Abstract
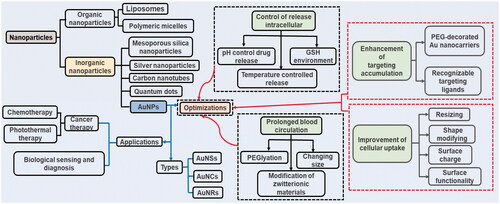
Gold nanoparticles (AuNPs), as a kind of inorganic nanoparticle, have been gradually recognized as one of the most promising nanomaterials, which is attributed to their unique optical, electronic, sensing and biochemical characteristics. Due to such unique characteristics, AuNPs have been widely applied in biomedical fields such as diagnosis, biosensing and drug delivery. Except for their use in cancer treatment alone with their photothermal ablation of solid tumours, when used with anticancer drugs, AuNPs can exert a dual role in treating cancer. With the advantages of protecting drugs from degradation and leakage in the physiological environment, tuneable modification in size, surface and shape, and biocompatibility, AuNPs can be used as promising drug carriers in anticancer drug design. However, there are still many aspects that need to be improved during the usage of drug carriers in pharmacology including the following aspects: prolongation in the plasma, enhancement in targeting accumulation, improvement in cellular uptake and the control of intracellular release. AuNPs are important drug carriers.
Introduction
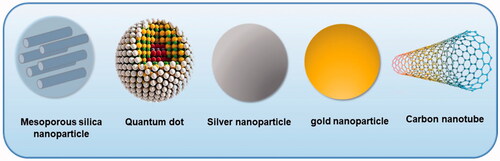
Nanotechnology is becoming trending topic involving many academic disciplines such as chemistry, biology and medicines [Citation1]. The size of nanoparticles, which have important activity compared to the same material in bulk form, is one billionth of a metre [Citation2]. There are many types of common nanomaterials such as nanoparticles, nanocapsules and nanotubes [Citation3]. Nanoparticles, as the most familiar nanomaterials, can be divided into two types, of which the most common one in pharmacology is organic nanoparticles, such as liposomes and polymeric micelles, and the other one is inorganic nanoparticles such as quantum dots (QDs), gold nanoparticles (AuNPs), silver nanoparticles (AgNPs), carbon nanotubes (CNTs) and mesoporous silica nanoparticles (MSNs) [Citation4].
Organic nanoparticles have already been used extensively in the clinical setting. However, adjusting the size and geometry to maintain the organic aggregates below a certain size threshold is challenging, especially in living systems [Citation5]. In contrast, inorganic nanoparticle-based drug carrier systems have more tuneable surface modification and adjustable size strategies. In addition, inorganic nanoparticles exhibit inherent physicochemical properties to transfer irradiated energy to heat or toxic radicals for high temperature, photothermal or photodynamic therapy for solid tumours, and are not susceptible to the environment in the body. Due to these unique properties, inorganic nanoparticles play a significant role in many fields, such as sensing, bioimaging and drug loading [Citation6] and are receiving increasingly more attention from researchers and undergoing extensive research in pharmacy. Inorganic nanoparticles are difficult to degrade in vivo and cause damage to normal human cells, which has limited their usage in pharmacology, but their broad application prospects in the pharmaceutical field should still be recognized.
AuNPs are one type of inorganic nanoparticle which play an important role in pharmacology. When adjusting to a suitable size and shape, AuNPs are also safe and have low phototoxicity. Additionally, they show a unique photophysical property named localized surface plasmon resonance (LSPR), which is caused by the free electron resonance of the free electron cloud's resonance of its metal lattice when irradiated by resonant frequency photons [Citation7]. Depending on their unique optical versatility and other characteristics, such as tuneable size and shape, convenient surface modification, biocompatibility, flexibility in fabricating different morphological forms, and ease to functionalise with active ligands via Au–S chemical bonds, have made AuNPs as promising drug carriers in cancer theranostics [Citation8–10].
To better employed AuNPs in pharmacology, some surface modification, such as conjugation with polyethylene glycol (PEG) or surface-capped mesoporous silica nanoparticles, should be applied [Citation10]. Through these changes, an ideal nanoparticle drug delivery system, which is inert or undetectable in the plasma while are activated after accumulating at the targeted site to be recognized by tumour cells, could be realised [Citation11]. Due to the development of inorganic nanoparticles in pharmaceutics, AuNPs are becoming a desired approach either in stand-alone therapeutics or combined with drugs as carriers for therapeutics [Citation12]. From this perspective, we summarize the current state of knowledge of how inorganic nanoparticles, especially AuNPs, are applied in pharmacology and how AuNPs are optimized to contribute to drug carriers ().
Inorganic nanoparticles
Inorganic nanoparticles are particles of metal oxide or metallic composition with an inorganic core and organic shell. Some inorganic nanomaterials have been used for diagnosis, treatment and cancer immunotherapy [Citation13]. Common inorganic nanomaterials applicable in biomedical applications include MSNs, AgNPs, CNTs, QDs and AuNPs () [Citation14].
MSNs
MSNs are a kind of nanomaterials with pore channels synthesized by silica particles [Citation15]. MSNs with a pore size ranging from 2 nm to 50 nm are employed as excellent candidates in drug carrier and biomedical applications, which is attributed to their unique properties [Citation16,Citation17].
First, the properties of their large surface area and pore volume supplied promising vehicle character for drugs or biological molecules to be loaded into the pore channels. The tuneable pore size and controlled mesoporous structure facilitate the dissolution of drugs and then prevent the crystallization of drugs. Moreover, the exterior and interior surfaces of MSNs are easily modified due to the high density of silanol, which may provide better control of drug loading and release kinetics. Surface modifications may improve the capability to gain hybrid multifunctional smart silica nanoparticles. When equipped with hydrophobic materials, the stability of drugs, especially water insoluble drugs can be extensively improved. The premature release of drug can be limited, and the delivered amount of drug can be elevated. In addition, controlled and targeted transporting manner can be designed and modified to improve the therapeutic efficacy and minimize the adverse and toxic effects [Citation17]. Finally, MSNs have been increasingly employed as ideal carriers due to their high chemical, thermal and mechanical stabilities over broad ranges of pH and temperature under physiological conditions [Citation18–21].
AgNPs
AgNPs are particles composed of silver atoms, usually ranging in size from 1 nm to 100 nm [Citation22]. Many synthetic methods have been developed for the manufacture of AgNPs [Citation23]. The reduction of silver salts by reducing agents is the most common synthetic method [Citation24]. For example, Sood et al. used Ocimum sanctum leaf extract both as reducing agent and as stabilizer. Then, they added the extract to silver nitrate solution dropwise under certain reaction conditions and AgNPs were synthesized [Citation25].
Due to remarkable optical properties, AgNPs have broad application prospects in biomedical and diagnostic testing [Citation26]. Local surface plasma resonance properties of AgNPs are commonly used for electromagnetic enhancement of spectral signals or for ultrasensitive detection of chemicals. For example, Cholula-Díaz et al. used AgNP colloids to detect ascorbic acid in SERS experiments in aqueous solution [Citation24]. In addition, AgNPs have antibacterial effect. Therefore, AgNPs can be used in the preparation of various antibacterial drugs [Citation25,Citation27].
CNTs
CNTs are tube skeleton material mainly composed of carbons [Citation28]. The arrangement of atom in a CNT is in a hexagonal form like that of graphite [Citation29]. These atoms in a CNT aggregated to CNTs can be obtained by applying various methods, such as laser ablation method [Citation30].
CNTs are widely applied in biological detection and analysis based on their remarkable intrinsic physical properties such as superior optical, electrical and thermal properties [Citation28,Citation29,Citation31,Citation32]. Apart from taking advantage of their intrinsic physical properties, one can also benefit from the presence of their inner hollow core and a chosen drug can be simultaneously encapsulated in their interior, while for the biocompatible, dispersible and targeting purposes, the external walls can be modified with various organic materials, such as peptides and substrates. The inner cavity of CNTs can serve as a nanocarrier that preserves the cargo from oxidation or any other unwanted interaction of cargo molecules with biological milieu [Citation33–35]. For example, Zhao et al. [Citation36] designed 300 nm PEGylated multi-walled CNTs which possessed a high doxorubicin (DOX)-loading capacity with high cumulative release within 24 h and a low premature leakage of 10%.
QDs
QDs are a kind of nanosized small semiconductor particle equipped with a heavy metal core-shell and an organic coating. Due to their high optical properties and potential toxicity. QDs are not typically used for drug delivery but are generally used as bioimaging agents to monitor certain substances, such as ATP, Cu2+, glutathione (GSH) and prostate protein antigen [Citation37–44].
Graphene quantum dots (GQDs), as an evolutionary grapheme relative to semiconductor QDs, show booming applications and potential prospects in terms of bioimaging and photovoltaic devices [Citation45]. Especially in the field of biomedical, GQDs are demonstrated to be excellent nanodrug carriers with the advantage of high stability and low toxicity [Citation46].
AuNPs
AuNPs are tiny particles of gold with diameters ranging from 1 to 100 nm. The colour of AuNPs varies from red to purple depending on the size. Typically, the controlled size and shape of AuNPs is synthesized by various physical, chemical and biological means.
Chemical synthesis usually uses chemicals and solvents, such as the citrate reduction method using gold chloride and trisodium citrate [Citation47]. Bio-nanoparticle synthesis is a relatively new, eco-friendly and promising area [Citation48]. Many plants have shown potential for the preparation of AuNPs and microorganisms are also capable of participating in the enzymatic reduction of gold ions by secreting many enzymes to prepare AuNPs [Citation49]. AuNPs have generated interest from different research fields, especially in the biopharmaceutical fields. Ease in preparation and modification, precision in controlling the physicochemical properties of particles, unique tuneable optics, unique electronic and SPR characteristics are distinctiveness properties of AuNPs [Citation48,Citation50]. These clear physical and chemical properties make AuNP a great scaffold for many applications in therapeutics, diagnostics, biomarkers, drug carrier and imaging [Citation51].
In addition, AuNPs of various shapes (nanospheres, nanorods, nanoshells and nanocages) have been synthesized. Different shapes of AuNPs usually have various properties, which characterize specific functions in numerous fields. The following is a detailed description of the different shapes of AuNPs.
Different shapes of AuNPs
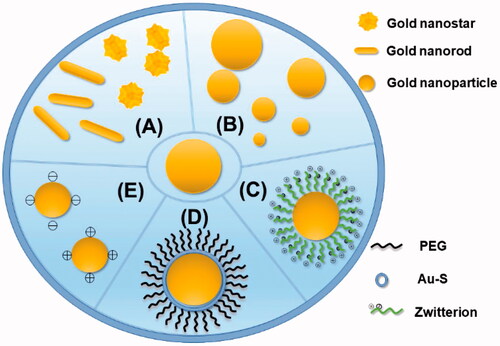
AuNPs with different morphologies could result in distinct uses in biomedicine. The possibility to tune the LSPR towards the near-infra-red region and improve the drug-loading capacity by adjusting the shape of AuNPs is one of the reasons why AuNPs are widely used [Citation51]. The morphologies of AuNPs include gold nanospheres, gold nanorods, gold nanocages and so on [Citation52]. They play an important role in pharmacology ().
Figure 3. Several modifications of AuNPs. (A) Change the shape of the AuNPs to become gold nanostars and gold nanorods. (B) Change the diameter of the AuNPs to make them different in size. (C) Zwitterionic surface engineering of AuNPs. (D) PEGlyation with surface of AuNPs. (E) Different surface charge of AuNPs.
Gold nanosphere
Gold nanospheres (AuNSs), which have received the most attention due to the ease of their synthesis, are AuNPs in the form of spheres [Citation53]. The most common method for synthesizing spherical AuNPs is based on the nucleation and growth process of citrate reduction of HAuCl4 in aqueous solution [Citation54]. However, this approach has certain limitations such as cytotoxicity and long duration. Many researchers have designed a green method to synthesize AuNSs. For example, Trouiller et al. designed a simple, non-physical method to synthesise small well-defined spherical AuNPs, without any additional reducing chemistry, to achieve rapid synthesis in 30 min [Citation55].
Because of the low cytotoxicity, ease of synthesis, spherical shape with uniform size, good biocompatibility, high tissue permeability and relatively simple surface modifications, spherical AuNPs are very popular [Citation56]. To efficiently deliver therapeutic agents to human lung cancer cells, Wang et al. produced gemcitabine (Gem)-loaded AuNSs. The results showed that the obtained nanospheres had good biocompatibility, had low cytotoxicity and showed obvious effects, and the efficiency was significantly higher than that of free drugs [Citation57].
Gold nanorod
Gold nanorods (AuNRs) are a kind of elongated AuNPs, which can usually be synthesized by the seed-mediated growth method [Citation53]. The preparation method usually consists of mixing the prepared seed solution and the growth solution at room temperature [Citation58].
Due to the anisotropy of AuNRs, two independent SPR bands have attracted considerable research interest. Transversal band and longitudinal bands appear in the visible and near infra-red region (NIR), respectively [Citation59]. The SPR band in the NIR region of AuNRs is converted into heat via the photothermal effect, causing photothermally induced high temperatures and irreversible damage to cancer cells [Citation60,Citation61].
Furthermore, AuNRs are known to exhibit greater fluorescence enhancement than Au nanospheres. Since the LSPR band of AuNRs can be adjusted to match the spectrum of the red/NIR dye, it is more effective in improving the fluorescence of red and NIR dyes to achieve optimal fluorescence enhancement. AuNR-based nanostructures with enhanced red/NIR emission are excellent in bioimaging [Citation62].
Gold nanocage
Gold nanocages (AuNCs) are a kind of AuNP that have unique hollow interiors and porous well structures. A common method of preparing AuNCs is a template etching method which still requires further improvement to avoid collapse of the gold shell after removal of the core [Citation63]. Easy preparation of AuNCs by displacement reaction using HAuCl4 [Citation64–66].
Small molecules can be encapsulated in AuNCs, and the pores on the surface are suitable for small molecules to enter the nanocage. Therefore, AuNCs can be applied to a target site [Citation67]. For instance, Park et al. devised a drug carrier system that contains an anticancer drug in its core loaded with a phase change material in its sheath. The heat generated by NIR causes the phase change of the load to melt, resulting in a rapid release of drug molecules [Citation68]. In addition, functional groups can easily modify their surface to specifically interact with the biological system. Tumour-targeting molecules can readily bind to AuNCs to enhance their tumour accumulation.
The surface plasmon resonance of the NIR region of AuNCs generates heat through the photothermal effect, which causes photothermal-induced hyperthermia and destroy cancer cells. Thus, AuNCs could be drug carriers and photothermal conversion agents. The combination of NIR light-triggered drug release and photothermotherapy shows excellent therapeutic efficacy [Citation69].
Application of AuNPs
AuNPs are suitable for several biomedical applications due to their relative inertness in biological environments. Furthermore, AuNPs can be used as drug carriers since several methods can be used to readily modify the AuNP surface for attachment of a ligand, drug or other targeting molecule [Citation70]. Previously, AuNPs have made great progress in biological sensing, diagnosis and cancer therapy.
Biological sensing and diagnosis
AuNPs have been widely used to develop various biosensors for molecular diagnostics. The optical properties of AuNPs allow for the expression of intense colours under light, which can be adjusted by varying their size and shape [Citation71]. AuNP-based biosensors may play an important role in future clinical diagnosis [Citation72]. For instance, Pannico et al. reported that AuNPs can be used as a surface-enhanced Raman spectroscopy (SERS) sensor for in vivo cellular uptake and localization. SERS experiments have shown that tumour cells take up a significant number of nanoparticles compared to normal cells. This largely different behaviour may be used for diagnosis [Citation73].
In addition to AuNP-based optical biosensor, there are non-optical biosensor, including electrochemical biosensors and piezoelectric biosensors [Citation74–77].
Cancer therapy
Chemotherapy
AuNPs can also be applied in pharmacology as drug carriers due to their many unique properties [Citation78,Citation79]. In addition, there are some specific morphologies of AuNPs such as gold nanocages, hollow gold nanoshells and hollow nanosphere [Citation80]. Thus, pores on the surface of the hollow nanostructures are suitable for small molecules to enter and to encapsulate small molecules. For example, Xin et al. synthesized Al(III) phthalocyanine chloride tetra sulphonic acid (AlPcS4) delivery systems with AuNRs to improve the limited PDT effect. AlPcS4, which binded to AuNRs, had a significant anticancer effect, since AuNRs are not only fit for AlPcS4 delivery but also exhibit enhanced singlet oxygen production effects and photothermal effects directly induce apoptosis [Citation81].
Khutale and Casey developed a gold nanoparticle drug carrier system for intracellular delivery of DOX, which is composed of a multifunctional gold nanoparticle-based drug delivery system (Au-PEG-PAMAM-DOX) [Citation82]. The combination of DOX and the AuNP drug carrier system can provide a promising platform for intracellular delivery of various anticancer drugs. Ren et al. designed a synergistic delivery system using NIR responsive hollow AuNPs to realize sequential release of inhibitor/DOX [Citation83].
Photothermal therapy
AuNPs convert light energy (NIR) into heat due to their unique surface plasmon resonance, which causes high temperatures. Therefore, photothermal conversion therapy, called plasma photothermotherapy, has emerged because it can be used to ablate cancer cells [Citation84]. Singh et al. synthesised a biodegradable liposome gold nanoparticle for photothermal therapy. The AuNPs enabled the system to specifically absorb NIR light (780 nm) by SPR and convert light energy into heat. The photothermal transduction efficacy of Au-liposome nanoparticles showed an obvious temperature rise, causing irreversible cell damage [Citation85].
Drug carriers and photothermal therapeutics also require AuNPs to participate in the synthesis. Therefore, AuNPs could be designed for chemo-photothermal synergistic therapy [Citation86]. Chuang et al. synthesized a nanocomposite coated with human serum albumin (HSA) showing promising combined effects of photothermal and chemical treatments without adverse side effects [Citation87].
Optimization of AuNPs in pharmacology
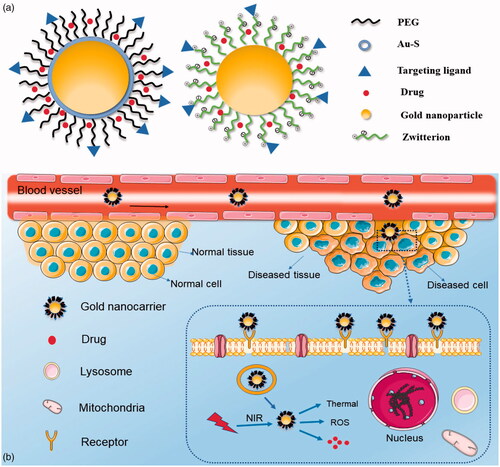
Over the past few decades, immense amounts of research have proven that the drug delivery process of AuNPs could be summarized in the following order: An ideal nanoparticle drug delivery process should be inert or undetectable in the plasma but activated upon tumour site accumulation to be recognised by tumour cells [Citation11]. However, when AuNPs are used as drug carriers, there are many things that can be improved. It is well known that the in vivo manner of various AuNPs is affected by their surface and size [Citation88]. To make AuNPs function better as drug carriers, various optimisation strategies of AuNPs have been developed. The following aspects will be described: prolongation in the plasma, enhancement in targeting accumulation, improvement in cellular uptake and the control of intracellular release ().
Figure 4. Delivery process of optimized gold nanocarriers in vivo. (a) Two types of optimized gold nanocarriers with drugs. (b) Ideal delivery process of optimized gold nanocarriers with drugs in vivo: prolonged blood circulation, enhancement of targeting accumulation, improvement of cellular uptake and control of intracellular release.
Prolonged plasma circulation
AuNPs as drug carriers have been restricted by their short circulating half-lives in the bloodstream. It has been reported that such limitation is due to the clearance of AuNPs by macrophages. The clearance is accepted to be primarily attributed to opsonin adsorption on the surfaces of AuNPs, leaving them susceptible to cells of the mononuclear phagocyte system [Citation89]. Therefore, a number of studies on long-circulating AuNPs developed in the past have concentrated on decreasing their clearance by macrophages and the reticuloendothelial system (RES) [Citation90–92] ().
PEGylation
In regard to prolonging time in the plasma, PEGylation (poly(ethylene glycol) conjugation) immediately comes to mind [Citation93]. PEGylation is a maturation strategy for improving the plasma circulation of AuNPs [Citation94], which provides a steric barrier against conditioning [Citation91,Citation95]. For instance, when DOX was loaded onto hybrid nanoparticles (HN-DOX), in vivo pharmacokinetics indicated that HN-DOX had a much longer cycle time than free DOX. The long cycle times in the plasma can be attributed to effective PEG shielding [Citation96]. However, it has been thought in recent years that PEG will experience accelerated plasma clearance after repeated administration [Citation97,Citation98].
Modification of zwitterionic materials
Here, zwitterionic materials can replace PEG to extend the cycle time of the nanoparticles without triggering an immune response [Citation99]. Zwitterions, which are promising ligands for the fabrication of non-interacting nanodrugs, are totally neutral under physiological conditions and can minimise the interaction with biological compositions, such as proteins and cells, and decrease their clearance [Citation100]. Moreover, compared to PEGylated AuNPs, it is reported that AuNPs that have undergone zwitterionic surface engineering exhibited prolonged plasma circulation [Citation98,Citation99]. Thapa et al. designed a mixed droplet containing zwitterionic chitosan (ZC), gold-graphene oxide (Au-GO) and DOX which is very beneficial for prolonging the plasma circulation time of the drug. Furthermore, zwitterionic AuNPs of different types may have different effects [Citation101]. Guével et al. found a significant increase in the plasma half-life using bidentate zwitterionic molecule AuNPs compared to common zwitterionic (GSH) AuNPs [Citation102]. In addition, adjusting the size of the AuNPs is also a great way to prolong the plasma circulation of AuNPs [Citation103].
Changing size
Generally, smaller AuNPs have longer plasma circulation time due to the fact that larger AuNPs were more easily cleared by macrophages and the RES [Citation104–106]. Terentyuk et al. selected 15, 50 and 160 nm AuNPs and measured their respective plasma concentrations. The results indicated that 15 nm AuNPs showed significant capacity during long circulation time and concentrations in the plasma were higher than those of 50 and 160 nm AuNPs 24 h after injection [Citation107]. In addition, it was reported that size seemed to be a major factor in the biodistribution in the body and metabolism whether the AuNPs were PEGylated or not [Citation88,Citation105]. Thus, strategies for prolonging the plasma circulation of AuNPs need to consider a combination of surface functional attachments and sizes.
Enhancement of targeting accumulation
In some cases, AuNPs can accumulate in a non-disease site, such as the liver and spleen, after which treatment effectiveness and particle toxicity become problems [Citation108]. Thus, enhancing targeting accumulation of AuNPs becomes a significant issue. There are two ways to deal with this matter.
PEG-decorated Au nanocarriers
One of the two ways is that PEG-decorated Au nanocarriers can passively target the lesion location via an improved permeation and retention (EPR) mechanism [Citation109,Citation110] (). However, PEG-modified Au nanocarriers have been questioned for their inability to accumulate in several cancerous tissues. Then, use of the other approach is generally used to enhance targeting accumulation of Au nanocarriers. As known to all, specific receptors overexpressed on the surface of certain diseased tissues can be selectively identified by binding the corresponding targeting agent to the surface of the nanoparticles [Citation111,Citation112].
Recognizable targeting ligands
Hence, the other way is to modify the surface of Au nanocarriers with identifiable targeting ligands, such as lectins or antibodies, low molecular weight molecules such as folate moieties or targeting peptides [Citation113].
Human galectin-3 (Gal-3) recognizes and binds to the β-D-galactoside moiety, which is a particularly attractive lectin. Aykac et al. envisaged a dually functionalized AuNP loaded with the anticancer drug methotrexate and multiple copies of the β-D-lactose unit as a targeting ligand for Gal-3 and β-CD macrocycles as an encapsulating moiety for methotrexate and yielded satisfactory results empirically [Citation114].
AuNPs bind to cancer cell surface receptors and have been used to specifically bind cancer cells [Citation115]. Penon et al. synthesized a gold nanoparticle combined with an anti-erbB2 [Citation111]. Then, they concluded that the anti-erbB2 antibody was an ideal ligand targeted to breast cancer tissues and realised the PDT treatment of AuNPs [Citation116].
Small molecules, such as peptides can be used as an alternative to targeting agents. Targeting peptides have significant advantages over antibodies, such as lower cost, lower conditioning and lower immunogenicity [Citation117]. Peptides are specific for their target tissues [Citation118]. A structure in which drug was loaded onto the AuNPs and peptides were conjugated holds great potential to increase the target efficacy of chemotherapeutic drugs [Citation49]. For example, the coupling of the gold compound to the targeting peptide (PVCAM-1) enhanced its targeting of the inflammatory synovium during cycling [Citation49].
Folate receptors are overexpressed in malignant tumour sites [Citation119]. Therefore, using folic acid as a targeted ligand can increase accumulation of AuNPs. There are plenty of studies demonstrating that AuNPs conjugate with folic acid to enhance targeting capability [Citation120]. For instance, AuNR, DOX and folic acid were polymerized by a series of physical chemical reactions. The experimental results showed that the FA targeting Au-HNTs-DOX@BSA had decent accumulation around cancer tissues. In addition, there are also many targeting ligands that are designed according to the specific structure of targeting locations [Citation113,Citation119,Citation121,Citation122], such as the strong binding affinity of bisphosphonate ligands for calcium [Citation113]. The discovery or synthesis of more targeting ligands through the special structure of the target site is indispensable to the enhancement of targeting accumulation of AuNPs.
Improvement in cellular uptake
The cellular uptake of AuNPs is found mainly to be related to size [Citation123], shape [Citation124], surface charge [Citation125], surface functionality [Citation126], and the interaction of these characteristics. Therefore, improving cellular uptake of AuNPs must start from the above factors ().
Resizing
Among these influencing factors, size can be the major contributor to cellular uptake [Citation127]. However, there is no consensus on maximizing the optimal particle size for cellular uptake levels [Citation128]. For example, Wong et al. observed that cellular uptake of AuNPs is inversely proportional to particle size [Citation127]. While Yue et al. believed that the cell uptake of 50 nm and 40 nm AuNPs is higher compared to that of 13 nm AuNPs [Citation124]. Moreover, Kumar et al. reported that AuNPs with sizes of 18 nm and 80 nm showed higher gold absorption than those with a size of 40 nm [Citation129].
Shape modification
The shape of AuNPs is also an important factor affecting the rate of cellular uptake [Citation130]. It is well known that shapes of AuNPs include gold nanospheres [Citation53], gold nanorods [Citation53], gold nanostar [Citation131], gold nanocage [Citation63] and gold nanoshells. For instance, rod-shaped gold nanoparticles displayed lower cellular uptake efficiency compared to gold nanospherical particles, with the percentage of cellular uptake decreasing with the increase in the aspect ratio [Citation132]. Similarly, cellular uptake of nanospheres increased on a higher scale compared with nanostars [Citation124]. Xie et al. synthesized three different shapes of AuNPs, stars, rods and triangles and then concluded that the cellular uptake efficiency of AuNP was, in order from lowest to highest, stars, rods and triangles [Citation133].
Surface charge
In addition to sizes and shapes of AuNPs, many studies have shown that the surface charge of AuNPs played a critical role in modulating cellular uptake [Citation134]. The surface charge of AuNPs includes positively charges [Citation135], negatively charges [Citation136] and neutral charges [Citation137]. Positively charged surfaces preferentially increase cellular uptake due to the negatively charged surface of the cells. For example, Li et al. synthesized AuNPs modified by carboxyl, amine and hydroxyl functional groups with different surface charge treated human bone marrow-derived mesenchymal stem cells (hMSCs) [Citation138]. The results indicated that positively charged AuNPs promoted high uptake of hMSCs. In the same way, Bai et al. reported that the AuNPs significantly reduced cellular uptake with a high-surface negative-charge density [Citation139]. We can infer that the surface charge of targeting cells influences cellular uptake to a certain extent. Liu et al. investigated the cellular uptake behaviours of AuNPs on both positively and negatively charged surfaces in cells. The results indicated that the choice of surface charge of AuNPs to improve cellular uptake should take targeting cell properties into account [Citation134].
Surface functionality
In addition, surface functionality of AuNPs is also a significant element in improving cellular uptake. Generally, surface modification of AuNPs to improve cellular uptake is similar to the targeting ligand. Yi et al. reported that the glucose-installed targeted AuNPs showed higher cellular uptake compared to glucose-unbound AuNPs [Citation140]. By the same principle, bovine serum albumin is a commonly used ligand as well which can bind to glycoprotein present on the targeting cell membrane [Citation141,Citation142]. Moreover, some studies implied that the interaction of sizes, shapes and surface functionalization can influence cellular uptake of AuNPs [Citation124,Citation125]. In summary, improving cellular uptake depends not only on changes in sizes, shapes and so on but also on the nature of the cells.
Control of intracellular release
Ideal drug release of AuNPs should be designed as such a model that drug released from the drug delivery system at physiological conditions is negligible, but drug release was significantly increased and sustained at a specific milieu [Citation143].
pH control drug release
The pH of plasma and normal tissue are neutral, while that of the extracellular environment of the tumour is acidic (pH ≈ 6.5–7.0), and the acidity of the endosomes and lysosomes is greater (pH ≈ 5.5–4.5) [Citation144]. In the past several years, many pH-responsive drug carrier systems have been synthesized to initiate drug release in cancer cells due to this particular acidic environment of the tumour site [Citation145]. For example, some acid-sensitive bonds such as amide bonds [Citation146–148] and hydrazone bonds [Citation149,Citation150], are introduced into the AuNPs due to their cleavage in an acidic environment. Khutale et al. designed a new drug carrier system. Under physiological conditions, DOX released in this system is negligible. In contrast, due to amide bond cleavage between DOX and dendrimers, much DOX was continuously released from the drug carrier system after 96 h at pH 4.0 [Citation82]. Wei et al. built the pH-responsive nanodrug Au-DOX. At physiological pH (pH = 7.4), the Au-DOX nanocomposites are relatively stable. At pH = 4.5 (similar to the intracellular pH of cancer cells), rapid release of DOX could be observed due to the rapid rate of hydrolysis of the hydrazone bond [Citation151].
GSH environment
GSH is more abundant in tumour cells compared to extracellular cells; thus, an intracellular drug release system is developed based on the combination of GSH-responsiveness [Citation152]. Drug-conjugated AuNPs, which are typically modified by a number of molecular thiol-terminated PEG drugs via a thiol-Au covalent bond, are promising effective nanodrugs; then GSH can be applied to regulate gold-thiol-mediated drug-release [Citation143]. For example, tiopronin-conjugated AuNPs (TPN @ GNPs) have GSH-reactive drug release properties and have been developed for the treatment of acute liver injury [Citation153].
Temperature-controlled release
AuNPs can be modified with heat-sensitive materials to realise drug release [Citation154]. For example, Lajunen et al. [Citation155] developed liposomal drug carriers which were formulated using a heat-sensitive composition with star- or rod-shaped AuNPs. AuNPs convert light energy into heat and release it into the lipid bilayer, causing an increase in the local temperature that causes double leakage of the liposome and triggers drug release. Phase change material (PCM) is a material with a large latent heat of fusion that melts and solidifies at a certain temperature. There are three forms of PCM: liquid-gas, solid-solid, and solid-liquid. Solid-liquid PCM is now widely used in basic research and practical application [Citation156]. The PCM which is applied in a drug release system should have good biocompatibility and a precise critical solution temperature with a slightly higher melting point than physiological temperature [Citation157]. lauric acid [Citation158], fatty acid and 1-tetradecanol [Citation156] are frequently used PCMs. Poudel et al. [Citation159] reported a new strategy in which hollow silver-gold nanoshells are encapsulated in hollow mesoporous silica as an effective platform for the release of anticancer drugs. The mesopores were blocked with the heat-sensitive PCM lauric acid to achieve drug-controlled release by photothermal action. In addition, there are also many dual-responsive drug release systems such as pH/near-infra-red dual-triggered drug release [Citation160], GSH/near-infra-red dual-triggered drug release [Citation161], GSH/pH dual-triggered drug release [Citation146] and other responsive drug release systems [Citation162,Citation163].
Conclusion
AuNPs have a wide range of applications in current medical and biological research, including diagnostics, therapy, biosensors, immunoassay, targeted delivery of drugs, and bioimaging. In this review, we have described the feature of inorganic nanoparticles and introduced AuNPs, including their shapes, their application in pharmacology and improvement in the drug delivery process. In recent years, little research has investigated the potential of AuNPs to act as stand-alone therapeutic agents. Thus, AuNPs should undergo some surface modification to play an important role in the drug delivery process.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Jain AK, Thareja S. In vitro and in vivo characterization of pharmaceutical nanocarriers used for drug delivery. Artif Cells Nanomed Biotechnol. 2019;47(1):524–539.
- Patil MP, Kim GD. Eco-friendly approach for nanoparticles synthesis and mechanism behind antibacterial activity of silver and anticancer activity of gold nanoparticles. Appl Microbiol Biotechnol. 2017;101(1):79–92.
- Chang T. Artificial cell evolves into nanomedicine, biotherapeutics, blood substitutes, drug delivery, enzyme/gene therapy, cancer therapy, cell/stem cell therapy, nanoparticles, liposomes, bioencapsulation, replicating synthetic cells, cell encapsulation/scaffold, biosorbent/immunosorbent haemoperfusion/plasmapheresis, regenerative medicine, encapsulated microbe, nanobiotechnology, nanotechnology. Artif Cells Nanomed Biotechnol. 2019;47(1):997–1013.
- Collier MA, Bachelder EM, Ainslie KM. Electrosprayed myocet-like liposomes: an alternative to traditional liposome production. Pharm Res. 2017;34(2):419–426.
- Dante S, Petrelli A, Petrini EM, et al. Selective targeting of neurons with inorganic nanoparticles: revealing the crucial role of nanoparticle surface charge. ACS Nano. 2017;11(7):6630–6640.
- Wang F, Li C, Cheng J, et al. Recent advances on inorganic nanoparticle-based cancer therapeutic agents. IJERPH. 2016;13(12):1182.
- Kim T, Hyeon T. Applications of inorganic nanoparticles as therapeutic agents. Nanotechnology. 2014;25(1):012001.
- Hu Y, Liu Y, Xie X, et al. Surfactant-regulated fabrication of gold nanostars in magnetic core/shell hybrid nanoparticles for controlled release of drug. J Colloid Interface Sci. 2018;529:547–555.
- Fokkema J, Fermie J, Liv N, et al. Fluorescently labelled silica coated gold nanoparticles as fiducial markers for correlative light and electron microscopy. Sci Rep. 2018;8(1):13625.
- Lee S, Kwon JA, Park KH, et al. Controlled drug release with surface-capped mesoporous silica nanoparticles and its label-free in situ Raman monitoring. Eur J Pharm Biopharm. 2018;131:232–239.
- Du JZ, Li HJ, Wang J. Tumor-acidity-cleavable maleic acid amide (TACMAA): a powerful tool for designing smart nanoparticles to overcome delivery barriers in cancer nanomedicine. Acc Chem Res. 2018;51(11):2848–2856.
- Melamed JR, Riley RS, Valcourt DM, et al. Using gold nanoparticles to disrupt the tumor microenvironment: an emerging therapeutic strategy. ACS Nano. 2016;10(12):10631–10635.
- Riley RS, June CH, Langer R, et al. Delivery technologies for cancer immunotherapy. Nat Rev Drug Discov. 2019;18(3):175–196.
- Bayda S, Hadla M, Palazzolo S, et al. Inorganic nanoparticles for cancer therapy: a transition from lab to clinic. CMC. 2018;25(34):4269–4303.
- Yan Y, Fu J, Wang T, et al. Controlled release of silyl ether camptothecin from thiol-ene click chemistry-functionalized mesoporous silica nanoparticles. Acta Biomater. 2017;51:471–478.
- Li X, Xie C, Xia H, et al. pH and ultrasound dual-responsive polydopamine-coated mesoporous silica nanoparticles for controlled drug delivery. Langmuir. 2018;34(34):9974–9981.
- Gounani Z, Asadollahi MA, Pedersen JN, et al. Mesoporous silica nanoparticles carrying multiple antibiotics provide enhanced synergistic effect and improved biocompatibility. Colloids Surf B Biointerfaces. 2019;175:498–508.
- Li H, Wu X, Yang B, et al. Evaluation of biomimetically synthesized mesoporous silica nanoparticles as drug carriers: Structure, wettability, degradation, biocompatibility and brain distribution. Mat Sci Eng C Mater. 2019;94(undefined):453–464.
- Gupta B, Poudel BK, Ruttala HB, et al. Hyaluronic acid-capped compact silica-supported mesoporous titania nanoparticles for ligand-directed delivery of doxorubicin. Acta Biomater. 2018;80:364–377.
- Khattabi AM, Talib WH, Alqdeimat DA. The effect of polymer length on the in vitro characteristics of a drug loaded and targeted silica nanoparticles. Saudi Pharm J. 2018;26(7):1022–1026.
- Tang X, Jing F, Lin B, et al. pH-responsive magnetic mesoporous silica-based nanoplatform for synergistic photodynamic therapy/chemotherapy. ACS Appl Mater Interfaces. 2018;10(17):15001–15011.
- Liu L, Cai R, Wang Y, et al. Polydopamine-assisted silver nanoparticle self-assembly on sericin/agar film for potential wound dressing application. IJMS. 2018;19(10). doi:10.3390/ijms19102875
- Rzayev ZMO, Bunyatova U, Lovell JF, et al. Ag-carried CMC/functional copolymer/ODA-Mt wLED-treated NC and their responses to brain cancer cells. Mater Sci Eng C, Mater. 2018;92:463–476.
- Cholula-Diaz JL, Lomeli-Marroquin D, Pramanick B, et al. Synthesis of colloidal silver nanoparticle clusters and their application in ascorbic acid detection by SERS. Colloids Surf B, Biointerfaces. 2018;163:329–335.
- Sood R, Chopra DS. Optimization of reaction conditions to fabricate Ocimum sanctum synthesized silver nanoparticles and its application to nano-gel systems for burn wounds. Materials Sci Eng C, Mater. 2018;92:575–589.
- Tang Y, Liang J, Wu A, et al. Co-delivery of trichosanthin and albendazole by nano-self-assembly for overcoming tumor multidrug-resistance and metastasis. ACS Appl Mater Interfaces. 2017;9(32):26648–26664.
- Al-Dhabi NA, Ghilan AM, Arasu MV, et al. Green biosynthesis of silver nanoparticles produced from marine Streptomyces sp. Al-Dhabi-89 and their potential applications against wound infection and drug resistant clinical pathogens. J Photochem Photobiol B, Biol. 2018;189:176–184.
- Tang L, Sun L, Zhao P, et al. Effect of activated carbon nanoparticles on lymph node harvest in patients with colorectal cancer. Colorectal Dis. (2018). doi:10.1111/codi.14538
- Kittana N, Assali M, Abu-Rass H, et al. Enhancement of wound healing by single-wall/multi-wall carbon nanotubes complexed with chitosan. IJN. 2018;13:7195–7206.
- Sajid MI, Jamshaid U, Jamshaid T, et al. Carbon nanotubes from synthesis to in vivo biomedical applications. Int J Pharm. 2016;501(1–2):278–299.
- Hemasa AL, Naumovski N, Maher WA, et al. Application of carbon nanotubes in chiral and achiral separations of pharmaceuticals, biologics and chemicals. Nanomaterials. 2017;7(7):186.
- Singh RP, Sharma G, Sonali , et al. Chitosan-folate decorated carbon nanotubes for site specific lung cancer delivery. Mater Sci Eng C, Mater. 2017;77:446–458.
- Suzuki Y, Tada-Oikawa S, Hayashi Y, et al. Single- and double-walled carbon nanotubes enhance atherosclerogenesis by promoting monocyte adhesion to endothelial cells and endothelial progenitor cell dysfunction. Part Fibre Toxicol. 2015;13(1):54.
- Reinholds I, Jansons M, Pugajeva I, et al. Recent applications of carbonaceous nanosorbents in solid phase extraction for the determination of pesticides in food samples. Crit Rev Anal Chem. 1-20 (2018).
- Martincic M, Tobias G. Filled carbon nanotubes in biomedical imaging and drug delivery. Expert Opin Drug Deliv. 2015;12(4):563–581.
- Zhao X, Tian K, Zhou T, et al. PEGylated multi-walled carbon nanotubes as versatile vector for tumor-specific intracellular triggered release with enhanced anti-cancer efficiency: optimization of length and PEGylation degree. Colloids Surf B Biointerfaces. 2018;168:43–49.
- Meng HM, Zhao D, Li N, et al. A graphene quantum dot-based multifunctional two-photon nanoprobe for the detection and imaging of intracellular glutathione and enhanced photodynamic therapy. Analyst. 2018;143(20):4967–4973.
- Yan ZY, Yao CX, Wan DY, et al. A sensitive and simple method for detecting Cu(2+) in plasma using fluorescent Bacillus amyloliquefaciens containing intracellularly biosynthesized CdSe quantum dots. Enzyme Microb Technol. 2018;119:37–44.
- Song ZL, Dai X, Li M, et al. Biodegradable nanoprobe based on MnO2 nanoflowers and graphene quantum dots for near infrared fluorescence imaging of glutathione in living cells. Mikrochim Acta. 2018;185(10):485
- Tian C, Wang L, Luan F, et al. An electrochemiluminescence sensor for the detection of prostate protein antigen based on the graphene quantum dots infilled TiO2 nanotube arrays. Talanta. 2019;191:103–108.
- Chowdhury AD, Ganganboina AB, Park EY, et al. Impedimetric biosensor for detection of cancer cells employing carbohydrate targeting ability of Concanavalin A. Biosens Bioelectron. 2018;122:95–103.
- Qu Z, Na W, Nie Y, et al. A novel fluorimetric sensing strategy for highly sensitive detection of phytic acid and hydrogen peroxide. Analytica Chimica Acta. 2018;1039:74–81.
- Liu JH, Li RS, Yuan B, et al. Mitochondria-targeting single-layered graphene quantum dots with dual recognition sites for ATP imaging in living cells. Nanoscale. 2018;10(36):17402–17408.
- Son J, Yi G, Yoo J, et al. Light-responsive nanomedicine for biophotonic imaging and targeted therapy. Adv Drug Deliv Rev. (2018). doi:10.1016/j.addr.2018.10.002
- Liu Q, Zhang J, He H, et al. Green preparation of high yield fluorescent graphene quantum dots from coal-tar-pitch by mild oxidation. Nanomaterials. 2018;8(10):844.
- Hong GL, Zhao HL, Deng HH, et al. Fabrication of ultra-small monolayer graphene quantum dots by pyrolysis of trisodium citrate for fluorescent cell imaging. IJN. 2018;13:4807–4815.
- Zabielska-Koczywas K, Wojtalewicz A, Uzarowska E, et al. Distribution of glutathione-stabilized gold nanoparticles in feline fibrosarcomas and their role as a drug delivery system for doxorubicin-preclinical studies in a murine model. IJMS. 2018;19(4). doi:10.3390/ijms19041021
- Singh P, Pandit S, Mokkapati V, et al. Gold nanoparticles in diagnostics and therapeutics for human cancer. IJMS. 2018;19(7):1979.
- Kalimuthu K, Lubin B-C, Bazylevich A, et al. Gold nanoparticles stabilize peptide-drug-conjugates for sustained targeted drug delivery to cancer cells. J Nanobiotechnol. 2018;16(1):34.
- Elahi N, Kamali M, Baghersad MH. Recent biomedical applications of gold nanoparticles: a review. Talanta. 2018;184:537–556.
- Kohout C, Santi C, Polito L. Anisotropic gold nanoparticles in biomedical applications. IJMS. 2018;19(11):3385.
- Zhang P, Li B, Du J, et al. Regulation the morphology of cationized gold nanoparticles for effective gene delivery. Colloids Surf B, Biointerfaces. 2017;157:18–25.
- Su X, Wang Y, Wang W, et al. Phospholipid encapsulated AuNR@Ag/Au nanosphere SERS tags with environmental stimulus responsive signal property. ACS Appl Mater Interfaces. 2016;8(16):10201–10211.
- Thies S, Simon P, Zelenina I, et al. In situ growth and size regulation of single gold nanoparticles in composite microgels. Small. 2018;14(51):1803589.
- Trouiller AJ, Bere E, Kalaani J, et al. Biocompatible spherical gold nanoparticles synthesis in aqueous tetraethylene oxide solution and their cellular uptake. J Nanosci Nanotechnol. 2019;19(7):3744–3754.
- Park JS, Ahn EY, Park Y. Asymmetric dumbbell-shaped silver nanoparticles and spherical gold nanoparticles green-synthesized by mangosteen (Garcinia mangostana) pericarp waste extracts. IJN. 2017;12:6895–6908.
- Wang Z, Chen L, Chu Z, et al. Gemcitabine-loaded gold nanospheres mediated by albumin for enhanced anti-tumor activity combining with CT imaging. Mater Sci Eng C, Mater. 2018;89:106–118.
- Battogtokh G, Gotov O, Ko YT. Chitosan-ceramide coating on gold nanorod to improve its physiological stability and reduce the lipid surface-related toxicity. Arch Pharm Res. 2017;40(3):356–363.
- Lakhani PM, Rompicharla SV, Ghosh B, et al. An overview of synthetic strategies and current applications of gold nanorods in cancer treatment. Nanotechnology. 2015;26(43):432001.
- Abadeer NS, Fulop G, Chen S, et al. Interactions of bacterial lipopolysaccharides with gold nanorod surfaces investigated by refractometric sensing. ACS Appl Mater Interfaces. 2015;7(44):24915–24925.
- Rao L, Bu LL, Ma L, et al. Platelet-facilitated photothermal therapy of head and neck squamous cell carcinoma. Angew Chem Int Ed. 2018;57(4):986–991.
- Zhang T, Gao N, Li S, et al. Single-particle spectroscopic study on fluorescence enhancement by plasmon coupled gold nanorod dimers assembled on DNA origami. J Phys Chem Lett. 2015;6(11):2043–2049.
- Xu Q, Wan J, Bie N, et al. A biomimetic gold nanocages-based nanoplatform for efficient tumor ablation and reduced inflammation. Theranostics. 2018;8(19):5362–5378.
- Xu N, Li J, Gao Y, et al. Apoptotic cell-mimicking gold nanocages loaded with LXR agonist for attenuating the progression of murine systemic lupus erythematosus. Biomaterials. 2019;197:380–392.
- Liang R, Xie J, Li J, et al. Liposomes-coated gold nanocages with antigens and adjuvants targeted delivery to dendritic cells for enhancing antitumor immune response. Biomaterials. 2017;149:41–50.
- Pang B, Yang X, Xia Y. Putting gold nanocages to work for optical imaging, controlled release and cancer theranostics. Nanomedicine (London, England). 2016;11(13):1715–1728.
- Battogtokh G, Gotov O, Kang JH, et al. Glycol chitosan-coated near-infrared photosensitizer-encapsulated gold nanocages for glioblastoma phototherapy. Nanomedicine . 2019;18:315–325.
- Park JH, Seo H, Kim DI, et al. Gold nanocage-incorporated poly(epsilon-caprolactone) (PCL) fibers for chemophotothermal synergistic cancer therapy. Pharmaceutics. 2019;11(2):60.
- Zhou G, Xiao H, Li X, et al. Gold nanocage decorated pH-sensitive micelle for highly effective photothermo-chemotherapy and photoacoustic imaging. Acta Biomater. 2017;64:223–236.
- Jiang P, Wang Y, Zhao L, et al. Applications of gold nanoparticles in non-optical biosensors. Nanomaterials. 2018;8(12):977.
- Lee JH, Cho HY, Choi HK, et al. Application of gold nanoparticle to plasmonic biosensors. IJMS. 2018;19(7). doi:10.3390/ijms19072021
- Coutinho C, Somoza A. MicroRNA sensors based on gold nanoparticles. Anal Bioanal Chem. 2019;411(9):1807–1824.
- Pannico M, Calarco A, Peluso G, et al. Functionalized gold nanoparticles as biosensors for monitoring cellular uptake and localization in normal and tumor prostatic cells. Biosensors. 2018;8(4):87.
- Hou W, Xia F, Alfranca G, et al. Nanoparticles for multi-modality cancer diagnosis: Simple protocol for self-assembly of gold nanoclusters mediated by gadolinium ions. Biomaterials. 2017;120:103–114.
- Shawky SM, Awad AM, Abugable AA, et al. Gold nanoparticles – an optical biosensor for RNA quantification for cancer and neurologic disorders diagnosis. IJN. 2018;13:8137–8151.
- Wang L, Zhang H, Wang C, et al. Poly-adenine-mediated spherical nucleic acids for strand displacement-based DNA/RNA detection. Biosens Bioelectron. 2019;127:85–91.
- Hamdy ME, Del Carlo M, Hussein HA, et al. Development of gold nanoparticles biosensor for ultrasensitive diagnosis of foot and mouth disease virus. J Nanobiotechnol. 2018;16(1):48.
- Suarasan S, Focsan M, Potara M, et al. Doxorubicin-incorporated nanotherapeutic delivery system based on gelatin-coated gold nanoparticles: formulation, drug release, and multimodal imaging of cellular internalization. ACS Appl Mater Interfaces. 2016;8(35):22900–22913.
- Zhang X. Gold nanoparticles: recent advances in the biomedical applications. Cell Biochem Biophys. 2015;72(3):771–775.
- Wang H, Han J, Lu W, et al. Facile preparation of gold nanocages and hollow gold nanospheres via solvent thermal treatment and their surface plasmon resonance and photothermal properties. J Colloid Interface Sci. 2015;440:236–244.
- Xin J, Wang S, Wang B, et al. AlPcS4-PDT for gastric cancer therapy using gold nanorod, cationic liposome, and Pluronic((R)) F127 nanomicellar drug carriers. IJN. 2018;13:2017–2036.
- Khutale GV, Casey A. Synthesis and characterization of a multifunctional gold-doxorubicin nanoparticle system for pH triggered intracellular anticancer drug release. Eur J Pharm Biopharm. 2017;119:372–380.
- Ren Y, Wang R, Gao L, et al. Sequential co-delivery of miR-21 inhibitor followed by burst release doxorubicin using NIR-responsive hollow gold nanoparticle to enhance anticancer efficacy. J Controlled Release. 2016;228:74–86.
- Parida S, Maiti C, Rajesh Y, et al. Gold nanorod embedded reduction responsive block copolymer micelle-triggered drug delivery combined with photothermal ablation for targeted cancer therapy. Biochim Biophys Acta Gen Subj. 2017;1861(1):3039–3052.
- Singh SP, Alvi SB, Pemmaraju DB, et al. NIR triggered liposome gold nanoparticles entrapping curcumin as in situ adjuvant for photothermal treatment of skin cancer. Int J Biol Macromol. 2018;110:375–382.
- Yang Y, Lin Y, Di D, et al. Gold nanoparticle-gated mesoporous silica as redox-triggered drug delivery for chemo-photothermal synergistic therapy. J Colloid Interface Sci. 2017;508:323–331.
- Chuang CC, Cheng CC, Chen P-Y, et al. Gold nanorod-encapsulated biodegradable polymeric matrix for combined photothermal and chemo-cancer therapy. IJN. 2018;14:181–193.
- Wang J, Bai R, Yang R, et al. Size- and surface chemistry-dependent pharmacokinetics and tumor accumulation of engineered gold nanoparticles after intravenous administration. Metallomics. 2015;7(3):516–524.
- Tsoi KM, Macparland SA, Ma XZ, et al. Mechanism of hard-nanomaterial clearance by the liver. Nat Mater. 2016;15(11):1212–1221.
- Zhou H, Fan Z, Li PY, et al. Dense and dynamic polyethylene glycol shells cloak nanoparticles from uptake by liver endothelial cells for long blood circulation. ACS Nano. 2018;12(10):10130–10141.
- Nunes SS, Fernandes RS, Cavalcante CH, et al. Influence of PEG coating on the biodistribution and tumor accumulation of pH-sensitive liposomes. Drug Deliv and Transl Res. 2019;9(1):123–130.
- Yang C, Bromma K, Chithrani D. Peptide mediated in vivo tumor targeting of nanoparticles through optimization in single and multilayer in vitro cell models. Cancers. 2018;10(3):84.
- Kumar P, Shenoi RA, Lai BF, et al. Conjugation of aurein 2.2 to HPG yields an antimicrobial with better properties. Biomacromolecules. 2015;16(3):913–923.
- Ma J, Hu Z, Wang W, et al. pH-sensitive reversible programmed targeting strategy by the self-assembly/disassembly of gold nanoparticles. ACS Appl Mater Interfaces. 2017;9(20):16767–16777.
- Wang J, Asghar S, Jin X, et al. Mitoxantrone-loaded chitosan/hyaluronate polyelectrolyte nanoparticles decorated with amphiphilic PEG derivates for long-circulating effect. Colloids Surf B, Biointerfaces. 2018;171:468–477.
- Zhong Y, Wang C, Cheng R, et al. cRGD-directed, NIR-responsive and robust AuNR/PEG–PCL hybrid nanoparticles for targeted chemotherapy of glioblastoma in vivo. J Controlled Release. 2014;195:63–71.
- Rajendrakumar SK, Chang NC, Mohapatra A, et al. A lipophilic IR-780 dye-encapsulated zwitterionic polymer-lipid micellar nanoparticle for enhanced photothermal therapy and NIR-based fluorescence imaging in a cervical tumor mouse model. IJMS. 2018;19(4):1189.
- Zhao J, Qin Z, Wu J, et al. Zwitterionic stealth peptide-protected gold nanoparticles enable long circulation without the accelerated blood clearance phenomenon. Biomaterials Sci. 2017;6(1):200–206.
- Ou H, Cheng T, Zhang Y, et al. Surface-adaptive zwitterionic nanoparticles for prolonged blood circulation time and enhanced cellular uptake in tumor cells. Acta Biomater. 2018;65:339–348.
- Shuaidong H, Ying J, Ziwen J, et al. Stable and oxidant responsive zwitterionic nanoclusters. Nanoscale. 2018;10(16):7382–7386.
- Thapa RK, Ku SK, Choi HG, et al. Vibrating droplet generation to assemble zwitterion-coated gold-graphene oxide stealth nanovesicles for effective pancreatic cancer chemo-phototherapy. Nanoscale. 2018;10(4):1742–1749.
- Le Guevel X, Henry M, Motto-Ros V, et al. Elemental and optical imaging evaluation of zwitterionic gold nanoclusters in glioblastoma mouse models. Nanoscale. 2018;10(39):18657–18664.
- Chen J, Chen Q, Liang C, et al. Albumin-templated biomineralizing growth of composite nanoparticles as smart nano-theranostics for enhanced radiotherapy of tumors. Nanoscale. 2017;9(39):14826–14835.
- Liu H, Liu T, Li L, et al. Size dependent cellular uptake, in vivo fate and light-heat conversion efficiency of gold nanoshells on silica nanorattles. Nanoscale. 2012;4(11):3523–3529.
- Tong X, Wang Z, Sun X, et al. Size dependent kinetics of gold nanorods in EPR mediated tumor delivery. Theranostics. 2016;6(12):2039–2051.
- Schmid G, Kreyling WG, Simon U. Toxic effects and biodistribution of ultrasmall gold nanoparticles. Arch Toxicol.. 2017;91(9):3011–3037.
- Terentyuk GS, Maslyakova GN, Suleymanova LV, et al. Circulation and distribution of gold nanoparticles and induced alterations of tissue morphology at intravenous particle delivery. J Biophoton.. 2009;2(5):292–302.
- Kong FY, Zhang JW, Li RF, et al. Unique roles of gold nanoparticles in drug delivery, targeting and imaging applications. Molecules. 2017;22(9). doi:10.3390/molecules22091445.
- Kang JW, Cho HJ, Lee HJ, et al. Polyethylene glycol-decorated doxorubicin/carboxymethyl chitosan/gold nanocomplex for reducing drug efflux in cancer cells and extending circulation in blood stream. Int J Biol Macromol. 2019;125:61–71.
- Madhusudhan A, Reddy G, Venkatesham M, et al. Efficient pH dependent drug delivery to target cancer cells by gold nanoparticles capped with carboxymethyl chitosan. IJMS. 2014;15(5):8216–8234.
- Penon O, Marin MJ, Russell DA, et al. Water soluble, multifunctional antibody-porphyrin gold nanoparticles for targeted photodynamic therapy. J Colloid Interface Sci. 2017;496:100–110.
- Daraee H, Eatemadi A, Abbasi E, et al. Application of gold nanoparticles in biomedical and drug delivery. Artif Cells Nanomed Biotechnol. 2016;44(1):410–422.
- Liu J, Detrembleur C, De Pauw-Gillet MC, et al. Gold nanorods coated with mesoporous silica shell as drug delivery system for remote near infrared light-activated release and potential phototherapy. Small. 2015;11(19):2323–2332.
- Aykac A, Martos-Maldonado MC, Casas-Solvas JM, et al. beta-Cyclodextrin-bearing gold glyconanoparticles for the development of site specific drug delivery systems. Langmuir. 2014;30(1):234–242.
- Van Der Heide S, Russell DA. Optimisation of immuno-gold nanoparticle complexes for antigen detection. J Colloid Interface Sci. 2016;471:127–135.
- Yan GH, Wang K, Shao Z, et al. Artificial antibody created by conformational reconstruction of the complementary-determining region on gold nanoparticles. Proc Natl Acad Sci USA. 2018;115(1):E34–E43.
- Biscaglia F, Rajendran S, Conflitti P, et al. Enhanced EGFR targeting activity of plasmonic nanostructures with engineered GE11 peptide. Adv Healthcare Mater. 2017;6(23):1700596.
- Zhang L, Liu C, Gao Y, et al. ZD2-engineered gold nanostar@metal-organic framework nanoprobes for T1-weighted magnetic resonance imaging and photothermal therapy specifically toward triple-negative breast cancer. Adv Healthcare Mater. 2018;7(24):1801144.
- Zhang N, Zhang S, Xu C, et al. Decoy oligodeoxynucleotides, polysaccharides, and targeted peptide-functionalized gold nanorods for the combined treatment of rheumatoid arthritis. Adv Healthcare Mater. 2018;7(23):1800982.
- Nguyen VD, Min HK, Kim CS, et al. Folate receptor-targeted liposomal nanocomplex for effective synergistic photothermal-chemotherapy of breast cancer in vivo. Colloids Surf B, Biointerfaces. 2019;173:539–548.
- Johnsen KB, Bak M, Melander F, et al. Modulating the antibody density changes the uptake and transport at the blood-brain barrier of both transferrin receptor-targeted gold nanoparticles and liposomal cargo. J Controlled Release. 2019;295:237–249.
- Penninckx S, Heuskin AC, Michiels C, et al. The role of thioredoxin reductase in gold nanoparticle radiosensitization effects. Nanomedicine. 2018;13(22):2917–2937.
- Vyas SP, Goswami R. Size-dependent cellular uptake and TLR4 attenuation by gold nanoparticles in lung adenocarcinoma cells. Nanomedicine. 2019;14(3):229–253.
- Yue J, Feliciano TJ, Li W, et al. Gold nanoparticle size and shape effects on cellular uptake and intracellular distribution of siRNA nanoconstructs. Bioconjugate Chem. 2017;28(6):1791–1800.
- Jiang Y, Huo S, Mizuhara T, et al. The interplay of size and surface functionality on the cellular uptake of sub-10 nm gold nanoparticles. ACS Nano. 2015;9(10):9986–9993.
- Aengenheister L, Dietrich D, Sadeghpour A, et al. Gold nanoparticle distribution in advanced in vitro and ex vivo human placental barrier models. J Nanobiotechnol. 2018;16(1):79.
- Wong AC, Wright DW. Size-dependent cellular uptake of DNA functionalized gold nanoparticles. Small. 2016;12(40):5592–5600.
- Ding L, Yao C, Yin X, et al. Size, shape, and protein corona determine cellular uptake and removal mechanisms of gold nanoparticles. Small. 2018;14(42):1801451.
- Kumar D, Mutreja I, Chitcholtan K, et al. Cytotoxicity and cellular uptake of different sized gold nanoparticles in ovarian cancer cells. Nanotechnology. 2017;28(47):475101.
- Kinnear C, Rodriguez-Lorenzo L, Clift MJ, et al. Decoupling the shape parameter to assess gold nanorod uptake by mammalian cells. Nanoscale. 2016;8(36):16416–16426.
- Favi PM, Gao M, Johana Sepulveda Arango L, et al. Shape and surface effects on the cytotoxicity of nanoparticles: Gold nanospheres versus gold nanostars. J Biomed Mater Res. 2015;103(11):3449–3462.
- Bd C, Aa G, Wc C. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006;6(4):662–668.
- Xie X, Liao J, Shao X, et al. The effect of shape on cellular uptake of gold nanoparticles in the forms of stars, rods, and triangles. Sci Rep. 2017;7(1):3827.
- Liu X, Huang N, Li H, et al. Surface and size effects on cell interaction of gold nanoparticles with both phagocytic and nonphagocytic cells. Langmuir. 2013;29(29):9138–9148.
- Xie Y, Huang Y, Tang D, et al. A competitive colorimetric chloramphenicol assay based on the non-cross-linking deaggregation of gold nanoparticles coated with a polyadenine-modified aptamer. Microchim Acta. 2018;185(12). doi:10.1007/s00604-018-3067-0
- Ular N, Uzer A, Durmazel S, et al. Diaminocyclohexane-functionalized/thioglycolic acid-modified gold nanoparticle-based colorimetric sensing of trinitrotoluene and tetryl. ACS Sens. 2018;3(11):2335–2342.
- Milewska-Hendel A, Zubko M, Karcz J, et al. Fate of neutral-charged gold nanoparticles in the roots of the Hordeum vulgare L. cultivar Karat. Sci Rep. 2017;7(1). doi:10.1038/s41598-017-02965-w
- Li JJ, Kawazoe N, Chen G. Gold nanoparticles with different charge and moiety induce differential cell response on mesenchymal stem cell osteogenesis. Biomaterials. 2015;54:226–236.
- Bai X, Zhang J, Chang YN, et al. Nanoparticles with high-surface negative-charge density disturb the metabolism of low-density lipoprotein in cells. IJMS. 2018;19(9):2790.
- Yi Y, Kim HJ, Zheng M, et al. Glucose-linked sub-50-nm unimer polyion complex-assembled gold nanoparticles for targeted siRNA delivery to glucose transporter 1-overexpressing breast cancer stem-like cells. J Controlled Release. 2019;295:268–277.
- Kumari N, Mathe VL, Dongre PM. Albumin nanoparticles conjugates binding with glycan – a strategic approach for targeted drug delivery. Int J Biol Macromol. 2019;126:74–90.
- Wang X, Li J, Kawazoe N, et al. Photothermal ablation of cancer cells by albumin-modified gold nanorods and activation of dendritic cells. Materials. 2018;12(1):31.
- Cui T, Liang JJ, Chen H, et al. Performance of doxorubicin-conjugated gold nanoparticles: regulation of drug location. ACS Appl Mater Interfaces. 2017;9(10):8569–8580.
- Rao KM, Kumar A, Suneetha M, et al. pH and near-infrared active; chitosan-coated halloysite nanotubes loaded with curcumin-Au hybrid nanoparticles for cancer drug delivery. Int J Biol Macromol. 2018;112:119–125.
- Song J, Zhou J, Duan H. Self-assembled plasmonic vesicles of SERS-encoded amphiphilic gold nanoparticles for cancer cell targeting and traceable intracellular drug delivery. J Am Chem Soc. 2012;134(32):13458–13469.
- Fu Y, Feng Q, Chen Y, et al. Comparison of two approaches for the attachment of a drug to gold nanoparticles and their anticancer activities. Mol Pharmaceutics. 2016;13(9):3308–3317.
- Guegain E, Tran J, Deguettes Q, et al. Degradable polymer prodrugs with adjustable activity from drug-initiated radical ring-opening copolymerization. Chem Sci. 2018;9(43):8291–8306.
- Santiago T, Devaux RS, Kurzatkowska K, et al. Surface-enhanced Raman scattering investigation of targeted delivery and controlled release of gemcitabine. IJN. 2017;12:7763–7776.
- Rajkumar S, Prabaharan M. Multi-functional core-shell Fe3O4@Au nanoparticles for cancer diagnosis and therapy. Colloids Surf B, Biointerfaces. 2019;174:252–259.
- Wang L, Hu Y, Hao Y, et al. Tumor-targeting core-shell structured nanoparticles for drug procedural controlled release and cancer sonodynamic combined therapy. J Controlled Release. 2018;286:74–84.
- Wei R, Xi W, Wang H, et al. In situ crystal growth of gold nanocrystals on upconversion nanoparticles for synergistic chemo-photothermal therapy. Nanoscale. 2017;9(35):12885–12896.
- Chen WH, Lei Q, Luo GF, et al. Rational design of multifunctional gold nanoparticles via host-guest interaction for cancer-targeted therapy. ACS Appl Mater Interfaces. 2015;7(31):17171–17180.
- Bao QY, Geng DD, Xue JW, et al. Glutathione-mediated drug release from Tiopronin-conjugated gold nanoparticles for acute liver injury therapy. Int J Pharm. 2013;446(1–2):112–118.
- Wang H, Cao G, Gai Z, et al. Magnetic/NIR-responsive drug carrier, multicolor cell imaging, and enhanced photothermal therapy of gold capped magnetite-fluorescent carbon hybrid nanoparticles. Nanoscale. 2015;7(17):7885–7895.
- Lajunen T, Viitala L, Kontturi LS, et al. Light induced cytosolic drug delivery from liposomes with gold nanoparticles. J Controlled Release. 2015;203:85–98.
- Li J, Hu Y, Hou Y, et al. Phase-change material filled hollow magnetic nanoparticles for cancer therapy and dual modal bioimaging. Nanoscale. 2015;7(19):9004–9012.
- Zhang Z, Liu C, Bai J, et al. Silver nanoparticle gated, mesoporous silica coated gold nanorods (AuNR@MS@AgNPs): low premature release and multifunctional cancer theranostic platform. ACS Appl Mater Interfaces. 2015;7(11):6211–6219.
- Cheng H, Huo D, Zhu C, et al. Combination cancer treatment through photothermally controlled release of selenous acid from gold nanocages. Biomaterials. 2018;178:517–526.
- Poudel BK, Soe ZC, Ruttala HB, et al. In situ fabrication of mesoporous silica-coated silver-gold hollow nanoshell for remotely controllable chemo-photothermal therapy via phase-change molecule as gatekeepers. Int J Pharm. 2018;548(1):92–103.
- Xu W, Qian J, Hou G, et al. Hyaluronic acid-functionalized gold nanorods with pH/NIR dual-responsive drug release for synergetic targeted photothermal chemotherapy of breast cancer. ACS Appl Mater Interfaces. 2017;9(42):36533–36547.
- Ting-Ting Z, Cong-Hui X, Wei Z, et al. A redox-activated theranostic nanoagent: toward multi-mode imaging guided chemo-photothermal therapy. Chem Sci. 2018;9(33):6749–6757.
- Wang Y, Zhang Z, Xu S, et al. pH, redox and photothermal tri-responsive DNA/polyethylenimine conjugated gold nanorods as nanocarriers for specific intracellular co-release of doxorubicin and chemosensitizer pyronaridine to combat multidrug resistant cancer. Nanomedicine. 2017;13(5):1785–1795.
- Cao H, Yang Y, Xin C, et al. Intelligent Janus nanoparticles for intracellular real-time monitoring of dual drug release. Nanoscale. 2016;8(12):6754–6760.