Abstract
Immune hepatic injury is a liver disease closely related to an immune imbalance of T cells and macrophages. Our previous series of studies have demonstrated that taraxasterol isolated from Taraxacum possesses great anti-inflammatory and immunomodulatory effects in vivo and in vitro. In this study, we explored the preventive effects of taraxasterol and its underlying mechanisms on concanavalin A (Con A)-induced acute hepatic injury in mice. It was found that treatment with taraxasterol significantly decreased the Con A-induced increase of liver index, serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) and hepatic malondialdehyde (MDA) levels, and increased the Con A-induced decrease of hepatic glutathione (GSH) and superoxide dismutase (SOD) production. Taraxasterol also significantly inhibited the release of pro-inflammatory cytokines tumour necrosis factor-α (TNF-α), interleukin-6 (IL-6), IL-1β, interferon-γ (IFN-γ) and IL-4. In addition, treatment with taraxasterol alleviated the hepatic histopathological injury and apoptosis induced by Con A. Furthermore, taraxasterol dramatically down-regulated the expressions of T toll-like receptor (TLR2), TLR4 and nuclear factor-κappaB (NF-κB) p65, and decreased the expression ratio of Bax/Bc1-2 in hepatic tissues. These findings suggest that taraxasterol prevents Con A-induced acute hepatic injury in mice by inhibiting TLRs/NF-κB inflammatory signalling pathway and promoting Bax/Bc1-2 anti-apoptotic signalling pathway.
Introduction
Immune-mediated hepatic injury is a liver disease closely related to immune imbalance and mainly induced by autoimmune attack and viral infections of hepatocytes. Recently, the incidence of immune hepatic injury has increased rapidly as a global health problem; it can progress into cirrhosis or even death. Concanavalin A (Con A), a lectin extracted from the fresh seeds of jack bean, can activate T cells and macrophages. The activation of T cells and macrophages leads to the release of a large number of inflammatory cytokines including tumour necrosis factor-α (TNF-α), interleukin-6 (IL-6), interferon-γ (IFN-γ) and IL-4. These inflammatory cytokines involve in hepatitis development and cause apoptosis and necrosis of hepatocytes [Citation1]. Thus, Con A is widely used to establish an experimental mouse model of immune-mediated hepatic injury that mimics viral and autoimmune hepatitis in humans and study the pathogenesis and clinical treatment of human immune hepatitis [Citation2,Citation3].
Currently, drugs such as immunosuppressants and corticosteroids are widely used for the main treatments for immune-mediated hepatic injury, but these drugs are accompanied by the extensive tolerance and significant side effects [Citation4]. Accordingly, great attraction is paid to the use of natural products as new therapeutic and preventive drugs against immune-mediated hepatic injury due to their high efficiency, high safety, and low cost. Some compounds of natural products have been investigated to possess protective effects on immune hepatic injury [Citation5–7].
Taraxacum (dandelion) is the dry whole herb of Taraxacum mongolicum Hand.-Mazz., Taraxacum borealisinense Kitam. or same genus plants. It is an edible and medicinal composite plant and widely used in traditional oriental food and medicine for its beneficial free radical scavenging, anti-bacterial, antioxidant, anti-inflammatory, anti-neoplastic, anti-atherosclerotic and hepatoprotective activities [Citation8–11]. Taraxasterol is a pentacyclic-triterpene component isolated from Taraxacum. Our series of studies have shown that taraxasterol has great anti-inflammatory effects in vitro and in vivo. Namely, taraxasterol exerts the in vitro anti-inflammatory and immunomodulatory activity by inhibiting inflammatory cytokine and mediator production via regulating NF-κB and MAPKs signalling pathways [Citation12,Citation13], and exhibits the in vivo protective effects in various animal models of inflammation including LPS-induced mouse endotoxic shock [Citation14], ovalbumin-induced mouse allergic asthma [Citation15] and adjuvant-induced rat arthritis [Citation16]. Our recent study has also shown that taraxasterol possesses the protective effects on alcoholic liver injury in mice by exerting anti-oxidative stress and anti-inflammatory response via CYP2E1/Nrf2/HO-1 and NF-κB signalling pathways [Citation17]. However, it is unknown whether taraxasterol possesses protective effects against immune-mediated hepatic injury and its underlying mechanisms. Accordingly, the aim of this study was undertaken to explore the potential protective effects and mechanisms of taraxasterol against immune-mediated hepatic injury induced by Con A in mice.
Materials and methods
Animals
Male ICR mice (weighing 18–22 g) were purchased from Changchun Yisi Experiment Animals Co. Ltd. (Certificate no. SCXK (J) 2003–0008, Changchun, Jilin, China). All mice were kept under controlled experimental conditions (room temperature 23 ± 1 °C, relative humidity 50 ± 5%) with a normal day/night cycle, and maintained food and water ad libitum. Before this experiment, the mice were adapted to the experimental environment for at least 1 week. All animal experimental procedures were carried out in accordance with the guidelines of the Animal Care Committee of Yanbian University (Certificate no. SCXK (J) 2011–0007, Yanji, Jilin, China).
Drugs and reagents
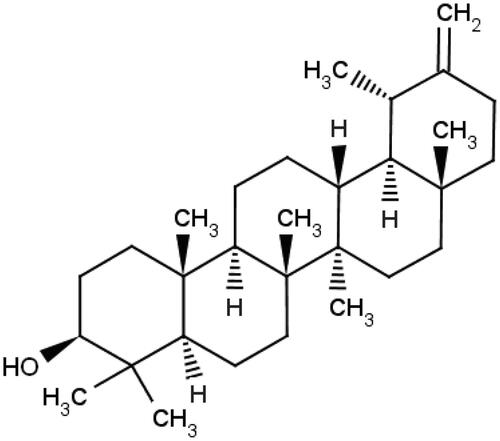
Taraxasterol () was supplied from Chengdu Fenruisi Biotechnology Co. Ltd. (Chengdu, Sichuan, China), and its purity was 99.5% based on HPLC analysis. Bifendate (Bif, No.036140404) was purchased from Xincang Pharmaceutical Co. Ltd. (Xincang, Zhejiang, China). Con A was purchased from Sigma–Aldrich (St. Louis, MO, USA). Alanine aminotransferase (ALT), aspartate aminotransferase (AST), malondialdehyde (MDA), glutathione (GSH) and superoxide dismutase (SOD) commercial reagent kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, Jiangsu, China). Mouse TNF-α, IL-6, IL-1β, IL-4 and IFN-γ ELISA kits were purchased from BioLegend Inc. (San Diego, CA, USA). Terminal deoxynucleotidy1 transferase (TdT)-mediated dUTP-biotin nick end labelling (TUNEL) apoptosis assay kit and chemiluminescent (ECL) kit were purchased from Beyotime Biotech (Shanghai, China). Antibodies against TLR2, TLR4, NF-κB p65, Bax and Bc1-2 were purchased from Cell Signaling Technology Inc. (Danvers, MA, USA). Antibodies against β-actin and peroxidase-conjugated secondary antibody were purchased from Santa Cruz (Santa Cruz, CA, USA).
Establishment of con A-induced hepatic injury model and experimental design
The model of Con A-induced acute hepatic injury in mice was established as previously described [Citation2,Citation18]. The mice were randomly divided into six groups as follows (n = 10 each group): normal group, Con A group, taraxasterol 10 mg/kg + Con A group, taraxasterol 5 mg/kg + Con A group, taraxasterol 2.5 mg/kg + Con A group and Bif 200 mg/kg + Con A group. The mice in taraxasterol groups were orally administered with taraxasterol at doses of 10, 5 and 2.5 mg/kg in 0.5% carboxymethyl cellulose sodium (CMC-Na) once a day for 7 d. The doses of taraxasterol used in this study were based on our series of in vivo studies [Citation14–17]. The mice in Bif group were orally administered with 200 mg/kg of Bif as a positive control. The mice in normal and Con A groups were orally administered with equal volume of 0.5% CMC-Na. On day 7, one hour after the last drug administration, the mice were injected a single dose of Con A (18 mg/kg) by tail vein except for normal group. After 8 h, the blood was collected from retro-orbital plexus; the mice were killed by cervical dislocation; the hepatic tissues were immediately collected. All samples were used to measure the following series of indicators.
Measurement of liver index
Eight hours after Con A injection, the mice were weighed, then the livers were quickly removed and weighed. The liver index was calculated by the formula: relative liver index (%) = liver weight/mouse body weight × 100%.
Measurement of serum enzyme ALT and AST
Blood samples were collected and sera were separated by centrifugation. The activities of serum enzyme ALT and AST were measured by using commercial kits according to the manufacturer’s instructions.
Measurement of hepatic MDA, GSH and SOD
Hepatic tissues were homogenized in normal saline and prepared 10% hepatic homogenate, and the supernatants were separated by centrifugation. The levels of hepatic MDA, GSH and SOD were measured by using commercial kits according to the manufacturer’s instructions. The protein concentrations in livers were measured with BCA protein assay reagent kit. The values were normalized to total protein in hepatic tissues.
Measurement of serum cytokines
Blood samples were collected and sera were separated by centrifugation. The levels of serum cytokines including TNF-α, IL-6, IL-1β, IFN-γ and IL-4 were determined by commercial sandwich ELISA kits according to the manufacturer’s instructions.
Observation of liver histology by H&E and hepatocyte apoptosis by TUNEL
The hepatic specimens were preserved in 10% neutral phosphate-buffered formalin solution and embedded in paraffin. The specimens were sectioned into 4 or 5 µm thickness and stained with haematoxylin and eosin (H&E). For TUNEL staining, the hepatic tissue sections were performed by using a commercial TUNEL apoptosis assay kit according to the manufacturer’s instructions. All sections were observed under a light microscope in a blinded manner.
Western blot analysis
The hepatic tissues were ground, lysed and centrifuged. The protein concentration was determined by BCA protein assay reagent kit according to the manufacturer’s instructions. The equal amounts of protein samples were separated by 10% SDS-PAGE gel electrophoresis, then transferred onto polyvinylidene fluoride (PVDF) membranes. The membranes were blocked with 5% non-fat milk for 2 h, and incubated with primary antibodies against TLR2, TLR4, NF-κB p65, Bax, Bc1–2 and β-actin in Tween 20/Tris-buffered saline (TTBS) for overnight at 4 °C, then incubated with secondary antibodies for 1 h at room temperature. The protein bands were visualized with the ECL reagent according to the manufacturer’s instructions. Densitometry analysis of all bands was performed using Image Lab software (Bio-Rad, Richmond, CA, USA).
Statistical analysis
All values are expressed as the means ± SEMs. One-way analysis of variance (ANOVA) and Student’s t-test were used to assess the differences between the groups. The statistical analyses were conducted using SPSS 17.0 software (SPSS, China). p Values <.05 was considered significant.
Results
Effect of taraxasterol on liver index in con A-induced acute hepatic injury
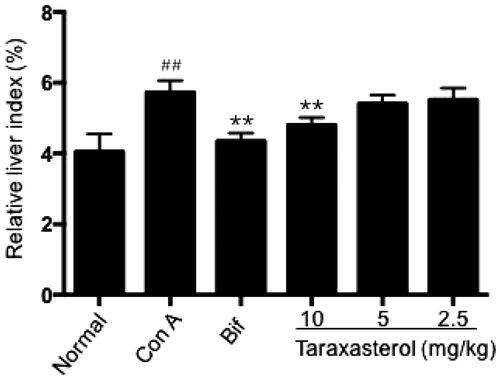
After Con A injection, the liver index of mice dramatically increased compared with normal group as shown in (p < .01); it indicated that Con A injection could induce hepatomegaly of mice. Treatment with taraxasterol at 10 mg/kg significantly decreased the liver index compared with Con A group and relieved Con A-induced hepatomegaly (p < .01). Taraxasterol at 5 and 2.5 mg/kg also decreased the liver index, but the decreasing trend of liver index had no significant difference compared with Con A group (p>.05). It showed that taraxasterol could alleviate hepatomegaly of mice with Con A-induced hepatic injury in a dose-dependent manner.
Figure 2. Effect of taraxasterol on liver index in Con A-induced acute hepatic injury. The mice were treated with taraxasterol (10, 5 and 2.5 mg/kg, respectively) or Bif and injected a single dose of Con A. The hepatic tissues were removed and weighed; the liver index was calculated. The values are expressed as the means ± SEMs. ##p < .01 vs. normal group; **p < .01 vs. Con A group.
Effects of taraxasterol on serum enzyme ALT and AST levels in con A-induced acute hepatic injury
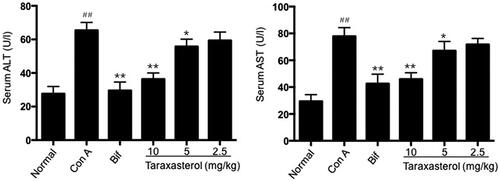
Serum enzyme ALT and AST were standard parameters for assessing hepatic function. As shown in , Con A injection resulted in a rapid increase of serum ALT and AST levels compared with normal group (p < .01). However, treatment with taraxasterol at 10 and 5 mg/kg significantly decreased serum ALT and AST levels compared with Con A group (p < .01 or p < .05). Treatment with taraxasterol at 2.5 mg/kg also decreased serum ALT and AST levels, but there was no significant difference compared with Con A group (p>.05). It indicated that treatment with taraxasterol could effectively inhibit the increase of serum ALT and AST levels induced by Con A in a dose-dependent manner.
Figure 3. Effects of taraxasterol on serum ALT and AST levels in Con A-induced acute hepatic injury. The mice were treated with taraxasterol (10, 5 and 2.5 mg/kg, respectively) or Bif and injected a single dose of Con A. Serum ALT and AST levels were determined by commercial reagent kits. The values represent the means ± SEMs and are expressed as U/l of sera. ##p < .01 vs. Normal group; *p < .05, **p < .01 vs. Con A group.
Effects of taraxasterol on hepatic MDA, GSH and SOD production in con A-induced acute hepatic injury
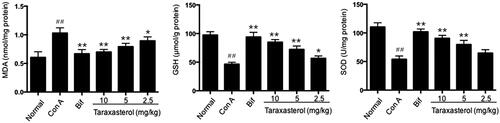
Antioxidant activity of taraxasterol against oxidative stress induced by Con A was assessed by testing the production of MDA, GSH and SOD in hepatic tissues. As shown in , Con A injection induced an obvious increase of MDA production, while an obvious decrease of GSH and SOD production in hepatic tissues compared with normal group (p < .01). Compared with Con A group, treatment with taraxasterol at 10, 5 and 2.5 mg/kg obviously reduced MDA production in a dose-dependent manner (p < .01 or p < .05). Compared with Con A group, treatment with taraxasterol at 10, 5 and 2.5 mg/kg obviously promoted GSH production in a dose-dependent manner (p < .01 or p < .05). Meantime, treatment with taraxasterol at 10 and 5 mg/kg obviously elevated SOD production compared with Con A group (p < .01), and taraxasterol at 2.5 mg/kg also elevated SOD production but had no significant difference compared with Con A group (p>.05). The data indicated that treatment with taraxasterol could improve antioxidant activity in Con A-induced hepatic injury by decreasing the Con A-induced increase of MDA production and promoting the Con A-induced decrease of GSH and SOD production.
Figure 4. Effects of taraxasterol on hepatic MDA, GSH and SOD production in Con A-induced acute hepatic injury. The mice were treated with taraxasterol (10, 5 and 2.5 mg/kg, respectively) or Bif and injected a single dose of Con A. Hepatic MDA, GSH and SOD production was determined by commercial reagent kits. The values represent the means ± SEMs and are expressed as nmol/mg of protein, μmol/g of protein and U/mg of protein, respectively. ##p < .01 vs. normal group; *p < .05, **p < .01 vs. Con A group.
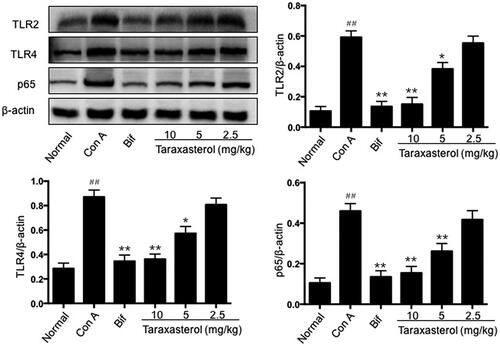
Effects of taraxasterol on serum cytokine release in con A-induced acute hepatic injury
The major pro-inflammatory cytokines involved in the progression of hepatic injury were detected by ELISA method. As shown in , Con A significantly promoted the release of serum cytokines TNF-α, IL-6, IL-1β, IFN-γ and IL-4 compared with the normal group (p < .01). However, treatment with taraxasterol at 10 and 5 mg/kg significantly suppressed TNF-α, IL-6, IL-1β and IL-4 release compared with Con A group (p < .01 or p < .05), and treatment with taraxasterol at 10, 5 and 2.5 mg/kg significantly suppressed IFN-γ release compared with Con A group (p < .01 or p < .05). Treatment with taraxasterol at 2.5 mg/kg also suppressed TNF-α, IL-6, IL-1β and IL-4 release, but there was no significant difference compared with Con A group (p>.05). It indicated that treatment with taraxasterol could effectively inhibit the release of the main pro-inflammatory cytokines induced by Con A in a dose-dependent manner.
Figure 5. Effects of taraxasterol on serum TNF-α, IL-6, IL-1β, IFN-γ and IL-4 release in Con A-induced acute hepatic injury. The mice were treated with taraxasterol (10, 5 and 2.5 mg/kg, respectively) or Bif and injected a single dose of Con A. Serum TNF-α, IL-6, IL-1β, IFN-γ and IL-4 release was determined by ELISA kits. The values represent the means ± SEMs and are expressed as pg/ml of sera. ##p < .01 vs. normal group; *p < .05, **p < .01 vs. Con A group.
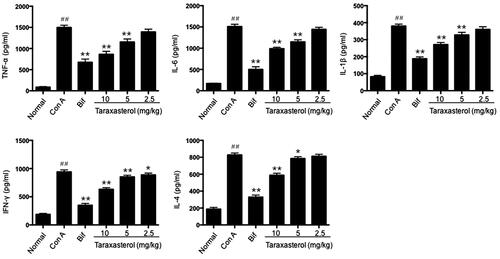
Effects of taraxasterol on hepatic histological alteration and hepatocyte apoptosis in con A-induced acute hepatic injury
To directly and further evaluate the preventive effects of taraxasterol on Con A-induced acute hepatic injury, hepatic histological alternation by H&E staining and hepatocyte apoptosis by TUNEL staining were determined. H&E staining showed that the structures of hepatic tissues were complete and hepatocytes were well-arranged in normal group (. In contrast, Con A group showed apparent inflammatory cell infiltration and massive areas of necrosis (. However, treatment with taraxasterol and Bif obviously alleviated these pathological changes to differing extent, especially in taraxasterol 10 mg/kg and Bif groups (. A small amount of inflammatory cell infiltration and necrosis were found in taraxasterol 5 mg/kg and 2.5 mg/kg groups (. TUNEL staining showed no hepatocyte apoptosis in normal group (. However, typical apoptotic cells were observed in the hepatic tissues in Con A group (, dark brown colour represented apoptotic cells). Treatment with drugs dramatically attenuated hepatocyte apoptosis induced by Con A, especially in taraxasterol 10 mg/kg, 5 mg/kg and Bif groups (), only a small amount of apoptosis was found in taraxasterol 2.5 mg/kg group (.
Figure 6. Effects of taraxasterol on hepatic histological alteration in Con A-induced acute hepatic injury (200×). The mice were treated with taraxasterol (10, 5 and 2.5 mg/kg, respectively) or Bif and injected a single dose of Con A. Hepatic histological alteration was observed under the optical microscope by H&E staining. (a) Normal group; (b) Con A group; (c) Bif + Con A group; (d) Taraxasterol 10 mg/kg + Con A group; (e) Taraxasterol 5 mg/kg + Con A group; (f) Taraxasterol 2.5 mg/kg + Con A group.
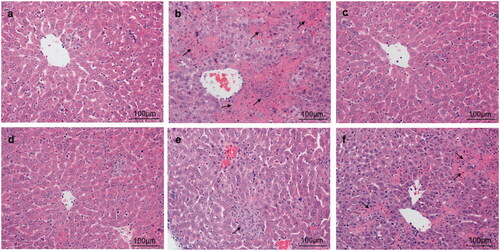
Figure 7. Effects of taraxasterol on hepatocyte apoptosis in Con A-induced acute hepatic injury (200×). The mice were treated with taraxasterol (10, 5 and 2.5 mg/kg, respectively) or Bif and injected a single dose of Con A. Hepatocyte apoptosis was observed under the optical microscope by TUNEL staining. (a) Normal group; (b) Con A group; (c) Bif + Con A group; (d) Taraxasterol 10 mg/kg + Con A group; (e) Taraxasterol 5 mg/kg + Con A group; (f) Taraxasterol 2.5 mg/kg + Con A group.
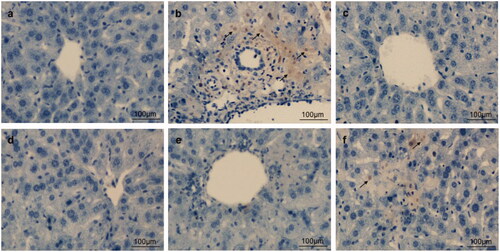
Effects of taraxasterol on hepatic TLR2, TLR4 and NF-kB p65 expressions in con A-induced acute hepatic injury
In TLRs/NF-κB inflammatory signalling pathway, the expressions of main protein TLR2, TLR4 and nuclear NF-kB p65 were analyzed by Western blot. The results were as shown in ; Con A injection remarkably enhanced the levels of hepatic TLR2, TLR4 and nuclear NF-kB p65 expressions compared with the normal group, respectively (p < .01). In contrast, treatment with taraxasterol at 10 and 5 mg/kg remarkably down-regulated the levels of TLR2, TLR4 and nuclear NF-kB p65 expressions compared with Con A group, respectively (p < .01 or p < .05). Treatment with taraxasterol at 2.5 mg/kg did not remarkably down-regulated the expressions of these proteins compared with Con A group (p > .05). It indicated that treatment with taraxasterol could suppress inflammatory response by down-regulating the expressions of TLR2, TLR4 and nuclear NF-kB p65 proteins in TLRs/NF-κB inflammatory signalling pathway.
Figure 8. Effects of taraxasterol on hepatic TLR2, TLR4 and NF-kB p65 expressions in Con A-induced acute hepatic injury. The mice were treated with taraxasterol (10, 5 and 2.5 mg/kg, respectively) or Bif and injected a single dose of Con A. Hepatic TLR2, TLR4 and NF-kB p65 expressions were measured by Western blot analysis. The relative TLR2, TLR4 and NF-kB p65 expressions were normalized to β-actin. The values represent the means ± SEMs. ##p < .01 vs. normal group; *p < .05, **p < .01 vs. Con A group.
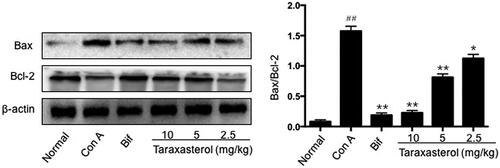
Effects of taraxasterol on hepatic Bax and Bc1-2 expression in con A-induced acute hepatic injury
The expressions of apoptotic protein Bax and anti-apoptotic protein Bc1-2 were analyzed by Western blot, and the ratio of Bax and Bc1-2 was calculated. As shown in , Con A injection caused a significant up-regulation of Bax expression and a down-regulation of Bc1-2 expression, and the ratio of Bax/Bc1-2 was significantly higher than that of normal group (p < .01). In contrast, treatment with taraxasterol at 10, 5 and 2.5 mg/kg suppressed the up-regulation of Bax expression and improved the down-regulation of Bc1-2 expression induced by Con A; the ratio of Bax/Bc1-2 was significantly lower than that of Con A group (p < .01 or p < .05). It indicated that treatment with taraxasterol could improve hepatic anti-apoptotic activity in Con A-induced hepatic injury by down-regulating the expression of apoptotic protein Bax and up-regulating the expression of anti-apoptotic protein Bc1-2.
Figure 9. Effects of taraxasterol on hepatic Bax and Bc1-2 expressions in Con A-induced acute hepatic injury. The mice were treated with taraxasterol (10, 5 and 2.5 mg/kg, respectively) or Bif and injected a single dose of Con A. Hepatic Bax and Bc1-2 expressions were measured by Western blot analysis; the ratio of Bax and Bc1-2 was calculated. The values represent the means ± SEMs. ##p < .01 vs. normal group; *p < .05, **p < .01 vs. Con A group.
Discussion
Immune-mediated hepatic injury is characterized by inflammatory response, immune dysfunction and impaired immune tolerance. It has a high tendency to develop into severe liver diseases such as hepatic fibrosis, cirrhosis and even hepatic cancer. Con A-induced acute hepatic injury model is a typical T cells-dependent model and resembles clinical immune hepatitis in humans. Thus, Con A animal model is considered as the most common and convenient model for investigating the mechanisms and developing the new therapeutic options of immune-mediated hepatic injury [Citation19,Citation20]. In this study, we explored the preventive effects and potential mechanisms of taraxasterol against immune-mediated hepatic injury by establishing Con A-induced acute hepatic injury in mice. Our findings show that treatment with taraxasterol exerts protective effects against hepatic injury induced by Con A in mice by inhibiting TLRs/NF-κB inflammatory signalling pathway and promoting Bax/Bc1-2 anti-apoptotic signalling pathway.
When Con A is injected intravenously into the body, the body produces abnormal immune responses, which leads to hepatic injury accompanied by highly elevated serum transaminases [Citation20]. Serum AST and ALT are the most intuitive and classical biochemical indicators for reflecting hepatic function and estimating the extent of hepatic damage [Citation21]. When the liver is injured, ALT and AST are released from the liver into the blood, which causes the abnormal elevation of serum ALT and AST [Citation22]. As shown in this study, Con A injection induced the obvious elevation of serum ALT and AST levels, while treatment with taraxasterol significantly decreased the elevated levels of serum ALT and AST induced by Con A.
Oxidative stress is a well-known cause of various hepatic damages. Hepatic function is directly correlated with the anti-oxidative capacity of hepatocytes. MDA is the end product of lipid peroxidation and an indicator representing oxidative damage of tissues. It can indirectly reflect the metabolism of oxygen free radicals and the severity of free radicals attack on the liver [Citation23]. When the liver is stimulated by Con A, lipid peroxidation occurs, and the production of MDA increases with the increase of oxygen free radicals. The excessive production of MDA causes hepatic damage, leading to necrosis and apoptosis of hepatocytes. In contrast, GSH and SOD are two important anti-oxidative parameters for assessing hepatic activity and antioxidant capacity [Citation24]. They can scavenge oxygen free radicals and lipid peroxides, and protect hepatocytes against oxidative damage. In the present study, Con A injection induced severe increase of hepatic MDA level and decrease of hepatic GSH and SOD levels, while treatment with taraxasterol showed significant reduction of MDA level and moderate restoration of GSH and SOD levels. It indicated that the preventive effects of taraxasterol on Con A-induced acute hepatic injury are associated with its antioxidant activity.
It is well-known that activated T cells and macrophages play an important role in the development of hepatic inflammation. The pathogenesis of immune-mediated hepatic injury is closely associated with the production of a large number of inflammatory cytokines. As a T cell mitogen, Con A can activate T cells and hepatic macrophages to secrete inflammatory cytokines, and promote inflammatory response and lead to hepatitis and apoptosis [Citation25]. The main inflammatory cytokines involved in the pathogenesis are TNF-α, IL-6, IL-1β, IFN-γ, IL-4, etc. The increased levels of these inflammatory cytokines are the feature of contributing to the development of immune hepatic injury [Citation19]. Among these inflammatory cytokines secreted during Con A-induced hepatic injury, TNF-α and IFN-γ are the dominant cytokines and play critical roles in the induction of hepatic inflammation and hepatocyte apoptosis [Citation19,Citation20,Citation26]. Another inflammatory cytokine IL-6 is found to play an important role in liver fibrosis [Citation27]. IL-4, which is secreted by Th 2 cell, is identified as an essential factor for triggering TNF-α production and inducing further hepatic damage in Con A-induced hepatic injury [Citation28]. Also, IL-1β is a central cytokine associated with hepatic injury and play a significant role in the necrosis of hepatic tissues [Citation29,Citation30]. Moreover, the secreted increase of inflammatory cytokines is the main reason for the further activation and accumulation of T cell chemokines and plays an important role in the development of hepatitis [Citation28]. Thus, suppressing the over-secretion of these inflammatory cytokines may be an effective therapeutic approach for the severe inflammatory response in Con A-induced acute hepatic injury. In this study, Con A injection remarkably induced the release of inflammatory cytokines TNF-α, IL-6, IL-1β, IFN-γ and IL-4, whereas pretreatment with taraxasterol reversed the significant increase of theses inflammatory cytokines. This result is line with the reduction of liver index and hepatomegaly (), and the restoration of pathological changes such as inflammatory cell infiltration and necrosis in hepatic tissues (). It is also consistent with our recent report that taraxasterol could inhibit inflammatory cytokines TNF-α and IL-6 production in mice with alcoholic liver injury [Citation17]. It indicated that taraxasterol could prevent Con A-induced acute hepatic injury by suppressing the secretion of pro-inflammatory cytokines.
TLRs are a group of immune receptors widely expressed in a variety of tissues and cells [Citation31]. TLRs, especially TLR2 and TLR4, are widely expressed in multiple hepatocytes and play critical roles in liver health [Citation32]. TLR2 and TLR4 were found to be involved in the pathogenesis of Con A-induced liver injury [Citation33]. After Con A injection, the levels of TLR2 and TLR4 protein in liver of mice increased, and the lack of functional TLR2 or TLR4 reduces the production of cytokines and results in cell damage [Citation34]. TLRs are involved in hepatic injury via promoting the activation of NF-κB signalling pathway [Citation35]. NF-κB complex is mainly composed of p50 and p65 dimers; it is retained in cytoplasm by binding to the inhibitory protein IκB in resting state. Once activated, NF-κB is released from cytoplasm and translocated to nucleus, and where it binds the κB site of target genes and induces the overproduction and release of inflammatory cytokines, which can damage the liver and eventually cause cell necrosis and apoptosis [Citation36]. It has demonstrated that Con A can up-regulate NF-κB expression in liver [Citation37], whereas liver injury induced by Con A is significantly attenuated by inhibiting NF-κB activation [Citation38,Citation39]. Therefore, inhibition of the expression of TLRs and the activation of NF-κB are the effective therapeutic targets for immune-mediated hepatic injury. As shown in this study, Con A strongly induced the expression of TLR2 and TLR4 receptors and the activation of NF-κB protein, whereas treatment with taraxasterol dramatically down-regulated the expressions of TLR2, TLR4 and NF-κB p65. The results illustrated that taraxasterol prevents Con A-induced hepatic injury in mice by inhibiting inflammatory cytokines production via TLRs/NF-κB signalling pathway.
Con A-induced immune hepatic injury is also associated with apoptosis or programmed cell death [Citation40]. Apoptosis, as a double-edged sword, can not only eliminate abnormal or dead cells and maintain tissue homeostasis, but can also cause tissue damage when over-activated and is another important manifestation of hepatic injury [Citation41]. Apoptosis can be activated by a variety of endogenous or exogenous factors; the study has shown that apoptosis is over-activated by Con A injection and leads to hepatic injury [Citation42]. Bax and Bcl-2 proteins are important markers and central regulators of apoptosis and play main roles in the apoptotic pathway. Bax is a pro-apoptotic protein, while Bcl-2 is an anti-apoptosis protein. Cell survival and apoptosis are determined by the balance or ratio between Bax and Bcl-2 [Citation43]. In this study, treatment with taraxasterol could down-regulate Bax expression and up-regulate Bcl-2 expression, thereby reducing Bax/Bcl-2 ratio and alleviating apoptosis of hepatocytes. It was consistent with the result of hepatocyte apoptosis by TUNEL staining (), which indicated that the preventive effects of taraxasterol on Con A-induced hepatic injury might be partially attributed to the anti-apoptotic ability through Bax/Bcl-2 apoptotic pathway.
Conclusions
In summary, the present study showed that taraxasterol isolated from Taraxacum has preventive effects on acute hepatic injury induced by Con A in mice. The preventive effects of taraxasterol are attributed to its ability in decreasing liver index, serum ALT and AST, and hepatic MDA levels, increasing hepatic GSH and SOD activities, inhibiting serum cytokines TNF-α, IL-6, IL-1β, IFN-γ and IL-4 production, and alleviating the hepatic histopathological injury and apoptosis induced by Con A. This potential preventive mechanism may be associated with inhibiting TLRs/NF-κB inflammatory signalling pathway and promoting Bax/Bc1-2 anti-apoptotic signalling pathway. These data support the potential application of taraxasterol in the prevention and treatment of immune-mediated hepatic injury.Author contributions
Xuemei Zhang designed the experiments; Rui Sang, Yifan Yu, Bingjie Ge, Lu Xu and Zheng Wang performed the experiments and analyzed the data; Xuemei Zhang and Rui Sang wrote the manuscript.
Disclosure statement
The authors declare that there is no conflict of interest.
Additional information
Funding
References
- Wimer BM, Mann PL. Mitogen information summaries. Cancer Biother Radiopharm. 2002;17(5):569–597.
- Tiegs G, Hentschel J, Wendel A. A T cell-dependent experimental liver injury in mice inducible by concanavalin A. J Clin Invest. 1992;90(1):196–203.
- Luo Q, Zhu L, Ding J, et al. Protective effect of galangin in concanavalin A-induced hepatitis in mice. Drug Des Devel Ther. 2015;9:2983–2992.
- Ichiki Y, Aoki CA, Bowlus CL, et al. T cell immunity in autoimmune hepatitis. Autoimmun Rev. 2005;4(5):315–321.
- Yuan Y, Gong X, Zhang L, et al. Chlorogenic acid ameliorated concanavalin A-induced hepatitis by suppression of Toll-like receptor 4 signaling in mice. Int Immunopharmacol. 2017;44:97–104.
- Li G, Chen MJ, Wang C, et al. Protective effects of hesperidin on concanavalin A-induced hepatic injury in mice. Int Immunopharmacol. 2014;21(2):406–411.
- Zhou Y, Weng X, Dou R, et al. Betulin from Hedyotis hedyotidea ameliorates concanavalin A-induced and T cell-mediated autoimmune hepatitis in mice. Acta Pharmacol Sin. 2017;38(2):201–210.
- Schütz K, Carle R, Schieber A. Taraxacum-a review on its phytochemical and pharmacological profile. J Ethnopharmacol. 2006;107(3):313–323.
- Jeon HJ, Kang HJ, Jung HJ, et al. Anti-inflammatory activity of Taraxacum officinale. J Ethnopharmacol. 2008;115(1):82–88.
- You Y, Yoo S, Yoon HG, et al. In vitro and in vivo hepatoprotective effects of the aqueous extract from Taraxacum officinale (dandelion) root against alcohol-induced oxidative stress. Food Chem Toxicol. 2010;48(6):1632–1637.
- Colle D, Arantes LP, Gubert P, et al. Antioxidant properties of Taraxacum officinale leaf extract are involved in the protective effect against hepatoxicity induced by acetaminophen in mice. J Med Food. 2012;15(6):549–556.
- Zhang XM, Xiong HZ, Liu LB. Effects of taraxasterol on inflammatory responses in lipopolysaccharide-induced RAW 264.7 macrophages. J Ethnopharmacol. 2012;14:206–211.
- Xiong HZ, Cheng Y, Zhang X, et al. Effects of taraxasterol on iNOS and COX-2 expression in LPS-induced RAW 264.7 macrophages. J Ethnopharmacol. 2014;155(1):753–757.
- Zhang XM, Xiong HZ, Li HY, et al. Protective effect of taraxasterol against LPS-induced endotoxic shock by modulating inflammatory responses in mice. Immunopharmacol Immunotoxicol. 2014;36(1):11–16.
- Liu JT, Xiong HZ, Cheng Y, et al. Effects of taraxasterol on ovalbumin-induced allergic asthma in mice. J Ethnopharmacol. 2013;148(3):787–793.
- Wang SS, Wang Y, Liu XY, et al. Anti-inflammatory and anti-arthritic effects of taraxasterol on adjuvant-induced arthritis in rats. J Ethnopharmacol. 2016;187:42–48.
- Xu L, Yu YF, Sang R, et al. Protective effects of taraxasterol against ethanol-induced liver injury by regulating CYP2E1/Nrf2/HO-1 and NF-κB signaling pathways in mice. Oxid Med Cell Longev. 2018;2018:1.
- Wang K, Song Z, Wang H, et al. Angelica sinensis polysaccharide attenuates concanavalin A-induced liver injury in mice. Int Immunopharmacol. 2016;31:140–148.
- Wang HX, Liu M, Weng SY, et al. Immune mechanisms of concanavalin A model of autoimmune hepatitis. World J Gastroenterol. 2012;18(2):119–125.
- Heymann F, Hamesch K, Weiskirchen R, et al. The concanavalin A model of acute hepatitis in mice. Lab Anim. 2015;49(1):12–20.
- Michalopoulos GH. Liver regeneration. J Cell Physiol. 2007;213(2):286–300.
- Tiegs G, Lohse AW. Immune tolerance: what is unique about the liver. J Autoimmun. 2010;34(1):1–6.
- Zhao J, Zhang S, You S, et al. Hepatoprotective effects of nicotiflorin from Nymphaea candida against concanavalin A-induced and D-galactosamine-induced liver injury in mice. IJMS. 2017;18(3):587.
- Grade MJ, Meguid MM. A glance at ethanol consumption, GSH suppression, and oxidative liver damage. Nutrition. 2017;33:199–203.
- Sass G, Heinlein S, Agli A, et al. Cytokine expression in three mouse models of experimental hepatitis. Cytokine. 2002;19:115–120.
- Mordue DG, Monroy F, La Regina M, et al. Acute toxoplasmosis leads to lethal overproduction of Th1 cytokines. J Immunol. 2001;67:4574–4584.
- Liang J, Zhang B, Shen RW, et al. Preventive effect of halofuginone on concanavalin A-induced liver fibrosis. PLoS One. 2013;8(12):e82232.
- Jakubowski A, Sternak M, Jablonski K, et al. 1-Methylnicotinamide protects against liver injury induced by concanavalin A via a prostacyclin-dependent mechanism: a possible involvement of IL-4 and TNF-α. Int Immunopharmacol. 2016;31:98–104.
- Weber A, Wasiliew P, Kracht M. Interleukin-1beta (IL-1beta) processing pathway. Sci Signal. 2010;3(105):cm2.
- Cui K, Yan G, Xu C, et al. Invariant NKT cells promote alcohol-induced steatohepatitis through interleukin-1β in mice. J Hepatol. 2015;62(6):1311–1318.
- Takeda K, Akira S. Toll-like receptors. Curr Protoc Immunol. 2015;109:14.12.1–14.12.10.
- Xu J, Zhang X, Monestier M, et al. Extracellular histones are mediators of death through TLR2 and TLR4 in mouse fatal liver injury. J Immunol. 2011;187(5):2626–2631.
- Tu CT, Han B, Yao QY, et al. Curcumin attenuates concanavalin A-induced liver injury in mice by inhibition of Toll-like receptor (TLR) 2, TLR4 and TLR9 expression. Int Immunopharmacol. 2012;12(1):151–157.
- Gong Q, Zhang H, Li JH, et al. High-mobility group box 1 exacerbates concanavalin A-induced hepatic injury in mice. J Mol Med. 2010;88(12):1289–1298.
- Gan F, Liu Q, Liu Y, et al. Lycium barbarum polysaccharides improve CCl4-induced liver fibrosis, inflammatory response and TLRs/NF-κB signaling pathway expression in Wistar rats. Life Sci. 2018;192:205–212.
- Liu XY, Xu L, Wang Y, et al. Protective effects of total flavonoids of Astragalus against adjuvant-induced arthritis in rats by regulating OPG/RANKL/NF-κB pathway. Int Immunopharmacol. 2017;44:105–114.
- Tiegs G, Kusters S, Kunstle G, et al. Ebselen protects mice against T cell-dependent, TNF-mediated apoptotic liver injury. J Pharmacol Exp Ther. 1998;287(3):1098–1104.
- Li Y, Wang ZL, He F, et al. Tp-58, a novel thienopyridine derivative, protects mice from concanavalin A-induced hepatitis by suppressing inflammation. Cell Physiol Biochem. 2012;29(1–2):31–40.
- Song B, Huang G, Tong C, et al. Gossypol suppresses mouse T lymphocytes via inhibition of NFκB, NFAT and AP-1 pathways. Immunopharmacol Immunotoxicol. 2013;35(5):615–621.
- Amin AR, Thakur VS, Gupta K, et al. Restoration of p53 functions protects cells from concanavalin A-induced apoptosis. Mol Cancer Ther. 2010;9(2):471–479.
- Chen K, Li JJ, Li SN, et al. 15-Deoxy-Δ12,14-prostaglandin J2 alleviates hepatic ischemia-reperfusion injury in mice via inducing antioxidant response and inhibiting apoptosis and autophagy. Acta Pharmacol Sin. 2017;38(5):672–687.
- Liu T, Xia Y, Li J, et al. Shikonin attenuates concanavalin A-induced acute liver injury in mice via inhibition of the JNK pathway. Mediators Inflamm. 2016;2016:1.
- Mao Y, Wang J, Yu F, et al. Ghrelin reduces liver impairment in a model of concanavalin A-induced acute hepatitis in mice. Drug Des Devel Ther. 2015;9:5385–5396.