility in allelic, homozygous, dominant and recessive models, suggesting its role in gastric cancer [Citation11]. NF-κB is activated in a variety of cancers as detected by phosphorylation and degradation of IκBα proteins, phosphorylation of RelA/p65, and elevated expression of NF-κB target genes. ZBTB20 enhances the innate immune responses induced by Toll-like receptor (TLR) through promoting NF-κB activation by repressing IκBα gene transcription [Citation12]. Moreover, the activation of NF-κB was significantly attenuated by ZBTB20 deficiency after partial hepatectomy and was associated with hepatocyte proliferation in mouse liver regeneration [Citation13]. However, whether and how IκBα/NF-κB signaling pathway involved in the ZBTB20-associated carcinogenesis in gastric cancer is still unknown.
Here, we determine the effect of ZBTB20 on the regulation of cell migration, invasion and proliferation of gastric cancer and explore the possible molecular mechanism involved in NF-κB signaling. Our findings reveal that ZBTB20 promotes cell migration and invasion of gastric cancer by inhibiting IκBα to induce NF-κB activation.
Materials and methods
Clinical samples
In total, the primary tumor samples were collected from 37 patients at Xuzhou Central Hospital who underwent surgery for gastric cancer between 2014 and 2017. None of the patients received preoperative radiotherapy or chemotherapy. All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethics Committee of Xuzhou Central Hospital and with the 1964 Helsinki declaration. This study protocol was approved by the Ethics Committee of Xuzhou Central Hospital. Written informed consents were provided prior to enrollment of patients.
Immunohistochemistry
Tissues were fixed in 4% paraformaldehyde, dehydrated and embedded in paraffin. Five-micrometer-thick slices were incubated in 3% H2O2 in methanol and 5% normal horse serum to minimize nonspecific staining. Sections were subsequently incubated overnight with the primary anti-ZBTB20 antibody (1:500; ab127702; Abcam, USA) at 4 °C, and then incubated with horseradish peroxidase (HRP)-labeled IgG secondary antibody (Beyotime Institute of Biotechnology, Shanghai, China) for half an hour at 25 °C. The section was then stained with diaminobenzidine (DAB) and hematoxylin for 3 min and washed in water for 10 min. Fields from each slide were examined and photographed under a light microscopy (×200). Quantitative data of positive area for immunocytochemistry (IHC) was analyzed using Image-pro Plus 6.0 software (Media Cybernetic, Silver Springs, MD, USA).
Cell culture and H. pylori infection
Human gastric cancer cell lines (SGC-7901, MKN-28, MKN-45 and BGC-823) and GES-1 human gastric epithelial mucosa cell line were obtained from the Shanghai Cell Bank (Shanghai, China) and cultured in RPMI-1640 supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin and 0.1 mg/ml streptomycin in an incubator at 37 °C with 5% CO2 atmosphere. BGC-823 and MKN-45 cells were infected with H. pylori (strain 43504, ATCC, Wesel, Germany) at a multiplicity of infection (MOI) of 25, 50 and 100 for 24 h.
Luciferase reporter gene assay
BGC-823 and MKN-45 cells were transfected with the pGL3-ZBTB20 promoter using Lipofectamine 2000 (Invitrogen Life Technologies) following the manufacturer's protocol. After 12 h transfection, cells were infected with H. pylori. Then, the cells were collected, and Luciferase activity was determined using a Luciferase assay kit (Promega).
Cell transfection
The RNAi (RNA interference) sequences targeting position 1163–1181 (siRNA-1; 5′-GGAGAUGGACAGCACUGUUUU-3′), position 1612–1630 (siRNA-2; 5′-CCAAACAGAACUACGUCAAUU-3′) or 1687–1705 (siRNA-3; 5′-CCUUCUCCUUAAAGGAUUAUU-3′) of the human ZBTB20 gene were transfected into MKN-45 and BGC-823 cells using Lipofectamine 2000 in accordance with the manufacturer's protocol. ZBTB20 and IκBα overexpression was constructed by integrating the coding sequence (CDS) of ZBTB20 and IκBα into the mammalian expression vector pCDNA3.1(+). The primers used to synthesize the CDS were shown as below: ZBTB20, forward primer: 5′-CCCAAGCTTATGACCGAGCGCATTCAC-3′ and reverse primer: 5′-CGGAATTCATCCGTCAGACACATGCATCC-3′; IκBα, forward primer: 5′-CCCAAGCTTATGTTCCAGGCGGCCGAG-3′ and reverse primer: 5′-CGGAATTCTCATAACGTCAGACGCTGGC-3′. 293 T cells obtained from American Type Culture Collection (ATCC, Manassas, VA, USA) were seeded in 6-well plate and transfected with pCDNA3.1(+)-ZBTB20 and pCDNA3.1(+)-IκBα at 37 °C for 5 h using Lipofectamine 2000 in accordance with the instruction of the manufacturer. 48 h after transfection recombined lentivirus were collected and used for infecting BGC-823 and MKN-45 cells. Cells transfected with scramble siRNA (siNC) or infected with blank pCDNA3.1(+) vector (blank vector) were used as negative controls.
CCK-8 assay
To assess cell proliferation, a Cell Counting Kit (CCK)-8 (Signalway Antibody LLC., College Park, MD, USA) assay was performed. Briefly, BGC-823 and MKN-45 cells (3 × 103 cells/well) were cultured in a 96-well plate, and cell proliferation was documented by administration of CCK-8 solution (10 μl per well) every 24 h in BGC-823 and MKN-45 cells transfected with ZBTB20-siRNA or infected with pCDNA3.1(+)-ZBTB20 and/or pCDNA3.1(+)-IκBα. The number of viable cells was determined at 450 nm cell′s absorbance readings using a DNM-9602 microplate reader (Pulang New Technology, Beijing, China).
Transwell assay
BGC-823 and MKN-45 cells (3 × 103 cells/well) were grown in a 6-well plate and maintained at 37 °C. After 24 h incubation, BGC-823 and MKN-45 cells transfected with ZBTB20-siRNA or infected with pCDNA3.1(+)-ZBTB20 and/or pCDNA3.1(+)-IκBα were serum-starved in basic medium for 24 h. Then, 300 μl cell suspension adjusted to 6 × 104 cells was filled in the upper Transwell chamber (8.0-µm with Size 24 Cluster Platel; Costar, USA) without or with 30 ml of 1 mg/ml Matrigel (BD Bioscience, San Jose, CA, USA), and 700 μl complete medium containing 10% FBS was added into the lower chamber. The cells were then cultured for 24 h (migration assay) or 48 h (invasion assay) at 37 °C, after which cells were fixed with 1 ml 4% methanol for 10 min and stained with 1 ml 0.5% crystal violet for 30 min. The BGC-823 and MKN-45 cells that migrated to the lower chamber and attached to the membrane were counted under a microscope (Shanghai Caikon Optical Instrument Factory, Shanghai, China).
Wound healing assay
BGC-823 and MKN-45 cells were seed in 35 mm2 tissue culture dishes at a density of 8 × 105 and further seeded until reached 100% confluence. Then confluence cultures were scratched using a pipette tip. After scratching, gently wash the well twice with medium to remove the detached cells. Scratched cultures were photographed under a microscope at 0 and 24 h. Migration of cells was established by measuring the width of the scratched area at each time point in the scratched area.
Gelatin zymography gel assay
To investigate the MMP-2 and MMP-9 activities in gastric cancer cell lines, proteins were separated on a 10% SDS-PAGE containing 1% gelatin (Sigma-Aldrich, München, Germany). The gels were incubated at 37 °C overnight, stained with Coomassie Blue, de-stained, and then scanned. Signal intensity was quantified by densitometry using Bio-1D software version 15.01 (Vilber Lourmat, Eberhardzell, Germany).
Real-time PCR assay
Total RNA from BGC-823 and MKN-45 cells was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Then Reverse Transcription System Kit (Takara Biomedical Technology (Beijing) Co., Ltd, Beijing, China) was used to synthesize the first-strand from 1 μg of total RNA according to the manufacturer′s protocols. Quantitative real-time PCR using Maxima SYBR Green/ROX qPCR Master Mix (Thermo scientific, Pittsburgh, PA, USA) was performed using an ABI Prism 7500 sequence detection PCR system (Applied Biosystems, Shanghai, China). The specific primers used in real-time PCR were shown as below: ZBTB20, forward primer: 5′-GCTTGGCTACAGCGACATC-3′ and reverse primer: 5′-GAACACATCGCCCACGTTC-3′; IκBα, forward primer: 5′-CTCCGAGACTTTCGAGGAAATAC-3′ and reverse primer: 5′-GCCATTGTAGTTGGTAGCCTTCA-3′; GAPDH, forward primer: 5′-AATCCCATCACCATCTTC-3′ and reverse primer: 5′-AGGCTGTTGTCATACTTC-3′. Expression levels are given as ratios to GAPDH, and each assay was performed in triplicate. The relative fold changes in messenger RNA expression were calculated using the 2−ΔΔCt method.
Western blotting
BGC-823 and MKN-45 cells were lysed by RIPA lysis buffer (Solarbio). After centrifugation, the supernatants were collected and BCA reagent was used to determine the concentration of protein. Next, 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was performed to isolate the proteins. After being transferred to polyvinylidene fluoride membranes, the protein was blocked with nonfat dry milk, added antibodies, and detected with supersignal west picochemiluminescent substrate. Primary antibodies include anti-ZBTB20 (1:1000; Abcam, Cambridge, MA, USA), anti-IκBα (1:2000; Abcam), anti-NF-κBp65 (1:1000; Cell Signaling Technology, Danvers, MA, USA), anti-p-NF-κBp65 (1:1000; Cell Signaling Technology), anti-MMP-2 (1:1000; Abcam), anti-MMP-9 (1:1000; Abcam) and anti-GAPDH (1:2000; Cell Signaling Technology) antibody. Secondary antibodies include HRP-labeled Goat Anti-Mouse or Goat Anti-Rabbit IgG antibody (1:1000; Beyotime Institute of Biotechnology).
Statistical analysis
All data were representative of experiments done in triplicate and were expressed as the mean ± SD. Samples were evaluated by the SPSS/Win11.0 software (SPSS, Inc., Chicago, IL, USA) using the Student′s t test for two groups and analysis of variance (ANOVA) for multiple groups. Overall survival of gastric cancer patients from Kaplan-Meier plotter database was analyzed by the Kaplan-Meier survival curves and log-rank nonparametric test. When p < .05, the difference between groups was statistically significant.
Results
ZBTB20 expression is increased in human gastric cancer tumors and correlated with overall survival
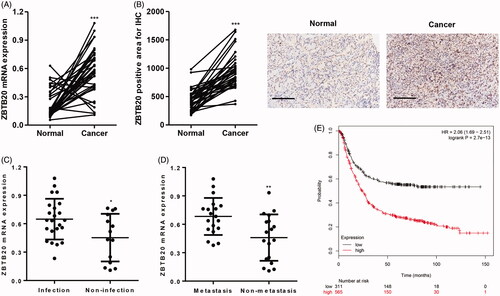
Analysis of tissues collected from our independent hospital demonstrated that ZBTB20 mRNA expression levels in human gastric cancer tumor samples (n = 37) were higher than those in the paired adjacent normal gastric tissue samples (n = 37) (). Similarly, ZBTB20 protein expression measured by immunohistochemistry was also upregulated in human gastric cancer tumor samples compared with the paired adjacent normal gastric tissue samples (). Mover, ZBTB20 mRNA expression levels were also increased in patients with H. pylori infection or metastasis (). According to the Kaplan Meier-plotter database, higher ZBTB20 expression also correlated with a poor overall survival of gastric cancer patients ().
Figure 1. ZBTB20 expression in gastric cancer tissues. ZBTB20 expression levels in gastric cancer tissues (n = 37) and their adjacent normal gastric tissues (n = 37) were determined by real-time PCR (A) and immunohistochemistry (B). Scale bars: 100 μm. (C, D) ZBTB20 expression levels in gastric cancer patients with different status of H. pylori infection and metastasis were determined by real-time PCR. (E) Overall survival of gastric cancer patients from Kaplan Meier-plotter database. *p < .05, **p < .01, ***p < .001 compared with adjacent normal, infection, or metastasis group.
ZBTB20 expression is increased by H. pylori infection in gastric cancer cell lines
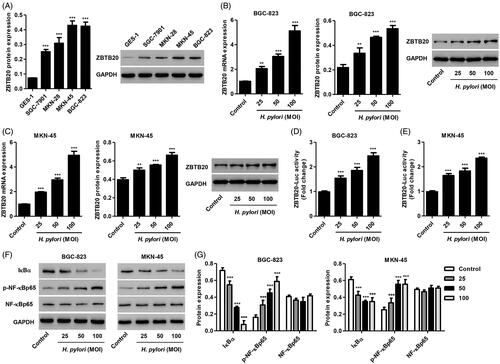
To assess the role of ZBTB20 in gastric cancer development, the expression of ZBTB20 in gastric cancer cell lines was measured. Our data showed that ZBTB20 expression was increased in gastric cancer cell lines compared with GES-1 cells, with the highest expression detected in BGC-823 and MKN-45 cells (). These two cell lines were therefore used for subsequent experiments. Given that H. pylori infection is considered a major risk factor for gastric cancer, the effect of H. pylori infection on the expression of ZBTB20 was further examined in BGC-823 and MKN-45 gastric cancer cell lines. As shown in , H. pylori infection significantly increased the mRNA and protein expression of ZBTB20 in BGC-823 and MKN-45 cells in a dose-dependent manner. Moreover, the transcriptional activation of ZBTB20 upon H. pylori infection was measured by the luciferase reporter gene assay (. To investigate the role of IκBα/NF-κB signaling pathway in gastric cancer, the effect of H. pylori infection on the expression of IκBα, p-NF-κB and NF-κB was also measured. We found that H. pylori infection significantly decreased IκBα expression, increased p-NF-κB levels, but had no effect on NF-κB expression in BGC-823 and MKN-45 cells (). Taken together, these data support the notion that ZBTB20 and IκBα/NF-κB signaling pathway may participant in the occurrence and development of gastric cancer.
Figure 2. Effect of H. pylori infection on the expression of IκBα, p-NF-κBp65 and NF-κBp65 in gastric cancer cells. (A) ZBTB20 expression levels in gastric cancer cell lines and GES-1 human gastric epithelial mucosa cell line were determined by western blot. ZBTB20 expression levels (B, C), transcriptional activation of ZBTB20 (D, E), and the protein expression of IκBα, p-NF-κBp65 and NF-κBp65 (F, G) upon H. pylori infection in BGC-823 and MKN-45 gastric cancer cell lines were determined by real-time PCR, western blot or luciferase reporter gene assay. **p < .01, ***p < .001 compared with control group.
ZBTB20 silencing represses cell proliferation of gastric cancer
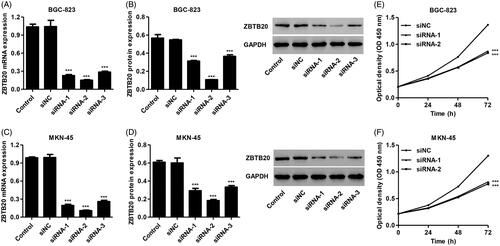
To examine the effect of ZBTB20 on the tumorigenesis of gastric cancer in vitro, BGC-823 and MKN-45 cells were transfected with three siRNAs targeting human ZBTB20 or a scramble siRNA (siNC). Silence of ZBTB20 significant decrease in the expression of ZBTB20 at both mRNA and protein levels. siRNA-1, siRNA-2 and siRNA-3 transfection in BGC-823 cells inhibited ZBTB20 mRNA expression by 78.3%, 85.5% and 72.6% and its protein expression by 42.9%, 80.4% and 33.4%, respectively, compared with siNC (). siRNA-1, siRNA-2 and siRNA-3 transfection in MKN-45 cells inhibited ZBTB20 mRNA expression by 80.5%, 88.9% and 73.8% and its protein expression by 51.3%, 69.4% and 44.7%, respectively, compared with siNC (). Therefore, siRNA-1 and siRNA-2 were used in our in vitro studies.
Figure 3. Effect of ZBTB20 silencing on the proliferation of gastric cancer cell lines. BGC-823 (A, B) and MKN-45 cells (C, D) were transfected with three siRNAs targeting human ZBTB20, and the expression levels of ZBTB20 were determined by real-time PCR and western blot analysis. (E, F) BGC-823 and MKN-45 cells were transfected with siRNA-1 and siRNA-2 targeting human ZBTB20, and cell proliferation was determined by CCK-8. ***p < .001 compared with siNC.
BGC-823 and MKN-45 cells were transfected with siRNA-1 and siRNA-2, and cell proliferation was measured by CCK-8 assay. We found that siRNA-1 and siRNA-2 significantly suppressed the proliferation of BGC-823 cells by 11.2% and 13.6% at 24 h, by 24.9% and 25.6% at 48 h, and by 36.0% and 38.5% at 72 h, respectively, compared with siNC (). siRNA-1 and siRNA-2 also significantly suppressed the proliferation of MKN-45 cells by 12.7% and 15.6% at 24 h, by 25.2% and 27.6% at 48 h, and by 37.6% and 40.4% at 72 h, respectively, compared with siNC ().
ZBTB20 silencing represses cell invasion and migration of gastric cancer
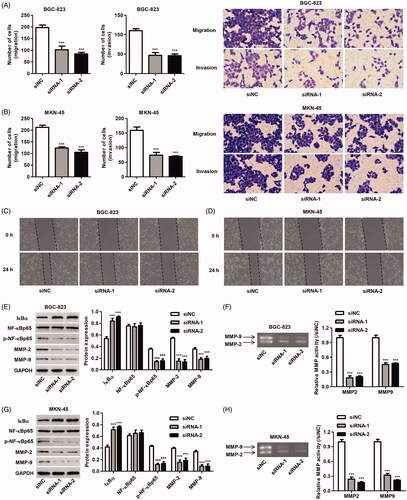
Furthermore, siRNA-1 and siRNA-2 also significantly inhibited the migration of BGC-823 cells by 48.2% and 57.2% and the invasion of BGC-823 cells by 57.4% and 58.3%, respectively, compared with siNC (). siRNA-1 and siRNA-2 also significantly inhibited the migration of MKN-45 cells by 41.8% and 50.5% and the invasion of MKN-45 cells by 53.3% and 56.1%, respectively, compared with siNC (). Compared with siNC, the scratch wound was wider with fewer migrating cells at 24 h after transfected with siRNA-1 and siRNA-2 (). Moreover, the expression of IκBα, p-NF-κBp65 and NF-κBp65 as well as the expression and activity of MMP-2 and MMP-9 were measured by western blotting and Gelatin zymography gel assay. It showed that siRNA-1 and siRNA-2 transfection in BGC-823 and MKN-45 cells significantly reduced the phosphorylation of NF-κBp65 and the expression and activity of MMP-2 and MMP-9, but induced IκBα expression compared with siNC ()).
Figure 4. Effect of ZBTB20 silencing on migration and invasion of gastric cancer cell lines. BGC-823 and MKN-45 cells were transfected with siRNA-1 and siRNA-2 targeting human ZBTB20. Cell migration and invasion were determined by Transwell (A, B) and Wound healing assay (C, D); the protein expression of IκBα, p-NF-κBp65, NF-κBp65, MMP-2 and MMP-9 was determined by western blotting (E, G); and the activity of MMP-2 and MMP-9 was determined by Gelatin zymography gel assay (F, H). ***p < .001 compared with siNC.
ZBTB20 overexpression promotes cell proliferation, invasion and migration of gastric cancer
In addition, recombinant pCDNA3.1(+)-ZBTB20 or blank pCDNA3.1(+) as a negative control was also transduced into BGC-823 and MKN-45 cells. Overexpression of ZBTB20 markedly increased the mRNA and protein expression of ZBTB20 by 3.98-fold and 1.10-fold in BGC-823 cells and by 7.12-fold and 1.61-fold in MKN-45 cells, respectively, compared with the blank vector ()).
Figure 5. Effect of ZBTB20 overexpression on the proliferation of gastric cancer cell lines. BGC-823 and MKN-45 cells were infected with pCDNA3.1(+)-ZBTB20 or blank vector, the expression levels of ZBTB20 were determined by real-time PCR (A, C) and western blot analysis (B, D), and cell proliferation was determined by CCK-8 (E, F). ***p < .001 compared with blank vector.
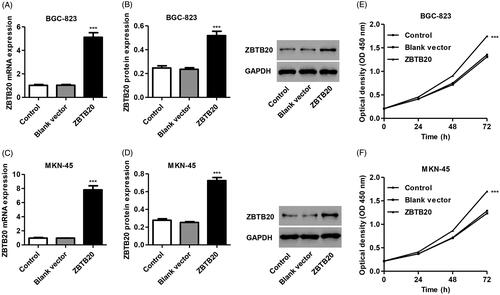
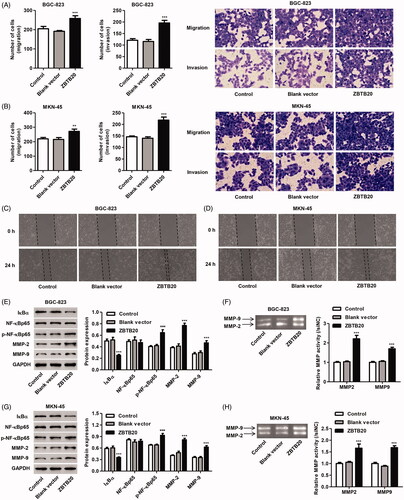
We found that pCDNA3.1(+)-ZBTB20 infection significantly promoted the proliferation of BGC-823 cells by 10.5%, 20.7% and 29.3% and that of MKN-45 cells by 10.4%, 20.6% and 32.3% at 24, 48 and 72 h, respectively, compared with blank vector (). Moreover, pCDNA3.1(+)-ZBTB20 infection significantly promoted the migration and invasion of BGC-823 cells by 26.0% and 62.2% and that of MKN-45 cells by 23.7% and 49.2%, respectively, compared with blank vector (). Compared with blank vector, the scratch wound was closer with more migrating cells at 24 h after infected with pCDNA3.1(+)-ZBTB20 (). As shown in , pCDNA3.1(+)-ZBTB20 infection in BGC-823 and MKN-45 cells significantly increased the phosphorylation of NF-κBp65 and the expression and activity of MMP-2 and MMP-9, but reduced IκBα expression compared with the blank vector.
Figure 6. Effect of ZBTB20 overexpression on the migration and invasion of gastric cancer cell lines. BGC-823 and MKN-45 cells were infected with pCDNA3.1(+)-ZBTB20 or blank vector. Cell migration and invasion were determined by Transwell (A, B) and Wound healing assay (C, D); the protein expression of IκBα, p-NF-κBp65, NF-κBp65, MMP-2 and MMP-9 (E, G) was determined by western blotting; and the activity of MMP-2 and MMP-9 was determined by Gelatin zymography gel assay (F, H). **p < .01, ***p < .001 compared with blank vector.
The involvement of IκBα/NF-κB signaling in the function of ZBTB20
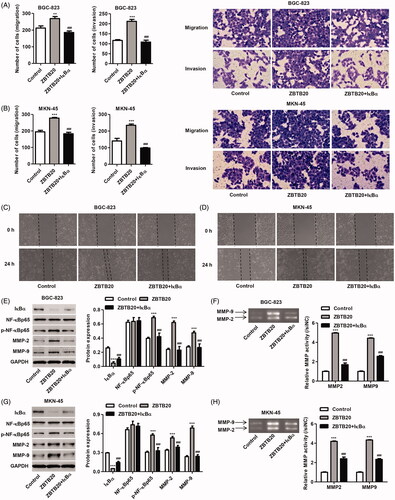
Give the role of ZBTB20 in the regulation of expression of IκBα, NF-κBp65, MMP-9 and MMP-2, we speculate that inhibition of IκBα expression to induce NF-κB activation may involve in the function of ZBTB20 in gastric cancer. Therefore, IκBα expressing vector (pCDNA3.1(+)-IκBα) was constructed in BGC-823 and MKN-45 cells and its effect on NF-κB activation, cell proliferation, migration and invasion was also measured. As shown in , overexpression of IκBα markedly increased the mRNA and protein expression of IκBα by 9.43-fold and 7.19-fold in BGC-823 cells and by 9.78-fold and 0.60-fold in MKN-45 cells, respectively, compared with the blank vector. Moreover, our data also demonstrated that IκBα overexpression significantly inhibited the proliferation, invasion and migration of MKN-45 and BGC-823 cells induced by ZBTB20 overexpression ( and ). We further checked up the phosphorylation of NF-κBp65 as well as the expression of IκBα, NF-κBp65, MMP-2 and MMP-9. As shown in , IκBα overexpression significantly inhibited the phosphorylation of NF-κBp65 and the expression and activity of MMP-2 and MMP-9 in BGC-823 and MKN-45 cells induced by ZBTB20 overexpression, suggesting the inactivation of NF-κB signaling. These data indicate that ZBTB20 promotes invasion, migration and proliferation of gastric cancer cell lines by inhibiting IκBα expression to induce NF-κB activation.
Figure 7. IκBα overexpression inhibits gastric cancer cell proliferation induced by ZBTB20. BGC-823 and MKN-45 cells were infected with pCDNA3.1(+)-IκBα or blank vector, and the expression levels of IκBα were determined by real-time PCR (A, C) and western blotting (B, D). BGC-823 and MKN-45 cells were infected with pCDNA3.1(+)-ZBTB20 and pCDNA3.1(+)-IκBα, and cell proliferation was determined by CCK-8 (E, F). ***p < .001 compared with control; ###p < .001 compared with pCDNA3.1(+)-ZBTB20.
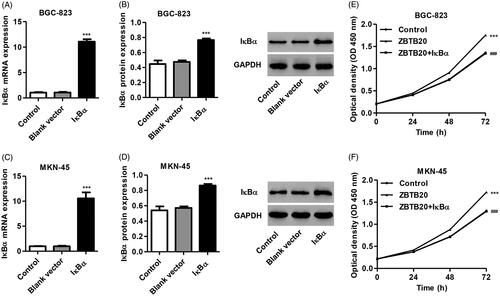
Figure 8. IκBα overexpression inhibits gastric cancer cell migration and invasion induced by ZBTB20. BGC-823 and MKN-45 cells were infected with pCDNA3.1(+)-ZBTB20 and pCDNA3.1(+)-IκBα. Cell migration and invasion were determined by Transwell (A, B) and Wound healing assay (C, D); the protein expression of IκBα, p-NF-κBp65, NF-κBp65, MMP-2 and MMP-9 (E, G) was determined by western blotting; and the activity of MMP-2 and MMP-9 was determined by Gelatin zymography gel assay (F, H). **p < .01, ***p < .001 compared with control; ###p < .001 compared with pCDNA3.1(+)-ZBTB20.
Discussion
Although increasing studies have focused on ZBTB20 and cancer [Citation8–10], the underlying mechanisms of ZBTB20 in cancer initiation remain largely unknown. In this study, we found that ZBTB20 expression is markedly upregulated in gastric cancer tissues and in gastric cancer cell lines infected with H. pylori. ZBTB20 knockdown significantly inhibits cell proliferation, invasion and migration as well as induces IκBα expression to inactivate NF-κB signaling in gastric cancer, while ZBTB20 overexpression induced the inverse effects were inhibited by IκBα overexpression.
Gastric cancer (GC) is the third leading cause of cancer-related deaths worldwide, and H. pylori infection is an important factor in the pathogenesis of gastric cancer. The major mechanisms of H. pylori-associated gastric cancer include perturbed multiple signaling pathways, induced mutations, and accumulated aberrant DNA methylation [Citation14]. In this study, we presented that ZBTB20 expression was significantly upregulated in gastric cancer tissues and in gastric cancer cell lines infected with H. pylori, which suggest that ZBTB20 may involve in the initiation and progression of gastric cancer. Our study evaluated for the first time correlations between H. pylori infection and ZBTB20 expression in gastric cancer cell lines. Similar to our findings, previous studies have demonstrated that ZBTB20 is significantly up-regulated in liver and lung cancer tissues compared with adjacent normal tissues [Citation9,Citation10].
ZBTB20 suppressed apoptosis and induced cell invasion, migration and proliferation of glioblastoma [Citation8]. ZBTB20 promoted cell cycle progression and cell proliferation in hepatocellular carcinoma and non-small cell lung cancer [Citation9,Citation10]. Gastric cancer is characterized by poor prognosis, strong invasion, and early metastasis. Identify new and specific markers for gastric cancer that contributes to this neoplasm at an early stage will improve the poor prognosis. A study showed that the rs9841504 polymorphism in ZBTB20 was statistically susceptible to gastric cancer [Citation15]. Consistently, our data showed that ZBTB20 knockdown in gastric cancer cell lines markedly suppressed cell proliferation, invasion and migration while ZBTB20 overexpression significantly promoted those. However, Shi et al., found a significant association between the rs9841504 polymorphism in ZBTB20 and decreased gastric cancer susceptibility, suggested that the ZBTB20 rs9841504 polymorphism is a protective factor for gastric cancer [Citation11]. Meanwhile, no statistical difference was found between the rs9841504 polymorphism in ZBTB20 and gastric cancer risk in other previous studies [Citation16–18]. It is likely that the contradictory results were due to the relatively small sample sizes and different genetic backgrounds. Therefore, the role of ZBTB20 in regulating gastric cancer process needs to be further investigated.
In cancer, NF-κB is proposed to contribute to oncogenesis through the induction of genes encoding proteins involved in promoting invasion and migration and suppressing apoptosis. Suppression of NF-κB activity through the inhibition of IκBα degradation contributes to the decreased cell migration in triple negative breast cancer [Citation19]. Activating the NF-κB signaling pathway also promoted cell invasion, migration and proliferation in gastric cancer [Citation20]. The NF-κB protein dimer is complexed with IκB protein under resting conditions. After stimulation with cytokines or lipopolysaccharide, IκBα is phosphorylated by upstream IKK (IκB kinases) and degradation, finally resulting in the activation of NF-κB [Citation21]. Consistently, our loss- and gain- of-function studies showed that ZBTB20 knockdown significantly induced IκBα expression, and then resulted in the decrease in phosphorylation of NF-κBp65 in gastric cancer cell lines. Consistent with our findings, and mechanistically, ZBTB20 promotes NF-κB activation through inhibiting IκBα gene transcription and governing IκBα protein expression [Citation12]. Moreover, ZBTB20 promotes hepatocyte proliferation and activation of NF-κB in regenerating liver after partial hepatectomy [Citation13].
In this study, we also found that the expression and activity of MMP-9 and MMP-2 were markedly downregulated in ZBTB20 knockdown cells but upregulated in ZBTB20 ectopic cells. Matrix metalloproteinases (MMPs) are of great importance in regulating cell migration and proliferation. Dysregulation of MMPs is correlated with many diseases including cancer [Citation22,Citation23]. NF-κB has been shown to directly regulate the transcription of MMP-2 and MMP-9 [Citation24]. The upregulation of MMP-2 and MMP-9 expression by activating NF-κB signaling pathway could promote metastasis in hepatocellular carcinoma [Citation25], induce cell proliferation, invasion and migration in gastric cancer in vitro, and promote tumor growth and metastasis in gastric cancer in vivo [Citation23]. Thus, it is indicates that ZBTB20 regulates cell invasion, migration and proliferation in gastric cancer through IκBα/NF-κB signaling pathway.
Conclusion
In summary, we have identified upregulated ZBTB20 expression in gastric cancer cell lines infected with H. pylori and in human gastric cancer tumor samples. ZBTB20 promotes cell invasion, migration and proliferation of gastric cancer through inhibiting IκBα to induce NF-κB activation. These findings demonstrated that ZBTB20 may be an appropriate target for gastric cancer prevention and treatment.
Disclosure statement
There are no competing financial interests in relation to this work.
Additional information
Funding
References
- Rugge M, Meggio A, Pravadelli C, et al. Gastritis staging in the endoscopic follow-up for the secondary prevention of gastric cancer: a 5-year prospective study of 1755 patients. Gut. 2018;68(1):11–17.
- Zhang E, He X, Yin D, et al. Increased expression of long noncoding RNA TUG1 predicts a poor prognosis of gastric cancer and regulates cell proliferation by epigenetically silencing of p57. Cell Death Dis. 2016;7:e2109.
- Juo YY, Gibbons MAM, Dutson E, et al. Obesity is associated with early onset of gastrointestinal cancers in California. J Obes. 2018;2018:7014073
- Hooi JKY, Lai WY, Ng WK, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 2017;153(2):420–429.
- Wang Q, Wang C, Chen J. NLRP6, decreased in gastric cancer, suppresses tumorigenicity of gastric cancer cells. CMAR. 2018;10:6431–6444.
- Wang F, Liu J, Zou Y, et al. MicroRNA-143-3p, up-regulated in H. pylori-positive gastric cancer, suppresses tumor growth, migration and invasion by directly targeting AKT2. Oncotarget. 2017;8:28711–28724.
- Doeppner TR, Herz J, Bahr M, et al. Zbtb20 regulates developmental neurogenesis in the olfactory bulb and gliogenesis after adult brain injury. Mol Neurobiol. 2019;56:567–582.
- Liu J, Jiang J, Hui X, et al. Mir-758-5p suppresses glioblastoma proliferation, migration and invasion by targeting ZBTB20. Cell Physiol Biochem. 2018;48(5):2074–2083.
- Kan H, Huang Y, Li X, et al. Zinc finger protein ZBTB20 is an independent prognostic marker and promotes tumor growth of human hepatocellular carcinoma by repressing FoxO1. Oncotarget. 2016;7(12):14336–14349.
- Zhao JG, Ren KM, Tang J. Zinc finger protein ZBTB20 promotes cell proliferation in non-small cell lung cancer through repression of FoxO1. FEBS Lett. 2014;588(24):4536–4542.
- Shi J, Li W, Ding X. Assessment of the association between ZBTB20 rs9841504 polymorphism and gastric and esophageal cancer susceptibility: a meta-analysis. Int J Biol Markers. 2017;32(1):96–e101.
- Liu X, Zhang P, Bao Y, et al. Zinc finger protein ZBTB20 promotes Toll-like receptor-triggered innate immune responses by repressing IkappaBalpha gene transcription. Proc Natl Acad Sci U S A. 2013;110(27):11097–11102.
- Zhang H, Shi JH, Jiang H, et al. ZBTB20 regulates EGFR expression and hepatocyte proliferation in mouse liver regeneration. Cell Death Dis. 2018;9(5):462.
- Maeda M, Moro H, Ushijima T. Mechanisms for the induction of gastric cancer by Helicobacter pylori infection: aberrant DNA methylation pathway. Gastric Cancer. 2017;20(S1):8–15.
- Shi Y, Hu Z, Wu C, et al. A genome-wide association study identifies new susceptibility loci for non-cardia gastric cancer at 3q13.31 and 5p13.1. Nat Genet. 2011;43(12):1215–1218.
- Sun Y, Gu J, Ajani JA, et al. Genetic and intermediate phenotypic susceptibility markers of gastric cancer in Hispanic Americans: a case-control study. Cancer. 2014;120(19):3040–3048.
- Song HR, Kim HN, Kweon SS, et al. Genetic variations in the PRKAA1 and ZBTB20 genes and gastric cancer susceptibility in a Korean population. Mol Carcinog. 2013;52(S1):155–160.
- Dong Y, Chen J, Chen Z, et al. Evaluating the association of eight polymorphisms with cancer susceptibility in a Han Chinese population. PloS one. 2015;10(7):e0132797.
- Woodcock CS, Woodcock SR, Cao C, et al. Electrophilic nitroalkenes inhibit triple negative breast cancer metastasis. FASEB J. 2016; 30 (1_supplement): 714.7-714.7.
- Wu Y, Zhang J, Zheng Y, et al. miR-216a-3p inhibits the proliferation, migration, and invasion of human gastric cancer cells via targeting RUNX1 and activating the NF-kappaB signaling pathway. Oncol Res. 2018;26(1):157–171.
- DiDonato JA, Mercurio F, Karin M. NF-κB and the link between inflammation and cancer. Immunol Rev. 2012;246(1):379–400.
- Zhang YH, Ma K, Liu JR, et al. Gamma-tocotrienol inhibits the invasion and migration of human gastric cancer cells through downregulation of cyclooxygenase-2 expression. Oncol Rep. 2018;40:999–1007.
- Liu J, Liu Q, Wan Y, et al. Osteopontin promotes the progression of gastric cancer through the NF-kappaB pathway regulated by the MAPK and PI3K. Int J Oncol. 2014;45(1):282–290.
- Kim MS, Park MJ, Kim SJ, et al. Emodin suppresses hyaluronic acid-induced MMP-9 secretion and invasion of glioma cells. Int J Oncol. 2005;27(3):839–846.
- Li J, Lau GK, Chen L, et al. Interleukin 17A promotes hepatocellular carcinoma metastasis via NF-kB induced matrix metalloproteinases 2 and 9 expression. PloS one. 2011;6(7):e21816.
