Abstract
Background
Ras-PI3K pathway aberrant activation plays an important role in the occurrence and development of osteosarcoma. This study investigated the functions of Ras-PI3K pathway specific activation on histone H2A phosphorylation at threonine 120 (H2AT120ph) in osteosarcoma cells, along with the possible internal molecular mechanisms.
Methods
Cell transfection was done to alter RasG12V/Y40C, H2AT120ph and vaccinia-related kinase 1 (VRK1) expression. Then, cell viability, proliferation, migration and cell cycle distribution were assessed, respectively. qRT-PCR was utilized to measure the VRK1 and Ras-PI3K pathway downstream genes (CYR61, IGFBP3, WNT16B, NT5E, GDF15 and CARD16) expression. Chromatin immunoprecipitation (ChIP) was conducted to evaluate the input levels of H2AT120ph and VRK1 in the promoter regions of Ras-PI3K pathway downstream genes.
Results
Ras-PI3K specific activation promoted histone H2AT120ph. H2AT120ph participated in the oncogenic functions of Ras-PI3K pathway on osteosarcoma by modulating the transcription of Ras-PI3K-targeted genes. Moreover, VRK1 contributed to the Ras-PI3K specific activation-induced up-regulation of H2AT120ph and osteosarcoma progression. Ras-PI3K pathway-specific activation-induced up-regulation of H2AT120ph was achieved by up-regulation of VRK1.
Conclusions
Ras-PI3K pathway activation promoted osteosarcoma progression might be via up-regulating VRK1-mediated H2AT120ph. We proposed that VRK1 and H2AT120ph could be the potential targets for osteosarcoma diagnosis and treatment.
H2AT120ph is specifically promoted by Ras-PI3K pathway activation.
H2AT120ph joins in the oncogenic effects of Ras-PI3K pathway on osteosarcoma.
H2AT120ph regulates the transcription of Ras-PI3K-targeted genes.
VRK1 takes part in the regulatory function of Ras-PI3K pathway on H2AT120ph.
Highlights
Background
Osteosarcoma, deriving from the aberrant growth of primitive bone-forming mesenchymal cells, is the most common malignant bone tumour in children and adolescents [Citation1,Citation2]. Ras-phosphatidylinositol 3-kinase (PI3K) pathway aberrant activation plays an important role in the occurrence and development of osteosarcoma [Citation3,Citation4]. It is proved that Ras-PI3K pathway takes part in the modulation of multiple osteosarcoma cellular biological processes [Citation4,Citation5]. A number of genes that exert critical regulatory activities in cell proliferation and metastasis are the downstream target genes of Ras-PI3K pathway in cells [Citation6,Citation7]. However, there is little information available about the definite effects of Ras-PI3K pathway activation on epigenetic changes of osteosarcoma cells. Histone modification is a common epigenetic change, which consists of methylation, acetylation, phosphorylation, ubiquitination and adenosine-diphosphate (ADP)-ribosylation [Citation8,Citation9]. There are generally five subtypes of histone in eukaryotic cells: H1, H2A, H2B, H3 and H4 [Citation10]. Histone H2A phosphorylated at threonine 120 (H2AT120ph) is a key histone phosphorylation pathway in cells [Citation11]. Kim et al. reported that H2AT120ph is involved in the regulation of multiple tumour suppressor genes transcriptions in many cancer cells, such as bladder cancer, breast cancer and prostate cancer [Citation12]. However, the effects of H2AT120ph on the gene transcription of osteosarcoma cells remain unclear.
Vaccinia-related kinase 1 (VRK1) is a nuclear serine-threonine kinase that can promote histone phosphorylation, including histone H2A phosphorylation [Citation13]. Increasing numbers of reports provided evidence that VRK1 is highly expressed in many cancer tissues and cells and exerts pro-proliferative and pro-migratory effects in cancer cells [Citation14,Citation15]. Molitor and Traktman indicated that overexpression of VRK1 promotes the proliferation and survival of cell lines derived from the normal or malignant mammary tissue, while the silence of VRK1 inhibits the proliferation and metastasis of MDA-MB-231 cells [Citation16]. The regulatory roles of VERK in osteosarcoma cells remain unclear yet. More researches are still needed.
MG63 cell line is an osteosarcoma cell line established from the bone tissue of a 14-year-old male, Caucasian boy [Citation17]. It is a hypotriploid cell line that the modal chromosome number is 66 occurring in 44% of cells and the rate of cells with higher ploidies is 2.0% [Citation17]. All cells have 18 to 19 marker chromosomes [Citation17]. U2OS cell line is another osteosarcoma cell line established from the bone tissue of a 15-year-old female Caucasian girl [Citation18]. It is chromosomally highly altered, with chromosome counts in the hypertriploid range and secretes a growth factor structurally related to a homodimer of platelet-derived growth factor (PDGF) A-chains [Citation18]. In this research, we investigated the effects of Ras-PI3K specific activation on H2AT120ph in osteosarcoma MG63 and U2OS cells. The regulatory roles of Ras-PI3K in VRK1, as well as VRK1 in H2AT120ph were also analyzed. This study will offer epigenetic evidence for further comprehending the occurrence and development of osteosarcoma.
Materials and methods
Plasmid construction and siRNA synthesis
The coding sequences of human K-Ras and H2A were amplified using polymerase chain reaction (PCR) and inserted into p-enhanced green fluorescent protein (p-EGFP)-N1 plasmid (Clontech Laboratories, Palo Alto, CA), respectively, which were referred as pEGFP-RasWT and pEGFP-H2A. The pEGFP-RasG12V was mutated using site-directed mutagenesis. pEGFP-RasG12V/Y40C was mutated using pEGFP-RasG12V as a template. pEGFP-H2AT120A and pEGFP-H2AT120D were generated using TaKaRa MutanBEST kit (TaKaRa Biotechnology, Japan). si-VRK1 and its negative control (siNC) were provided by Invitrogen (Carlsbad, CA).
Cell culture
MG63 and U2OS cells were obtained from the Cell Culture Centre of Peking Union Medical College (Beijing, China) and grown in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Carlsbad, CA) including 10% foetal bovine serum (FBS, Gibco) at 37 °C with 5% CO2.
Cell transfection
MG63 or U2OS cells were cultivated in a 6-well plate (5 × 105 cells/well) overnight. Then, plasmids or siRNAs were transfected alone or co-transfected using Lipofectamine 2000 reagent (Invitrogen) [Citation19]. Forty-eight hours later, transfection was stopped by changing culture medium.
qRT-PCR
Total RNAs were separated using TRIzolTM Plus RNA Purification kit (Invitrogen). cDNA was synthesized using SuperScriptTM IV First-Stand Synthesis System (Invitrogen) following with the instruction of manufacturer. Then, the VRK1, Cysteine-rich 61 (CYR61), Insulin-like growth-factor binding protein 3 (IGFBP3), WNT16B, ecto-5′-nucleotidase (NT5E), Growth/differentiation factor 15 (GDF15) and Caspase recruitment domain 16 (CARD16) mRNA levels were measured using SYBR Green ERTM qPCR SuperMix Universal (Invitrogen) and normalised to GAPDH expression. Data were calculated by 2−ΔΔCt method [Citation20].
Cell viability assay
Viability of MG63 and U2OS cells was detected using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltertrazolium bromide tetrazolium (MTT) assay (Beyotime Biotechnology, Shanghai, China) [Citation21]. Briefly, after relevant transfection, MG63 or U2OS cells were cultivated in a 96-well plate (5 × 103 cells/well) for 48 h. Then, 10 μL MTT (5 mg/mL) was mixed into the culture medium for 4 h. After centrifugation at 1500 g for 5 min, the culture supernatant was removed and 150 μL dimethyl sulfoxide (DMSO) was added into each well. Followed by agitating, the absorbance of each well was tested by Micro-plate reader (Bio-Tek Instruments, Winooski, VT) at 570 nm.
Soft-agar colony formation assay
The proliferation of MG63 and U2OS cells was assessed using a soft-agar colony formation assay [Citation22]. Briefly, after relevant transfection, 1 × 103 MG63 or U2OS cells were suspended in DMEM containing 0.35% low-melting agarose (Sigma-Aldrich, St Louis, MO) and seeded into a 6-well plate pre-coating with 0.6% low-melting agarose. After 3 weeks incubation, the number of colonies in each group was counted under microscope (40×, Nikon, Chiyoda, Japan).
Two-chamber migration assay
Migration of MG63 and U2OS cells was measured using a two-chamber migration assay (Corning Incorporated, New York, NY) with 8 μm pore filters as earlier described [Citation23]. The incubation time was 48 h. Migrated cells in the lower chamber were stained using 0.5% crystal violet solution (Beyotime Biotechnology) and counted under microscope (40×, Nikon). Then, the crystal violet adhered in the lower chamber was dissolved into 33% acetic acid, and the optical density (OD) value was measured at 570 nm using Micro-plate reader.
Cell cycle distribution assay
Cell cycle distribution of MG63 was evaluated using flow cytometric analysis as earlier described [Citation21]. Briefly, after relevant transfection, MG63 cells in each group were harvested, washed with ice-cold phosphate buffer saline (PBS) and fixed with 1% paraformaldehyde (Sigma-Aldrich) solution. After washing, the MG63 cells were re-suspended into 70% ethanol at 4 °C overnight. Then, cells were rehydrated in PBS, incubated using 100 μg/mL RNase A for 30 min at 37 °C and stained using 50 μg/mL propidium iodide (PI) for 30 min in the dark. Data were analyzed using flow cytometry (Guava Technologies, Hayward, CA).
Western blotting
After relevant transfection and/or treatment, total proteins in MG63 or U2OS cells were extracted using radio-immunoprecipitation assay (RIPA) Lysis Buffer (Beyotime Biotechnology). BCA Protein Assay kit was done to measure the concentration of total proteins. Western blotting was done as reported before [Citation21]. The nitrocellulose membranes were incubated with primary antibodies at 4 °C overnight, and then incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies for 1 h at 20–25 °C in the dark. The protein signals were tested by Western Blotting Luminol Reagent (Santa Biotechnology, Santa Cruz, CA). The densities of bands were quantified using Gel-Pro Analyser Version 4.0 software (Media Cybernetics, Bethesda, MD).
Chromatin immunoprecipitation (ChIP)
ChIP was carried out as previously described [Citation24]. Briefly, after different transfection, approximately 3 × 106 MG63 cells were fixed with 1% paraformaldehyde, rinsed twice with cold PBS and lysed using SDS Lysis Buffer (Upstate Biotechnology, Lake Placid, NY). Then, the lysates were sonicated in ultrasonic bath (Ultrasonic Processor VCX-750, 25% power, 4.5 s impact, 9 s gap) and centrifuged. The supernatants were mixed with ChIP Dilution Buffer (Upstate Biotechnology) and immunoprecipitated overnight with 2 μg Rabbit anti-H2AT120ph or Rabbit anti-VRK1 antibodies at 4 °C. Rabbit anti-IgG served as control. After that, the beads were rinsed using low-salt buffer, high-salt buffer, LiCl buffer (all Upstate Biotechnology) and 1 × TE solution (Upstate Biotechnology). The DNA was eluted from the beads in 100 mM NaHCO3 (containing 1% SDS) and precipitated with ethanol, mixed with 20 μg proteinase K and purified using QIAquick PCR Purification Columns (Qiagen, Germantown, MD). Finally, the amount of immunoprecipitated DNA and input DNA was analyzed using qRT-PCR.
Statistical analysis
All experiments were duplicated three times. Statistical analysis was performed using SPSS 19.0 software. Results were shown as mean ± standard deviation (SD). The p-value was analyzed using Student’s t-test. Statistical significance was set at p < .05.
Results
H2AT120ph was specifically up-regulated by the Ras-PI3K pathway
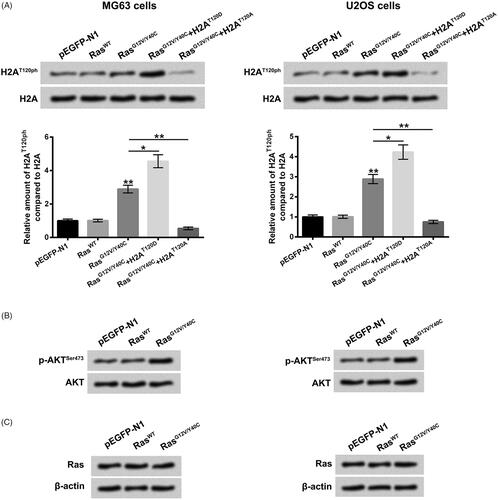
Firstly, to specific activate Ras-PI3K pathway in MG63 cells, the pEGFP-N1 plasmid inserted with Ras mutated at G12V and Y40C (RasG12V/Y40C) was transfected into MG63 cells. Empty pEGFP-N1 plasmid and pEGFP-N1 plasmid inserted with wild-type Ras (RasWT) severed as controls. showed that the relative amount of H2AT120ph compared to H2A was significantly enhanced in MG63 and U2OS cells after RasG12V/Y40C transfection, relative to pEGFP-N1 or RasWT transfection (p < .01), which suggested that RasG12V/Y40C specific activation could promote histone H2AT120ph. Moreover, to mimic the phosphorylation of H2AT120, the threonine at 120 sites of H2A was mutated to aspartic acid (D), which was referred as H2AT120D. To mimic the de-phosphorylation of H2AT120, the threonine in 120 sites of H2A was mutated to alanine (A), which was referred as H2AT120A. Results displayed that H2AT120D transfection notably enhanced the relative amount of H2AT120ph compared to H2A, while H2AT120A transfection obviously reduced the relative amount of H2AT120ph compared to H2A in MG63 and U2OS cells (, p < .05 or p < .01). Besides, the result of displayed that RasWT transfection had no significant effect on p-protein kinase B (AKT)Ser473 protein expression level, while RasG12V/Y40C transfection elevated the p-AKTSer473 protein level in MG63 and U2OS cells. The total Ras expression levels after RasWT or RasG12V/Y40C transfection were not changed in MG63 and U2OS cells (. Taken together, these above findings implied that RasG12V/Y40C specific activation could activate PI3K/AKT pathway and regulated H2AT120ph in osteosarcoma MG63 and U2OS cells.
Figure 1. H2AT120ph was specifically promoted by Ras-PI3K pathway activation. (A) Empty-pEGFP-N1, pEGFP-RasWT, pEGFP-RasG12V/Y40C and/or H2AT120D or H2AT120A plasmids were transfected into MG63 or U20S cells, respectively. The H2AT120ph expression was measured using western blotting followed by densitometric analysis. (B) Empty-pEGFP-N1, pEGFP-RasWT or pEGFP-RasG12V/Y40C plasmid was transfected into MG63 or U2OS cells, respectively. The phosphate-AKTSer473 expression was measured using western blotting. (C) Empty-pEGFP-N1, pEGFP-RasWT or pEGFP-RasG12V/Y40C plasmid was transfected into MG63 or U2OS cells, respectively. The Ras expression was assessed using western blotting. H2AT120ph: Histone H2A phosphorylated on threonine 120; PI3K: Phosphatidylinositol 3-kinase. *p < .05; **p < .01 (n = 3).
H2AT120ph joined in the oncogenic effects of Ras-PI3K pathway on osteosarcoma
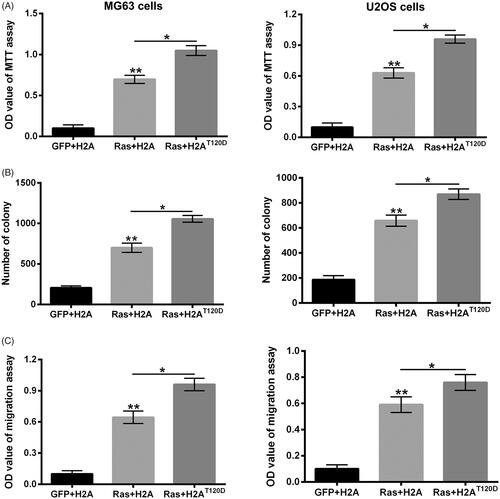
To confirm whether H2AT120ph joined in the oncogenic effects of Ras-PI3K pathway, the MG63 and U2OS cell viability, proliferation and migration were respectively detected after RasG12V/Y40C and/or H2AT120A or H2AT120D transfection. presented that RasG12V/Y40C transfection dramatically enhanced the OD value of MG63 and U2OS cells (p < .01), while H2AT120A transfection obviously alleviated the RasG12V/Y40C transfection-induced enhancement of OD value in a concentration-dependent pathway (p < .05 or p < .01), which suggested that H2AT120ph might participate in the promoting effects of RasG12V/Y40C specific activation on MG63 cell viability. Similar results were found in , which showed that RasG12V/Y40C transfection significantly promoted the proliferation and migration of MG63 and U2OS cells (p < .01), while H2AT120A transfection notably attenuated the RasG12V/Y40C transfection-induced enhancement of MG63 and U2OS cell proliferation and migration (p < .05). On the contrary, H2AT120D transfection remarkably promoted the RasG12V/Y40C transfection-induced MG63 and U2OS cell viability, proliferation and migration (, p < .05). These findings indicated that H2AT120ph joined in the oncogenic activity of Ras-PI3K pathway on osteosarcoma.
Figure 2. H2AT120ph joined in the oncogenic effects of Ras-PI3K on osteosarcoma. pEGFP-N1, pEGFP-H2A, pEGFP-RasG12V/Y40C and/or pEGFP-H2AT120A were indicated as GFP, H2A, Ras and H2AT120A, respectively. (A) The viabilities of MG63 and U2OS cells were detected using MTT assay after transfection with GFP, H2A, Ras and/or increasing amounts of H2AT120A (0.5, 1 or 2 μM). (B) The numbers of MG63 and U2OS cell colonies were counted after transfection with GFP, H2A, Ras and/or 2 μM H2AT120A. (C) The migration of MG63 and U2OS cells was assessed using two-chamber transwell assay after transfection with GFP, H2A, Ras and/or 2 μM H2AT120A. *p < .05; **p < .01 (n = 3).
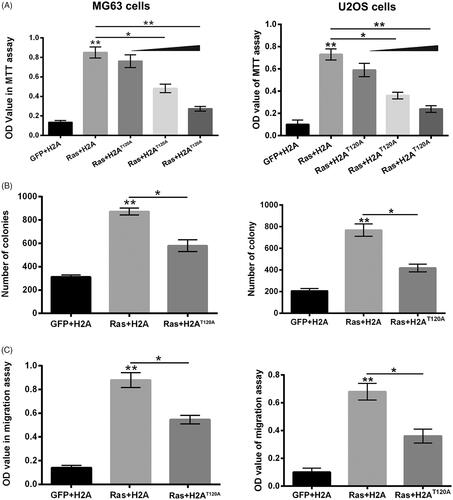
Figure 3. H2AT120D promoted the oncogenic effects of Ras-PI3K on osteosarcoma. pEGFP-N1, pEGFP-H2A, pEGFP-RasG12V/Y40C and/or pEGFP-H2AT120D were indicated as GFP, H2A, Ras and H2AT120D, respectively. (A) The viabilities of MG63 and U2OS cells were detected using MTT assay after transfection with GFP, H2A, Ras and/or 2 μM H2AT120D. (B) The numbers of MG63 and U2OS cell colonies were counted after transfection with GFP, H2A, Ras and/or 2 μM H2AT120D. (C) The migration of MG63 and U2OS cells was assessed using two-chamber transwell assay after transfection with GFP, H2A, Ras and/or 2 μM H2AT120D. *p < .05; **p < .01 (n = 3).
H2AT120ph regulated the transcription of Ras-PI3K-targeted genes in osteosarcoma cells
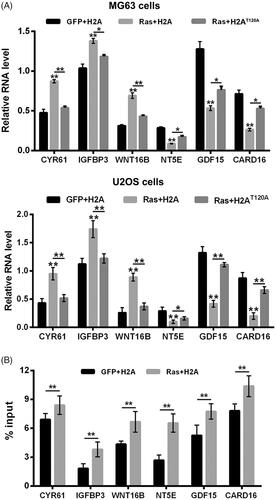
Next, the transcription levels of Ras-PI3K downstream genes in MG63 and U2OS cells were also assessed after RasG12V/Y40C and/or H2AT120A transfection. Results in displayed that RasG12V/Y40C transfection noticeably up-regulated the transcription levels of CYR61, IGFBP3 and WNT16B, and down-regulated the transcription levels of NT5E, GDF15 and CARD16 in MG63 and U2OS cells (p < .01). H2AT120A transfection obviously attenuated the RasG12V/Y40C transfection-induced up-regulation of CYR61, IGFBP3 and WNT16B transcription, as well as down-regulation of NT5E, GDF15 and CARD16 transcription in MG63 and U2OS cells (p < .05 or p < .01). These outcomes illustrated that H2AT120ph participated in the transcription regulation of Ras-PI3K-targeted genes. In addition, ChIP results in showed that RasG12V/Y40C transfection significantly enhanced the input levels of H2AT120ph in the promoter regions of CYR61, IGFBP3, WNT16B, NT5E, GDF15 and CARD16 in MG63 cells (p < .01) in MG63 cells, which evidenced that RasG12V/Y40C specific activation could promote the binding activity of H2AT120ph on the promoter regions of PI3K downstream genes in osteosarcoma cells.
Figure 4. H2AT120ph regulated the transcription of Ras-PI3K-targeted genes in osteosarcoma cells. pEGFP-N1, pEGFP-H2A, pEGFP-RasG12V/Y40C and pEGFP-H2AT120A were indicated as GFP, H2A, Ras and H2AT120A, respectively. (A) The CYR61, IGFBP3, WNT16B, NT5E, GDF15 and CARD16 mRNA expressions in MG63 and U2OS cells were tested using real-time PCR after transfection with GFP, H2A, Ras and/or 2 μM H2AT120A. (B) The input levels of H2AT120ph in promoter regions of CYR61, IGFBP3, WNT16B, NT5E, GDF15 and CARD16 in MG63 cells were evaluated using chromatin immunoprecipitation (ChIP) after transfection with GFP, H2A and/or Ras. *p < .05; **p < .01 (n = 3).
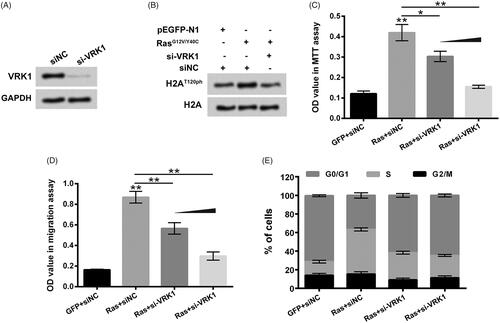
Suppression of VRK1 alleviated Ras-PI3K-induced up-regulation of H2AT120phand suppressed osteosarcoma progression
To analyze the roles of VRK1 in RasG12V/Y40C transfection-induced up-regulation of H2AT120ph, as well as enhancement of MG63 cell viability and migration, si-VRK1 was transfected into MG-63 cells. presented that si-VRK1 transfection decreased the VRK1 protein level in MG63 cells. showed that compared to single RasG12V/Y40C transfection, the H2AT120ph expression in MG63 cells was decreased after RasG12V/Y40C+si-VRK1 co-transfection, which suggested that VRK1 suppression could alleviate the RasG12V/Y40C specific activation-induced up-regulation of H2AT120ph. The results of displayed that similar to H2AT120A transfection, VRK1 suppression also significantly attenuated the RasG12V/Y40C transfection-induced enhancement of MG63 cell viability and migration in a concentration-dependent pathway (p < .05 or p < .01). Moreover, illustrated that RasG12V/Y40C transfection promoted the rate of MG63 cells in cell cycle S phase, while VRK1 suppression weakened the RasG12V/Y40C transfection-induced increases of MG63 cell in cell cycle S phase. These findings illustrated that suppression of VRK1 could alleviate Ras-PI3K pathway-specific activation-induced up-regulation of H2AT120ph and suppressed osteosarcoma progression.
Figure 5. Suppression of VRK1 alleviated Ras-PI3K-induced up-regulation of H2AT120ph and suppressed osteosarcoma progression. (A) After siNC or si-VRK1 transfection, the VRK1 protein level in MG63 cells was detected using western blotting. (B) After pEGFP-N1, pEGFP-RasG12V/Y40C, si-VRK1 and/or siNC transfection, the H2AT120ph expression in MG63 cells was measured using western blotting. pEGFP-N1, and pEGFP-RasG12V/Y40C were indicated as GFP and Ras, respectively. After GFP, Ras, siNC and/or si-VRK1 transfection, (C) the viability of MG63 cells was detected using MTT assay; (D) the migration of MG63 cells was measured using two-chamber transwell assay; (E) the cell cycle distribution of MG63 cells was assessed using flow cytometric analysis. VRK1: Vaccinia-related kinase 1. *p < .05; **p < .01 (n = 3).
Ras-PI3K pathway-specific activation-induced up-regulation of H2AT120ph was achieved by up-regulation of VRK1
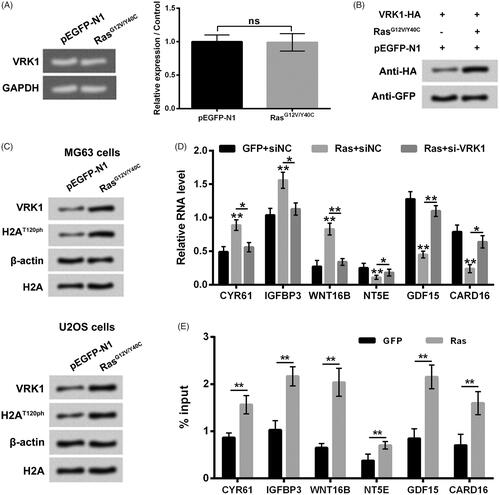
Finally, the regulatory effect of RasG12V/Y40C specific activation on VRK1 expression in osteosarcoma cells was investigated. showed that RasG12V/Y40C transfection had no significant effect on VRK1 mRNA expression levels in MG63 cells. presented that RasG12V/Y40C transfection enhanced the VRK1 protein level in MG63 cells. displayed that the up-regulation of VRK1 and up-regulation of H2AT120ph were synchronous in MG63 and U2OS cells after RasG12V/Y40C transfection, which implied that RasG12V/Y40C specific activation-induced up-regulation of H2AT120ph might be achieved by up-regulation of VRK1. The results of showed that similar to H2AT120A transfection, si-VRK1 transfection also mitigated the RasG12V/Y40C transfection-induced up-regulation of CYR61, IGFBP3 and WNT16B transcription, as well as down-regulation of NT5E, GDF15 and CARD16 transcription in MG63 cells. Moreover, RasG12V/Y40C transfection also notably enhanced the input levels of VRK1 in the promoter regions of CYR61, IGFBP3, WNT16B, NT5E, GDF15 and CARD16 in MG63 cells (p < .01). Taken together, these above findings indicated that Ras-PI3K pathway-specific activation-induced up-regulation of H2AT120ph was achieved by up-regulation of VRK1.
Figure 6. Ras-PI3K pathway specific activation-induced up-regulation of H2AT120ph was achieved by up-regulation of VRK1. (A, B) After pEGFP-N1 or pEGFP-RasG12V/Y40C transfection, the VRK1 mRNA and protein levels were detected using real-time PCR and western blotting, respectively. (C) After pEGFP-N1 or pEGFP-RasG12V/Y40C transfection, the VRK1 and H2AT120ph expression in MG63 and U2OS cells were measured using western blotting. (D) The CYR61, IGFBP3, WNT16B, NT5E, GDF15 and CARD16 mRNA expressions in MG63 cells were tested using real-time PCR after transfection with pEGFP-N1, pEGFP-RasG12V/Y40C and/or siNC or si-VRK1. (E) The input levels of VRK1 in promoter regions of CYR61, IGFBP3, WNT16B, NT5E, GDF15 and CARD16 in MG63 cells were evaluated using chromatin immunoprecipitation (ChIP) after transfection with pEGFP-N1 or pEGFP-RasG12V/Y40C. VRK1: Vaccinia-related kinase 1. **p < .01 (n = 3).
Discussion
With high rates of recurrence and metastasis, osteosarcoma has become one of the most common threats to the health and life of children and adolescents [Citation1]. Defining the pathogenesis of osteosarcoma will be helpful for osteosarcoma diagnosis and treatment [Citation25]. Herein, we revealed that Ras-PI3K specific activation could promote histone H2AT120ph. H2AT120ph participated in the oncogenic activity of Ras-PI3K pathway on osteosarcoma by modulating the transcription of Ras-PI3K-targeted genes. Moreover, we found that VRK1 contributed to the Ras-PI3K specific activation-induced up-regulation of H2AT120ph and osteosarcoma progression. Ras-PI3K pathway-specific activation-induced up-regulation of H2AT120ph might be achieved by up-regulation of VRK1.
As a well-known oncogenic pathway in cells, Ras-PI3K pathway has been proved to be closely related to the occurrence and progression of multiple cancers, including osteosarcoma [Citation3,Citation26]. The aberrant activation of Ras-PI3K pathway can promote osteosarcoma cell growth, proliferation, adhesion and metastasis [Citation27,Citation28]. Histone modification, a very important phenomenon of epigenetic changes, can affect the structure of chromatin and alter the expression of multiple genes that contribute to the regulation of a variety of cell biological processes [Citation8]. The enhancement of H2AT120ph has been demonstrated to be implicated in the progression of a number of cancers [Citation12]. In the present study, we found that RasG12V/Y40C transfection could activate the PI3K/AKT pathway and enhance the expression of H2AT120ph in osteosarcoma MG63 and U2OS cells. Moreover, blockade of H2AT120ph could alleviate the RasG12V/Y40C transfection-induced increases of MG63 cell viability, proliferation and migration, while up-regulation of H2AT120ph had opposite effects. These findings suggested that Ras-PI3K pathway activation-induced enhancement of H2AT120ph might be associated with the growth and metastasis of osteosarcoma cells. Earlier pieces of literature reported that Ras-PI3K-AKT pathway activation also could cause other histone modification in cancer cells. For example, Liu et al. and Li et al. indicated that Ras-PI3K pathway activation could reduce the acetylation of histone H3 at lysine 56 (H3K56ac) and then promote the proliferation and migration of HeLa cells, as well as the occurrence and development of uveal melanoma [Citation29,Citation30]. Moreover, Sang et al. discovered that Ras-AKT pathway activation could inhibit the phosphorylation of histone H1.5 at threonine 10 (H1.5T10ph) and then promote the development of glioma [Citation31]. We could propose that Ras-PI3K-AKT pathway activation might take part in the regulation of the number of histone modifications in cancer cells, which may form a very intricate regulatory network. More experiments are still needed in the future to comprehensive analyze this regulatory network in different cancer cells.
Histone modification, including histone phosphorylation, usually influences the expression of genes via modulating gene transcription [Citation32]. CYR61 is a growth factor-inducible early gene that can promote cell proliferation, migration and adhesion [Citation33]. IGFBP3 has been proved to be involved in cell growth and proliferation and play key roles in the aetiology of many cancers [Citation34,Citation35]. WNT16B is a member of Wnt family, which can promote tumour growth and acquired resistance to chemotherapy [Citation36]. NT5E, GDF15 and CARD16 have also been demonstrated to participate in the regulation of cell proliferation and migration [Citation37–39]. They are all the downstream genes of Ras-PI3K pathway. Herein, we discovered that Ras-PI3K pathway activation elevated the CYR61, IGFBP3 and WNT16B transcription, and reduced the NT5E, GDF15 and CARD16 transcription in osteosarcoma MG63 and U2OS cells. Blockade of H2AT120ph attenuated the Ras-PI3K pathway activation-induced up-regulation of CYR61, IGFBP3 and WNT16B transcription, as well as down-regulation of NT5E, GDF15 and CARD16 transcription. More importantly, we revealed that Ras-PI3K pathway activation promoted the binding activity of H2AT120ph on the promoter regions of CYR61, IGFBP3, WNT16B, NT5E, GDF15 and CARD16. These outcomes suggested that Ras-PI3K presented oncogenic activity in osteosarcoma might be via promoting H2AT120ph, enhancing H2AT120ph activity to bind to the promoter of downstream genes, and then modulating downstream genes transcription.
VRK1 is a key histone phosphorylation kinase in cells [Citation40]. Aihara et al. reported that VRK1 could promote H2AT120ph [Citation13]. Herein, we discovered that suppression of VRK1 could attenuate the Ras-PI3K activation-induced up-regulation of H2AT120ph. In addition, similarly to H2AT120ph blockade, suppression of VRK1 also could weaken the Ras-PI3K activation-induced increases of MG63 cell viability, proliferation and migration. These findings were consistent with the previous studies, which reported that knockdown of VRK1 reduced the proliferation and metastasis of breast cancer [Citation16], oesophageal squamous cell carcinoma [Citation41] and hepatocellular carcinoma [Citation42]. Besides, we pointed out that Ras-PI3K activation elevated the VRK1 protein level in MG63 cells but had no significant effect on VRK1 mRNA level. The up-regulations of VRK1 and H2AT120ph were synchronous in MG63 and U2OS cells after RasG12V/Y40C transfection. The depletion of VRK1 could mitigate the RasG12V/Y40C transfection-induced up-regulation of CYR61, IGFBP3 and WNT16B transcription, as well as down-regulation of NT5E, GDF15 and CARD16 transcription in MG63 cells. Moreover, Ras-PI3K pathway activation also promoted the binding activity of VRK1 on the promoter regions of CYR61, IGFBP3, WNT16B, NT5E, GDF15 and CARD16. These findings suggested that Ras-PI3K activation-induced up-regulation of H2AT120ph might be achieved by up-regulation of VRK1.
Conclusions
To sum up, this research revealed that Ras-PI3K pathway activation promoted osteosarcoma progression might be via up-regulating VRK1-mediated H2AT120ph. The findings of our study provide the epigenetic evidence for comprehending the pathogenesis of osteosarcoma further. Besides, we propose that VRK1 and H2AT120ph might be the potential targets for osteosarcoma diagnosis and treatment.
Disclosure statement
The authors declare that there is no conflict of interest.
References
- Marina N, Gebhardt M, Teot L, et al. Biology and therapeutic advances for pediatric osteosarcoma. The Oncol. 2004;9(4):422–441.
- Gajewska J, Ambroszkiewicz J, Chelchowska M, et al. PS-094 the levels of bone alkaline phosphatase and human epidermal growth factor receptor-2 in patients with osteosarcoma. Arch Dis Child. 2014;99(2):A144.
- Tsubaki M, Yamazoe Y, Yanae M, et al. Blockade of the Ras/MEK/ERK and Ras/PI3K/Akt pathways by statins reduces the expression of bFGF, HGF, and TGF-beta as angiogenic factors in mouse osteosarcoma. Cytokine. 2011;54(1):100–107.
- Zhang J, Yu XH, Yan YG, et al. PI3K/Akt signaling in osteosarcoma. Clinica Chimica Acta: Int J Clin Chem. 2015;444:182–192.
- Murillo MM, Zelenay S, Nye E, et al. RAS interaction with PI3K p110alpha is required for tumor-induced angiogenesis. J Clin Invest. 2014;124(8):3601–3611.
- Sheridan C, Downward J. Inhibiting the RAS-PI3K pathway in cancer therapy. Enzymes. 2013;34:107–136.
- Wu CL, Tsai HC, Chen ZW, et al. Ras activation mediates WISP-1-induced increases in cell motility and matrix metalloproteinase expression in human osteosarcoma. Cell Signal. 2013;25(12):2812–2822.
- Chrun ES, Modolo F, Daniel FI. Histone modifications: a review about the presence of this epigenetic phenomenon in carcinogenesis. Pathol, Res Pract. 2017;213(11):1329–1339.
- Izzo A, Schneider R. The role of linker histone H1 modifications in the regulation of gene expression and chromatin dynamics. Biochimica et Biophysica Acta. 2016;1859(3):486–495.
- Cavalcanti MC, Rizgalla M, Geyer J, et al. Expression of histone 1 (H1) and testis-specific histone 1 (H1t) genes during stallion spermatogenesis. Anim Reprod Sci. 2009;111(2–4):220–234.
- Wanner G, Schroeder-Reiter E, Ma W, et al. The ultrastructure of mono- and holocentric plant centromeres: an immunological investigation by structured illumination microscopy and scanning electron microscopy. Chromosoma. 2015;124(4):503–517.
- Kim K, Kim JM, Kim JS, et al. VprBP has intrinsic kinase activity targeting histone H2A and represses gene transcription. Mol Cell. 2013;52(3):459–467.
- Aihara H, Nakagawa T, Mizusaki H, et al. Histone H2A T120 phosphorylation promotes oncogenic transformation via upregulation of cyclin D1. Mol Cell. 2016;64(1):176–188.
- Li J, Wang T, Pei L, et al. Expression of VRK1 and the downstream gene BANF1 in esophageal cancer. Biomed Pharmacother: Biomed Pharmacother. 2017;89:1086–1091.
- Mon AM, MacKinnon AC Jr, Traktman P. Overexpression of the VRK1 kinase, which is associated with breast cancer, induces a mesenchymal to epithelial transition in mammary epithelial cells. PLoS One. 2018;13(9):e0203397.
- Molitor TP, Traktman P. Molecular genetic analysis of VRK1 in mammary epithelial cells: depletion slows proliferation in vitro and tumor growth and metastasis in vivo. Oncogenesis. 2013;2(6):e48.
- Billiau A, Edy VG, Heremans H, et al. Human interferon: mass production in a newly established cell line, MG-63. Antimicrob Agents Chemother. 1977;12(1):11–15.
- Heldin CH, Johnsson A, Wennergren S, et al. A human osteosarcoma cell line secretes a growth factor structurally related to a homodimer of PDGF A-chains. Nature. 1986;319(6053):511–514.
- Dalby B, Cates S, Harris A, et al. Advanced transfection with Lipofectamine 2000 reagent: primary neurons, siRNA, and high-throughput applications. Methods (San Diego, Calif). 2004;33(2):95–103.
- Ish-Shalom S, Lichter A. Analysis of fungal gene expression by real time quantitative PCR. Methods Mol Biol. 2010;638:103–114.
- Kong L, Wang X, Zhang K, et al. Gypenosides synergistically enhances the anti-tumor effect of 5-fluorouracil on colorectal cancer in vitro and in vivo: a role for oxidative stress-mediated DNA damage and p53 activation. PLoS one. 2015;10(9):e0137888.
- Horibata S, Vo TV, Subramanian V, et al. Utilization of the soft agar colony formation assay to identify inhibitors of tumorigenicity in breast cancer cells. JoVE. 2015;99:e52727.
- Zhang H, Li H, Liu Z, et al. Triptolide inhibits the proliferation and migration of medulloblastoma Daoy cells by upregulation of microRNA-138. J Cell Biochem. 2018;119(12):9866–9877.
- Luo H, Shenoy AK, Li X, et al. MOF acetylates the histone demethylase LSD1 to suppress epithelial-to-mesenchymal transition. Cell Rep. 2016;15(12):2665–2678.
- Broadhead ML, Clark JC, Myers DE, et al. The molecular pathogenesis of osteosarcoma: a review. Sarcoma. 2011;2011:1.
- Martini M, De Santis MC, Braccini L, et al. PI3K/AKT signaling pathway and cancer: an updated review. Annal Med. 2014;46(6):372–383.
- Tsubaki M, Satou T, Itoh T, et al. Reduction of metastasis, cell invasion, and adhesion in mouse osteosarcoma by YM529/ONO-5920-induced blockade of the Ras/MEK/ERK and Ras/PI3K/Akt pathway. Toxicol Appl Pharmacol. 2012;259(3):402–410.
- Wang T, Gong X, Jiang R, et al. Ferulic acid inhibits proliferation and promotes apoptosis via blockage of PI3K/Akt pathway in osteosarcoma cell. Am J Trans Res. 2016;8(2):968–980.
- Liu Y, Wang DL, Chen S, et al. Oncogene Ras/phosphatidylinositol 3-kinase signaling targets histone H3 acetylation at lysine 56. J Biol Chem. 2012;287(49):41469–41480.
- Li Y, Sun D, Sun W, et al. Ras-PI3K-AKT signaling promotes the occurrence and development of uveal melanoma by downregulating H3K56ac expression. J Cell Physiol. 2019;234(9):16032–16042.
- Sang B, Sun J, Yang D, et al. Ras-AKT signaling represses the phosphorylation of histone H1.5 at threonine 10 via GSK3 to promote the progression of glioma. Artific Cells, Nanomed, Biotechnol: An Int J. 2019;47(1):2882–2890.
- Castillo J, Lopez-Rodas G, Franco L. Histone post-translational modifications and nucleosome organisation in transcriptional regulation: some open questions. Adv Exp Med Biol. 2017;966:65–92.
- Kireeva ML, Mo FE, Yang GP, et al. Cyr61, a product of a growth factor-inducible immediate-early gene, promotes cell proliferation, migration, and adhesion. Mol Cell Biol. 1996;16(4):1326–1334.
- Slattery ML, Samowitz W, Curtin K, et al. Associations among IRS1, IRS2, IGF1, and IGFBP3 genetic polymorphisms and colorectal cancer. Cancer Epidemiol, Biomark Prevent. 2004;13(7):1206–1214.
- Al-Zahrani A, Sandhu MS, Luben RN, et al. IGF1 and IGFBP3 tagging polymorphisms are associated with circulating levels of IGF1, IGFBP3 and risk of breast cancer. Human Mol Genet. 2006;15(1):1–10.
- Johnson LM, Price DK, Figg WD. Treatment-induced secretion of WNT16B promotes tumor growth and acquired resistance to chemotherapy: implications for potential use of inhibitors in cancer treatment. Cancer Biol Ther. 2013;14(2):90–91.
- Xiong L, Wen Y, Miao X, et al. NT5E and FcGBP as key regulators of TGF-1-induced epithelial-mesenchymal transition (EMT) are associated with tumor progression and survival of patients with gallbladder cancer. Cell Tissue Res. 2014;355(2):365–374.
- Costa VL, Henrique R, Danielsen SA, et al. Three epigenetic biomarkers, GDF15, TMEFF2, and VIM, accurately predict bladder cancer from DNA-based analyses of urine samples. Clin Cancer Res: Offic J Am Assoc Cancer Res. 2010;16(23):5842–5851.
- Karasawa T, Kawashima A, Usui F, et al. Oligomerized CARD16 promotes caspase-1 assembly and IL-1beta processing. FEBS Open Bio. 2015;5(1):348–356.
- Lopez-Borges S, Lazo PA. The human vaccinia-related kinase 1 (VRK1) phosphorylates threonine-18 within the mdm-2 binding site of the p53 tumour suppressor protein. Oncogene. 2000;19(32):3656–3664.
- Liu ZC, Cao K, Xiao ZH, et al. VRK1 promotes cisplatin resistance by up-regulating c-MYC via c-Jun activation and serves as a therapeutic target in esophageal squamous cell carcinoma. Oncotarget. 2017;8(39):65642–65658.
- Huang W, Cui X, Chen Y, et al. High VRK1 expression contributes to cell proliferation and survival in hepatocellular carcinoma. Pathol, Res Pract. 2016;212(3):171–178.
