Abstract
Triple-negative breast cancer (TNBC) stands for a refractory subtype, which predicts poor prognosis and has no effective therapies yet for improving it. Given the restrictions of traditional treatments, novel therapeutic strategies need excavating to alleviate the intrinsic or acquired resistance. Ribociclib, a selective CDK4/6 inhibitor, has successfully prevented cancers from deteriorating by intervening the CDK4/6-cyclin D-Rb-E2F pathway, especially for estrogen receptor-positive (ER +) breast cancer. However, there still remains limited accessibility referring to TNBC. Performing experiments on MDA-MB-231 cells, we found that LEE011 could suppress cell proliferation, and this suppression tended to be dose-dependently. Western blotting analysis presented significant decrease with the expression of CDK4/6 after LEE011 treated, and other proteins associated with this axis such as cyclin D1, p-Rb, Rb, E2F1 showed aberrant changes. Moreover, LEE011 induced G0–G1 phase cell cycle arrest, promoted cell apoptosis, and reduced cell migration in vitro. In addition, tumor growth was remarkably impeded without obvious side-effects in MDA-MB-231 xenograft models. Our research has identified that LEE011 was not completely invalid for MDA-MB-231. Considering its pivotal status in TNBC, the CDK4/6-cyclin D-Rb-E2F pathway informed us the possibility and practicality of Ribociclib (LEE011) as pharmacological intervention, but challenges warrant further validation in prospective studies.
Introduction
Triple-negative breast cancer (TNBC) is characterized by the absence of estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor-2 (HER2), which accounts for 10–20% of all breast carcinomas [Citation1]. TNBC represents a minority but refractory hypotype with extremely finite response and extensive resistance to existing therapeutic schemes, neither endocrinotherapy nor combined chemotherapies have proven efficient [Citation2–4]. Moreover, accompany with improper interventions, both in timing and methods, come earlier relapses and worse outcomes [Citation5]. Hence, more available strategies desire to be explored.
Cell cycle progression undertakes a crucial role in cell proliferation, whose aberration has been acknowledged as a hallmark of cancer [Citation6,Citation7]. The cyclin-dependent kinase (CDK) 4/6-cyclin D-retinoblastoma (Rb)-E2F pathway participates in cell cycle control and mediates G1-S phase transition through CDK4/6-cyclin D complexes. Specifically, cyclin-dependent-kinase 4 (CDK4), together with its homolog cyclin-dependent- kinase 6 (CDK6), belongs to the subfamily of serine-threonine kinases. These CDKs function in a form of cyclin D1-bound compounds at cell cycle checkpoint, and they can promote cell cycle in G1/S phase transition by triggering hyperphosphorylation and deactivation of the tumor suppressor retinoblastoma protein (Rb), and then releasing the E2Fs. What's more, these transcription factors can regulate the downstream genes which link to cell cycle and anti-apoptosis [Citation8]. So it is a potential way to induce cell cycle arrest and apoptosis by targeting these CDKs [Citation9].
Amplification and overactivation of the CDK4/6-cyclin D-Rb-E2F pathway have been observed in various malignancies including breast cancer [Citation10–15]. Considering that the dysfunctional CDKs can drive unscheduled cell cycle progression, inhibitors targeting the combination between CDKs with D-type cyclins might be an imposing strategy [Citation16]. And it turns out to be validated by a great deal of experimental data practically. CDK4/6 inhibitors – the most representative ones – have experienced a vigorous development since first proposed [Citation17–19].
Ribociclib (LEE011) is a highly selective third-generation CDK4/6 inhibitor [Citation20], it works by competitively combining with the ATP-binding sites of CDK4/6 [Citation17]. This mechanism will help block CDK4/6-cyclin D-Rb-E2F axis, reverse uncontrolled cell proliferation and wild tumor growth. With the Food and Drug Administration (FDA) approving of it as first-line endocrine-based therapy for those postmenopausal women with HR-positive, HER2-negative advanced or metastatic breast cancer [Citation21–24], further expansion towards different carcinomas has witnessed its pervasive efficiency as well as endurable toxicity. Although large amounts of pre-clinical and clinical trials based on different TNBC subtypes were conducted, we have identified no explicit target yet [Citation24,Citation25]. For the purpose of solving the question whether Ribociclib can be applied as a cure for TNBC, we performed the current research to evaluated its anticancer impacts in vivo and in vitro and delved into the anti-proliferation mechanisms mediated by Ribociclib.
Materials and methods
Cell lines and cell culture
The non-triple-negative breast cancer (NTNBC) cell line MCF-7 were obtained from the Shanghai Life Science Institute Cell Library (Shanghai, China), the human triple-negative breast cancer (TNBC) cell line MDA-MB-231 and the non-tumorigenic mammary epithelial cell lines MCF-10A was purchased from Procell Life Science &Technology Company (Wuhan, China). These cells were maintained in the Key Laboratory of Tumor Biological Behaviors of Hubei Province. The MDA-MB-231 and MCF-7 cells were cultured in DMEM (Hyclone, USA) containing 10% fetal bovine serum (Wisent, Canada), 1% penicillin/streptomycin. The MCF-10A cells was cultured in DMEM/F12 (Procell, China) containing 5% horse serum, 20 ng/ml EGF, 0.5 μg/ml hydrocortisone, 10 μg/ml insulin, 1% NEAA, 1% penicillin/streptomycin. All cells were incubated in 5% CO2 at 37 °C.
Immunofluorescence assay
The immunofluorescence assay was performed to assess the background expression of CDK4, CDK6 and cyclin D1 between the normal mammary epithelial cells and breast cancer cells. Having MCF10A, MCF-7 and MDA-MB-231 cells cultured in 6-wells plates for 24 h, we processed them with a 15-min fixation 4% paraformaldehyde, 15-min permeabilization with 0.2% Triton X-100 in PBS, and 30-min block with bovine serum albumin (BSA) at room temperature in sequence. These cells were then incubated in a humidified chamber at 4 °C overnight, exposing to the CDK4 primary antibody (1:150 dilution, #A0366, ABclonal, USA) with cyclin D1 primary antibody(1:50 dilution, #AC853, Beyotime, China), or the CDK6 primary antibody (1:150 dilution, #A1545, ABclonal, USA) with cyclin D1 primary antibody, followed by incubation with CY3 (Red) conjugated goat anti-rabbit antibody (1:50 dilution, #AS-1109, Aspen, USA) and FITC (Green) conjugated goat anti-mouse antibody (1:150 dilution, #AS-1112, Aspen, USA) for 2 h. The coverslips were mounted in VECTASHIELD mounting medium with DAPI (#AS1075, Aspen, USA). Finally, cells were imaged on a Nikon Eclipse Ti-U fluorescence microscope (Diagnostic Instruments Inc., USA) equipped with a digital camera.
Pharmacologic treatment
10.0 mM stock solution was produced by dissolving 10 mg of LEE011 powder (M.W.: 434.54 g/mol, HY-15777, MCE) into 2.3013 ml of DMSO. Then the MDA-MB-231 cells were treated with LEE011 at various concentrations (0–20.0 µM) by stepwise dilution, the 0 µM-treatment used 0.1% DMSO as a substitute. For animal experiments, xenografts bearing engrafted tumors of 100–200 mm3 are randomized to oral treatment with 200 mg/kg of LEE011 in 0.5% methylcellulose or vehicle once daily in an 18-day cycle.
Morphological observation assay
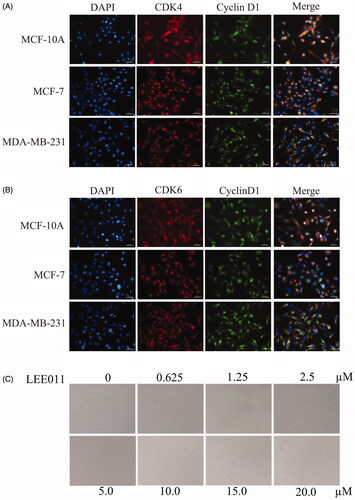
The effect of LEE011 on MDA-MB-231 cells was judged by morphologic changes. With MDA-MB-231 cells seeded into 6-well plates at a density of 30–40% per well and incubated at various concentrations (0, 0.625, 1.25, 2.5, 5.0, 10.0, 15.0, 20.0 µM) of LEE011 for 72 h, these cells were observed from quantity to morphology at the end of the exposure. Cells were finally imaged on a Nikon Eclipse Ti-U fluorescence microscope (Diagnostic Instruments Inc., USA) equipped with a digital camera.
Western blotting
Protein lysates were extracted from MCF-10A, MCF-7, MDA-MB-231 cells with RIPA lysis buffer (#P0013B, Beyotime Biotechnology, China) for 30 min, supplemented with 1× PMSF (#ST506, Beyotime Biotechnology, China) and phosphatase inhibitor tablets (#4906837001, Roche, USA). With concentrations quantified by BCA assay (#23227, Thermo, USA), these protein lysates were to be boiled at 100 °C for 10 min. Protein samples were separated by 10% SDS-PAGE and then transferred to PVDF membranes. After blocking with 5% skim milk in TBST for 2 h, the membranes were incubated with primary antibodies human Rb (1:1000 dilution, #25628–1-AP, Proteintech, China), pRBSer780 (1:1000 dilution, #9307, Cell Signaling Technology, USA), E2F1 (1:1000 dilution, #12171–1-AP, Proteintech, China), cyclin E1 (1:1000 dilution, #A14225, ABclonal, USA), CDK2 (1:1000 dilution, #2546, Cell Signaling Technology, USA), CDK4 (1:1000 dilution, #A0366, ABclonal, USA), CDK6(1:1000 dilution, #A1545, ABclonal, USA), cyclin D1 (1:5000 dilution, #60186–1-lg, Proteintech, China), P16 (1:1000 dilution, #10883–1-AP, Proteintech, China), Bax (1:5000 dilution #50599–2-Ig, Proteintech, China), BCL-2 (1:2000 dilution, #12789–1-AP, Proteintech, China), GAPDH (1:5000, #60004–1-Ig, Proteintech, China) at 4 °C overnight. Fully combined with primary antibodies, a 2-h incubation with HRP-conjugated goat anti-mouse antibody (1:10000, #ANT19, Antgene, China) or HRP-conjugated goat anti-rabbit antibody (1:10000, #ANT020, Antgene, China) was conducted after washing the membranes 5 times for 10 min each with TBST. Specific bands were visualized by ECL (Advansta, USA) and detected with the ChemiDoc XRS + system (Bio-Rad, Hercules, CA, USA). Moreover, the Image J program was performed on protein quantification.
Cell proliferation assay
The effect of LEE011 on cell proliferation and viability was assayed by CCK8 (Dojindo Laboratories, Kumamoto, Japan). The MDA-MB-231 cells were suspended in DMEM with 10% FBS and inoculated in 96-well plates (2000 cells/well). 24 h later, LEE011 was imposed on each well mixed with DMEM containing 2% FBS. Incubated for 0, 24, 48, 72, 96, 120, 144 h, wells were added with 10 µl of CCK8 solution. And another 0–4-h incubation was necessary for sufficient response before measuring OD450 with SpectraMax Absorbance Reader (Molecular Devices, USA).
Colony formation assay
Twenty-four hours prior to the exposure with LEE011/vehicle, 500 MDA-MB-231 cells were transplanted per well into 6-well plates. Wells were treated with indicated concentrations (0, 2.5, 5.0, 10.0 µM) of LEE011 at least for two weeks, and the medium was refreshed twice a week. Cells were then fixed and stained with a solution of 4% paraformaldehyde and 0.5% crystal violet for 30 min severally at room temperature. Colonies greater than or equal to 50 cells were visually identified and counted by two investigators. The surviving fraction of LEE011-treated cells was normalized to the plating efficiency of vehicle-treated cells. All colony formation assays were performed in triplicates.
Cell cycle analysis
After exposure to LEE011 (0, 2.5, 5.0, 10.0, 20.0 µM) for 72 h, the MDA-MB-231 cells were harvested and detected cell cycle instantly. Nuclear staining for cell cycle analysis was performed by propidium iodide (#CCS012, Multi Sciences, China) according to the manufacturer’s instructions. And samples were then detected with their data analyzed by flow cytometry (Cytoflex, Beckman, USA). The above procedures were performed three times in the same conditions.
Apoptosis assay
Cell apoptosis was detected and analyzed by flow cytometry (FACS Aria III, BD, USA) after incubated with LEE011 (0, 2.5, 5.0, 10.0, 20.0 µM) for 72 h. Adherent and suspended cells were both collected for apoptosis analysis, and FITC-conjugated Annexin V/propidium iodide method was used to perform the experiments (Annexin VFITC/PI Apoptosis Kit, Bestbio, China). Every treatment was replicated three times under the same condition.
Wound healing assay
MDA-MB-231 cells were seeded into 6-well plates at a density of 1.0 × 106 cells per well. After cells reached 100% confluency, the adherent cell layer was wounded by scraping three parallel lines with a sterile 10 µl tip. Then, fresh low-serum (2%) DMEM mixed with LEE011 (0, 2.5, 5.0, 10.0 µM) immediately replaced after 2 times of washing with PBS. Wounds were observed and photographed at 0, 24, 48 and 72 h after treatment under a Nikon Eclipse Ti-U fluorescence microscope (Diagnostic Instruments Inc., USA) equipped with a digital camera. The wound width was evaluated by measuring the distance between the two edges of the scratch at five sites in each image. Cell migration was determined using the following formula: Percentage of wound healing (%) = 100% × (wound width at the 0 h time point - wound width at the observed time point)/wound width at the 0 h time point.
In vivo tumor growth
For MDA-MB-231 cell lines xenograft models, 5 × 106 cells were suspended in 100 ml PBS and subcutaneously injected into male nude mice (aged 4–6 weeks). Two to four weeks later, mice bearing engrafted tumors of 100–200 mm3 were randomized to oral treatment with 200 mg/kg LEE011 in 0.5% methylcellulose (n = 4) or vehicle (n = 4) once daily and sustained for 18 days. The body weight of mice and tumor dimension were monitored for 3 weeks. The perpendicular tumor diameters were measured with calipers, and tumor volumes (V) were calculated according to the formula of the rotational ellipsoid: V = A × (B2/2), (A = longer diameter, B = smaller diameter). After treatment, the tumors, eyeball blood and several organs including heart, liver, kidney were totally preserved for next experiments respectively. The animal experiment was approved by the Institutional Animal Care and Use Committee of Wuhan University and executed as Institutional Guidelines and Protocols.
Blood biochemical analysis
Blood samples were prepared with the eyeball blood. Various biochemical indicators (ALT, AST, ALB, ALP, BUN, CR, LDH-L, CK) were detected by different kits (Changchun Huila Biotech Co., Ltd., China) and analyzed by an automatic biochemical analyzer (Chemray240, Rayto, China).
Hematoxylin-Eosin staining of tissues
The tissues sections were mounted on glass slides and treated with hematoxylin-eosin staining solution (#G1005, Servicebio, China) next as protocol read, which instructed staining with hematoxylin for 5 min after routine de-waxing and washing. When the color was separated and the sections were changed from back to blue, dyed with eosin for another 5 min, then dehydrated these samples with increasing concentrations of alcohol, hyalinized with dimethylbenzene, finally sealed with neutral gum for observation under the microscope.
Immunohistochemistry of tumor xenografts
Immunohistochemical staining was performed with primary antibodies on 4 mm-thick formalin-fixed paraffin-embedded (FFPE) tissue sections. The tissue sections were deparaffinized in a graded series of xylene and rehydrated in a graded series of ethanol. Antigens were retrieved in citrate buffer (PH 9.0) using heat-induced epitope retrieval method. To inactivate endogenous peroxidases, the tissue sections were incubated in 3% hydrogen peroxide for 25 min at room temperature and then blocked for 30 min with 3% bovine serum albumin (BSA). The primary antibodies used were CDK4 (1:100 dilution, #A0366, ABclonal, USA), CDK6 (1:100 dilution, #A1545, ABclonal, USA), cyclinD1 (1:100dilution, #A10757, ABclonal, USA), Rb (1:100 dilution, #10048–2-lg, Proteintech, China). Detection was performed under the automatic digital slide scanning and analysis system (Aperio VERSA 8, LEICA, Germany). Tissue slides were visualized in 200/400 × microscope vision. And protein quantification was performed using Image-Pro Plus 6.0 (Media Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
The Data were analyzed statistically by using Student’s t-test. A p values of <.05 was considered statistically significant. Statistical analyses were carried out with IBM SPSS Statistics v20.0 software.
Results
LEE011 suppresses cell proliferation and promotes cell apoptosis in MDA-MB-231
To investigate the impact of Ribociclib on triple-negative breast cancer, we firstly evaluated the expression of CDK4, CDK6, cyclin D1 in TNBC cells (MDA-MB-231), NTNBC cells (MCF-7), non-tumorigenic mammary epithelial cells (MCF-10A) by immunofluorescence. As shown, MDA-MB-231 possessed an abundant expression of CDK4/6 and cyclin D1 compared to MCF-10A, MCF7. ().
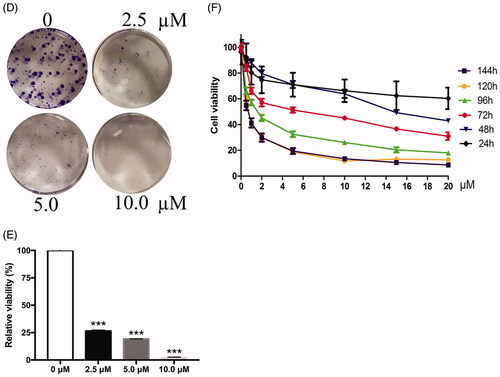
Figure 1. Targeting CDK4/6, LEE011 inhibited cell proliferation in MDA-MB-231. (A,B). Immunofluorescence was performed to evaluate the expression of CDK4, CDK6, cyclin D1 in TNBC cells – MDA-MB-231, NTNBC cells – MCF-7 and non-tumorigenic mammary epithelial cells – MCF-10A. Cells were imaged on a fluorescence microscope equipped with a digital camera: nuclear was dyed with DAPI (blue); CDK4/6 and cyclin D1 appeared in red and green respectively; merging images were listed in the last row. The magnification is 400× (scale bar, 50 µm). (C) MDA-MB-231 cells were treated with increasing concentrations of LEE011 and the morphologic changes of cells were observed by microscopy after 72h-treatment. The magnification is 200× (scale bar, 100 µm). (D) The inhibition of cell proliferation induced by LEE011 on MDA-MB-231 was reflected by cloning efficiency after incubation with diverse concentrations (0, 2.5, 5.0, 10.0 µM) for 2 weeks. (E). All colony formation assays were conducted in triplicates. ***p < .001 compared with the 0 µM group. (F) Cell viability was determined by CCK8 assay after exposure to an escalating dose of LEE011 for 0–144 hours.
With an attempt to testify the effect of LEE011 on MDA-MB-231 cells, obvious abnormity appeared both in cell number and morphology after treated by increasing doses of LEE011 for 72 h (). Visible signs of toxicity such as cells reduction and abnormal cell morphology (including unusual shape and appearance, cellular lysis, and destruction) could be observed in MDA-MB-231 cells after treated with 2.5 µM of LEE011, and changes became more prominent when drug concentration increased up to 20 µM. Further, Western blotting analysis suggested significant downregulation of the CDK4 and CDK6 (), which acted as the specific targets of LEE011. Moreover, the anti-proliferation effect of LEE011 was determined by CCK8 and clonogenic assays simultaneously. After exposure to diverse concentrations, cell viability (and colony formation decreased dose-dependently ().
Figure 2. LEE011 suppressed cell proliferation and promoted apoptosis by inhibiting the CDK4/6-cyclin D-Rb-E2F pathway in MDA-MB-231. CDK4, CDK6, cyclin D1 levels were detected by Western blotting after treatment with LEE011 for 72 hours and the expression of proteins which connected to CDK4/6-cyclin D-Rb-E2F axis, as well as apoptosis, was simultaneously assessed.
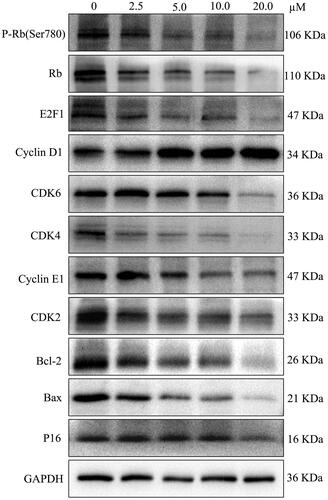
LEE011 inhibits the CDK4/6-cyclin D-Rb-E2F pathway of MDA-MB-231 in a dose-dependent manner
We traced the dynamic changes of protein levels associated with the CDK4/6-cyclin D-Rb-E2F pathway under an escalating dose (0, 2.5, 5.0, 10.0, 20.0 µM) of LEE011 imposed on MDA-MB-231 cells for 72 h. Through western blotting, CDK4, CDK6 and downstream regulators p-Rb, Rb, E2F1, as well as some indirect cell cycle targets CDK2 and cyclin E1, all presented dose-dependent reductions, while cyclin D1 altered in a converse tendency. Moreover, extremely limited changes in P16 proteins have been detected (. In general, these results indicate that LEE011’s antineoplastic activity depends on inhibiting the CDK4/6-cyclin D1-Rb-E2F pathway.
LEE011 induces G1 cell cycle arrest and cell apoptosis in MDA-MB-231
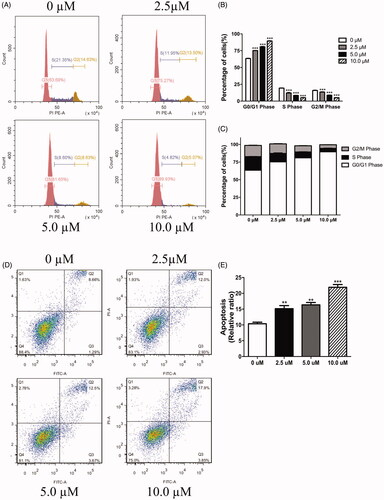
We further assessed the depression effect on cell cycle progression and apoptosis, followed by 72-h exposure to LEE011. After being incubated with different concentrations for 3 days, the FACS analysis of MDA-MB-231 cells witnessed evident anomalies in cell cycle distribution (). When treated with 2.5, 5.0, 10.0 µM of LEE011, G0/G1 portion increased with 11.58%, 17.96%, and 26.24% respectively, S portion decreased with 9.4, 12.72, 16.53%, and G2/M portion concurrently decreased with 1.13%, 5.72%, 9.56% compared with the control. Besides, LEE011-induced apoptosis rate presented a gradual accumulation after cultured at a gradient dose (). Concretely, LEE011 (2.5, 5.0, 10.0 µM) led to 1.5 times (14.93%), 1.6 times (16.17%), and 2.2 times (21.75%) of apoptosis separately than control (9.95%) in MDA-MB-231 cells (p < .01). As drug concentration escalated, cell cycle arrest and cell apoptosis peak increased subsequently.
Figure 3. LEE011 induced cell cycle G1 phase arrest and promoted cell apoptosis in MDA-MB-231. After culturing the MDA-MB-231 cells with increasing concentrations of LEE011 for 72 hours, we applied FACS to evaluate cell proliferation and cell apoptosis. (A–C) Representative images of cell cycle distribution in MDA-MB-231 were displayed after exposure to LEE011 for 72 hours. When treated with 2.5 µM of LEE011, G0/G1 portion increased with 11.58%, S portion decreased with 9.4%, and G2/M portion decreased with 1.13%. In 5.0 µM of LEE011 incubated group, G0/G1 portion increased with 17.96%, S portion decreased with 12.72%, and G2/M portion decreased with 5.72%. Besides, G0/G1 portion increased with 26.24%, S and G2/M portion decreased with 16.53%, 9.56%, respectively in 10.0 µM group compared to the control. Cell numbers of different cell cycle phases in different treatment groups were counted and analyzed statistically. Two-tailed Student’s t-test was utilized to determine statistical significance. Error bars indicate standard deviations. p < .05 was considered significant compared with the 0µM group (*p < .05, **p < .01, ***p < .001). (D,E) Representative images of cell apoptosis in MDA-MB-231 were displayed after 72 hours-LEE011 exposure. Alive cells are shown in the lower left part of the panel (Q4); early apoptotic cells are shown in the lower right part of the panel (Q3); late apoptotic cells are shown in the higher right part of the panel (Q2); necrotic cells are shown in the higher left part of the panel(Q1). LEE011 (2.5, 5.0, 10.0 µM) led to 1.5 times (14.93%), 1.6 times (16.17%), and 2.2 times (21.75%) of apoptosis separately than control (9.95%). Cell apoptosis of different treatment groups were counted and analyzed statistically. Two-tailed Student’s t-test was performed to determine statistical significance. Error bars indicate standard deviations. p < .05 was considered significant compared with the 0 µM group (**p < .01, ***p < .001).
Consistently, CDK2, CDK4, CDK6 and cyclin E1 which mediated G1-S phase transition indicated lower expression. And a dose-dependent reduction in the cell anti-apoptotic protein, Bcl-2, was also observed under LEE011 treatment (.
LEE011 reduces cell migration of MDA-MB-231 in vitro
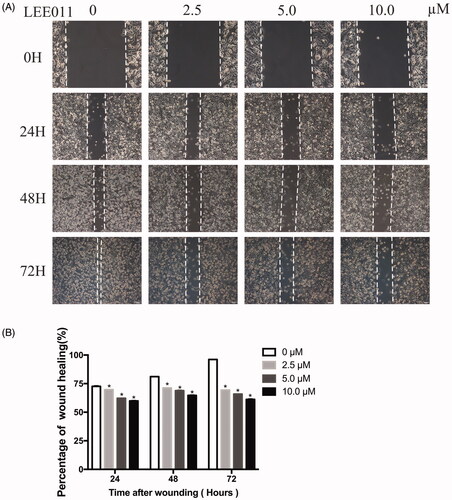
Next, we conducted a wound healing assay to evaluate its damage on migration capabilities caused by LEE011 treatment. After exposure to various concentrations of LEE011 for 24 h 48 h, and 72 h, the wound healing rate has obviously slowed down in a dose-dependent as well as time-dependent manner (). In other words, cell migration activities were significantly stifled when treated with this agent.
Figure 4. LEE011 reduces cell migration of MDA-MB-231 in vitro. Cell migration was determined by wound healing assays after an escalating dose (of LEE011 treatment imposed on MDA-MB-231 cells for the indicated time. (A) Representative images of MDA-MB-231 cell migration after 0, 2.5, 5.0, 10.0 µM LEE011 exposure for 24, 48, 72 hours. The magnification is 200× (scale bar, 100 µm). (B) Cell migration of MDA-MB-231 was measured after LEE011 exposure. Two-tailed Student’s t-test was utilized to determine statistical significance. Error bars indicate standard deviations. p < .05 was considered significant (*p < .05).
LEE011 effectively restrains tumor growth in vivo
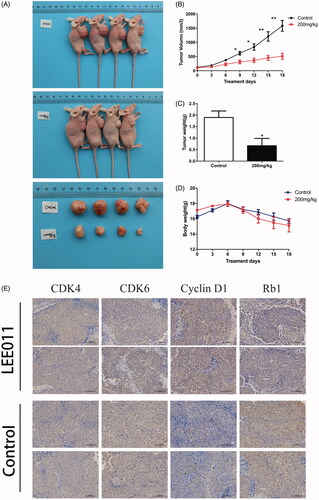
TNBC xenograft models have been established to verify the efficacy of LEE011 in vivo. The xenografts were treated with LEE011 (200 mg/kg) or vehicle once daily for 18 days. LEE011 effectively restrained tumor growth in xenograft models (). Expectedly, there came a striking reduction on tumor volume with LEE011 treated against the vehicle control () as well as tumor weight (). Moreover, a similar effect reappeared in the experimental group when referring to body weight, and a notable loss has been captured from the ninth day of administration (). For another, we demonstrated the inhibition on CDK4/6-cyclin D-Rb-E2F1 pathway by immunohistochemical staining for CDK4, CDK6, cyclin D1 and Rb. We found that the expression of CDK4, CDK6 and Rb was lower in LEE011 treatment group than the control group ().
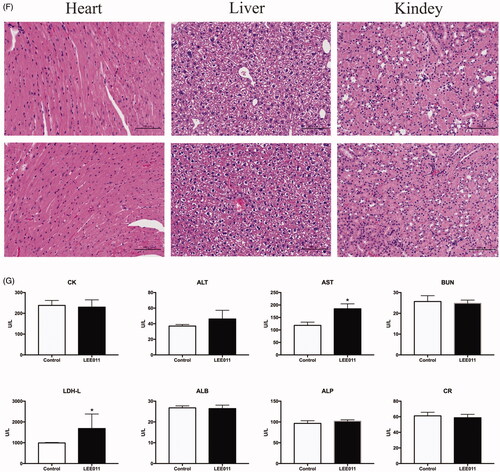
Figure 5. LEE011 effectively restrains tumor growth in vivo. Nude mice bearing engrafted tumors of 100–200 mm3 were randomly assigned to the control (n = 4) or 200 mg/kg-LEE011 treatment group (n = 4). Two-tailed Student’s t-test was performed to determine statistical significance. Error bars indicate standard deviations. p < .05 was considered significant (*p < .05, **p < .01). (A) After implemented euthanasia, tumors were extracted from both of control group and LEE011 treated group. The sizes of tumors extracted from LEE011 group were smaller than the control group in MDA-MB-231 xenograft models. (B–D) 200 mg/kg of LEE011 treatment significantly inhibited tumor growth in MDA-MB-231 xenograft models. (E). Suppression on the CDK4/6-cyclin D-RB-E2F pathway was confirmed by immunohistochemical staining for CDK4, CDK6, cyclin D1 and Rb (scale bar, 100 µm). (F,G) With morphology studied by HE staining of the heart, liver and kidney tissues and the biochemical indicators (ALT, AST, ALB, ALP, BUN, CR, LDH-L, CK) detected from blood samples, no serious adverse effects presented in xenograft models given LEE011 treatment.
Additionally, several blood biochemical indicators (ALT, AST, ALB, ALP, BUN, CR, LDH-L, CK) were encompassed within a safety assessment of LEE011, which involved a comprehensive estimation for heart, liver and kidney in MDA-MB-231 xenografts (). No obvious aberrations were detected in morphology and enzymatic marker of LEE011 treatment group. Above all, these results suggested that LEE011 was an eutherapeutic and secure inhibitor on MDA-MB-231 xenograft mice models.
Discussions
As a refractory type, triple-negative breast cancer has shown great resistance and incredible finiteness to existing treatment regimes. Radiotherapy, chemotherapy or combined endocrinotherapy, these traditional therapeutic strategies seem to be no more sufficient in cancer control. Consequently, many original target waits to be explored. With the approval of FDA and the prosperity of CDK4/6 inhibitors, the powerful effectiveness has been authenticated in various cancers both in preclinical and clinical researches (http://clinicaltrials.gov). However, only a fraction present certain effectiveness to Ribociclib in TNBC according to those ongoing clinical trials, while the majority remain unclear [Citation25,Citation26].
Cyclin D1, as well as cyclin E1, functions as a ‘weak’ oncogene in the mammary epithelium [Citation7]. There is evidence that the accumulation of cyclin D1 in the nucleus could trigger the formation of cyclin D1-CDKs complexes in proliferating cells during G1 phase [Citation27]. Present research based on molecular genetics suggested that CDK4, CDK6 and cyclin D1 prolifically expressed in MDA-MB-231 compared with MCF10A, which has informed us the possibility of Ribociclib - a selective dual CDK4/6 inhibitor - to make difference against TNBC. Then, we conducted a tentative experiment, treated MDA-MB-231 cells with LEE011 at a serious of different concentrations. As expected, cell proliferation was powerfully suppressed and cell apoptosis was strongly stimulated after treatment for 72 h. Furthermore, we have verified and affirmed its inhibition on CDK4/6 targets through western blotting. Therefore, to verdict the effect of LEE011 on CDK4/6-cyclin D1 complexes constituted the next step.
There have been not remarkable morphological or quantitative changes observed until incubated with LEE011 for 3 days. Consequently, we performed all subsequent experiments in vitro after treatment with LEE011 for 72 h. The CCK8 and colony formation assays reconfirmed its anti-proliferation impact. Especially, even at a 2.5 µM-dosage, MDA-MB-231 cells nearly died out. Whether does this mean a hysteresis effect with LEE011? To prolong the drug duration at a lower dosage may be a considerable strategy.
The 72-h treatment on MDA-MB-231 cells with escalating LEE011 led to a dose-dependent decrease in CDK4, CDK6, p-Rb, Rb, E2F1, CDK2 and cyclin E1 level, accompanied by synchronous rise of cyclin D1. View of their established significance in activating the Rb pathway, cyclin-CDK complexes have occupied a predominant status in cell cycle control [Citation7,Citation28]. And LEE011-induced alterations in CDK4/6-cyclin D-Rb-E2F1 axis supported our assumption. We incorporated cyclin E1 detected out of targeted effect, which means the inhibition on Rb-E2F pathway will decrease cyclin E1 and CDK2-cyclin E complexes level [Citation7]. Owing to the following dysregulation in E2F downstream genes FoxM1, CCNA1, Myc [Citation10], this impediment may facilitate G1-S cell cycle arrest and halts cell proliferation ultimately [Citation29]. Additionally, different from what we have presumed [Citation13,Citation30], tiny changes occurred with P16, which could be explained as limited necessity of P16 (CDNK2A) for TNBC [Citation31]. Disruptions upon the CDK4/6-cyclin D-P16-Rb pathway have emerged frequently in many human cancers [Citation32,Citation33], considering its highest negativity with TNBC group [Citation34], we may find it not too hard to contact this two events together.
The FACS analysis furtherly demonstrated its anti-proliferation effect in cell cycle progression with an increasing G1 phase fraction as well as a decreasing S phase fraction. But what confused us was the relative reduction in G1 phase fraction at 20.0 µM against lower dosage (Supplementary Figure). With pharmacologically admitting an optimum dosage in the effective doses level, it made sense that a higher dose didn’t bring a better outcome [Citation35]. A comprehensive assessment for a novel antitumor drug is as much about tolerable cytotoxicity as it is about maximum efficacy, at which several phase I studies on CDK4/6 inhibitors have been put into practice [Citation36–38].
Furthermore, earlier studies have shown that the overactive Rb-E2F pathway will contribute to cancer cell apoptosis [Citation39]. The increasing apoptotic cells through flow cytometry analysis and the decreasing Bcl-2 level (anti-apoptotic protein) by western blotting had verified it. However, the pro-apoptotic protein Bax didn’t express higher as we have observed in cervical cancer [Citation13], but gradually lessened on the contrary. It may be persuasive that LEE011 induced more cell autophagy and senescence than apoptosis in MDA-MB-231 [Citation30,Citation40]. Taken together, cell proliferation suppression and cell apoptosis accumulation induced by LEE011 rest partly on inhibiting the CDK4/6-cyclin D-Rb-E2F1 pathway in vitro.
Next, we examined the anti-tumor activity of LEE011 in MDA-MB-231 xenografts, which suggested a powerful delay in tumor growth. It was a pity that our untreated subjects died on the 18th day because of excessive tumor burden during an established 21-day plan. So, we performed various measurements and tests with samples treated for 18 days. Immunohistochemical analysis indicated the inhibition on CDK4/6-cyclin D-Rb pathway and anti-proliferation, while biochemical analysis showed great safety to the heart, liver, and kidney.
In conclusion, our data has proven that Ribociclib (LEE011) was not completely invalid for MDA-MB-231. LEE011 could suppress proliferation and induce apoptosis of the MDA-MB-231 cells in vitro and in vivo, and this anti-tumor effect, in some way, could be ascribed to the inhibition on CDK4/6-cyclin D-Rb-E2F pathway. Ribociclib may be a promising antineoplastic agent in the future, however, challenges warrant further validation in prospective studies.
Supplementary_figure.pdf
Download ()Acknowledgements
We appreciate all the assistance from Hubei Key Laboratory of Tumor Biological Behaviors in favour of this research.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Brown M, Tsodikov A, Bauer KR, et al. The role of human epidermal growth factor receptor 2 in the survival of women with estrogen and progesterone receptor-negative, invasive breast cancer: the California Cancer Registry, 1999–2004. Cancer. 2008;112(4):737–747.
- Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15):4429–4434.
- Lee A, Djamgoz M. Triple negative breast cancer: Emerging therapeutic modalities and novel combination therapies. Cancer Treat Rev. 2018;62:110–122.
- Gu G, Dustin D, Fuqua SA. Targeted therapy for breast cancer and molecular mechanisms of resistance to treatment. Curr Opin Pharmacol. 2016;31:97–103.
- Agarwal G, Nanda G, Lal P, et al. Outcomes of triple-negative breast cancers (TNBC) compared with non-TNBC: does the survival vary for all stages? World J Surg. 2016;40(6):1362–1372.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674.
- Sutherland RL, Musgrove EA. Cyclins and breast cancer. J Mammary Gland Biol Neoplasia.. 2004;9(1):95–104.
- Turner NC, Liu Y, Zhu Z, et al. Cyclin E1 expression and palbociclib efficacy in previously treated hormone receptor-positive metastatic breast cancer. JCO. 2019;37(14):1169–1178.
- Rader J, Russell MR, Hart LS, et al. Dual CDK4/CDK6 inhibition induces cell-cycle arrest and senescence in neuroblastoma. Clin Cancer Res. 2013;19(22):6173–6182.
- Lee HJ, Lee WK, Kang CW, et al. A selective cyclin-dependent kinase 4, 6 dual inhibitor, Ribociclib (LEE011) inhibits cell proliferation and induces apoptosis in aggressive thyroid cancer. Cancer Lett. 2018;417:131–140.
- Li XY, Seebacher NA, Garbutt C, et al. Inhibition of cyclin-dependent kinase 4 as a potential therapeutic strategy for treatment of synovial sarcoma. Cell Death Dis.. 2018;9(5):446–459.
- Iyengar M, O’Hayer P, Cole A, et al. CDK4/6 inhibition as maintenance and combination therapy for high grade serous ovarian cancer. Oncotarget. 2018;9(21):15658–15672.
- Xiong YD, Li TQ, Assani G, et al. Ribociclib, a selective cyclin D kinase 4/6 inhibitor, inhibits proliferation and induces apoptosis of human cervical cancer in vitro and in vivo. Biomed Pharmacother. 2019;112:108602
- Naz S, Sowers A, Choudhuri R, et al. Abemaciclib, a selective CDK4/6 inhibitor, enhances the radiosensitivity of non-small cell lung cancer in vitro and in vivo. Clin Cancer Res. 2018;24(16):3994–4005.
- Olmez I, Brenneman B, Xiao AZ, et al. Combined CDK4/6 and mTOR inhibition is synergistic against glioblastoma via multiple mechanisms. Clin Cancer Res.. 2017;23(22):6958–6968.
- Dean JL, McClendon AK, Knudsen ES. Modification of the DNA damage response by therapeutic CDK4/6 inhibition. J Biol Chem. 2012;287(34):29075–29087.
- Fang H, Huang D, Yang F, et al. Potential biomarkers of CDK4/6 inhibitors in hormone receptor-positive advanced breast cancer. Breast Cancer Res Treat. 2018;168(2):287–297.
- Rondla R, PadmaRao LS, Ramatenki V, et al. Selective ATP competitive leads of CDK4: Discovery by 3D-QSAR pharmacophore mapping and molecular docking approach. Comput Biol Chem. 2017;71:224–229.
- Rocca A, Schirone A, Maltoni R, et al. Progress with palbociclib in breast cancer: latest evidence and clinical considerations. Ther Adv Med Oncol.. 2017;9(2):83–105.
- Poratti M, Marzaro G. Third-generation CDK inhibitors: a review on the synthesis and binding modes of palbociclib, ribociclib and abemaciclib. Eur J Med Chem. 2019;172:143–153.
- Beaver JA, Amiri-Kordestani L, Charlab R, et al. FDA Approval: Palbociclib for the Treatment of Postmenopausal Patients with Estrogen Receptor-Positive, HER2-Negative Metastatic Breast Cancer. Clin Cancer Res. 2015;21(21):4760–4766.
- Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25–35.
- Walker AJ, Wedam S, Amiri-Kordestani L, et al. FDA approval of palbociclib in combination with fulvestrant for the treatment of hormone receptor-positive, HER2-negative metastatic breast cancer. Clinic Cancer Res. 2016;22(20):4968–4972.
- Kwapisz D. Cyclin-dependent kinase 4/6 inhibitors in breast cancer: palbociclib, ribociclib, and abemaciclib. Breast Cancer Res Treat. 2017;166(1):41–54.
- Asghar US, Barr AR, Cutts R, et al. Single-cell dynamics determines response to CDK4/6 Inhibition in triple-negative breast cancer. Clin Cancer Res. 2017;23(18):5561–5572.
- Finn RS, Dering J, Conklin D, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11(5):R77.
- Matsushime H, Quelle DE, Shurtleff SA, et al. D-type cyclin-dependent kinase-activity in mammalian-cells. Mol Cell Biol. 1994;14(3):2066–2076.
- Sherr CJ, Beach D, Shapiro GI. Targeting CDK4 and CDK6: from discovery to therapy. Cancer Discov. 2016;6(4):353–367.
- Zhang YX, Sicinska E, Czaplinski JT, et al. Antiproliferative effects of CDK4/6 inhibition in CDK4-amplified human liposarcoma in vitro and in vivo. Mol Cancer Ther. 2014;13(9):2184–2193.
- Tao YF, Wang NN, Xu LX, et al. Molecular mechanism of G1 arrest and cellular senescence induced by LEE011, a novel CDK4/CDK6 inhibitor, in leukemia cells. Cancer Cell Int. 2017;17(1):35.
- Russo AA, Tong L, Lee JO, et al. Structural basis for inhibition of the cyclin-dependent kinase Cdk6 by the tumour suppressor p16INK4a. Nature. 1998;395(6699):237–243.
- Arima Y, Hayashi N, Hayashi H, et al. Loss of p16 expression is associated with the stem cell characteristics of surface markers and therapeutic resistance in estrogen receptor-negative breast cancer. Int J Cancer. 2012;130(11):2568–2579.
- Tripathy D, Bardia A, Sellers WR. Ribociclib (LEE011): mechanism of action and clinical impact of this selective cyclin-dependent kinase 4/6 inhibitor in various solid tumors. Clin Cancer Res. 2017;23(13):3251–3262.
- Shin E, Jung WH, Koo JS. Expression of p16 and pRB in invasive breast cancer. Int J Clinic Exp Pathol. 2015;8(7):8209–8217.
- Wong HH, Halford S. Dose-limiting toxicity and maximum tolerated dose: still fit for purpose? Lancet Oncol. 2015;16(13):1287–1288.
- Seront E, Schmitz S, Papier M, et al. Phase 1 study evaluating the association of the cyclin-dependent kinase 4/6 inhibitor ribociclib and cetuximab in recurrent/metastatic p16-negative squamous cell carcinoma of the head and neck. Front Oncol. 2019;9:155.
- Doi T, Hewes B, Kakizume T, et al. Phase I study of single-agent ribociclib in Japanese patients with advanced solid tumors. Cancer Sci. 2018;109(1):193–198.
- Flaherty KT, LoRusso PM, DeMichele A, et al. Phase I, dose-escalation trial of the oral cyclin-dependent kinase 4/6 inhibitor PD 0332991, administered using a 21-day schedule in patients with advanced cancer. Clinic Cancer Res. 2012;18(2):568–576.
- Day ML, Foster RG, Day KC, et al. Cell anchorage regulates apoptosis through the retinoblastoma tumor suppressor/E2F pathway. J Biol Chem. 1997;272(13):8125–8128.
- Valenzuela CA, Vargas L, Martinez V, et al. Palbociclib-induced autophagy and senescence in gastric cancer cells. Exp Cell Res. 2017;360(2):390–396.