Abstract
Nano-hydroxyapatite is being investigated as vital components of implants and dental and tissue engineering devices. It is found as a bone replacement due to its non-toxicity and cytocompatibility with dental tissues and bone. The reality that nanocrystalline hydroxyapatite can be made of porous granules and scaffolds. Additionally, it has a massive loading potential indicating its use as a transporter for drugs or a regulated drug release mechanism in pharmaceutical research. This review aims to present existing nano-hydroxyapatite research developments as a drug carrier employed in bone tissue disorders locally and deliver poorly soluble drugs with reduced bioavailability. We have discussed the nano-hydroxyapatite role in the delivery of drugs (i.e. anti-resorptive, anti-cancer, and antibiotics), proteins, genetic material, and radionuclides.
Introduction
Nano-hydroxyapatite (nHA) with the general formula of Ca10(PO4)6(OH)2, is rapidly finding applications in regenerative medicines and biomaterials engineering [Citation1]. nHA is a principal constituent of the mineral fraction of dental tissues and bones and exhibits biocompatibility with the physiological environment. nHA with carbonated multi-substituted and crystalline nano-structure with calcium and OH groups are regarded as biological apatite [Citation2]. nHA is extensively employed as a constituent of bone supplements and fillers for its osteo-inductive and tissue-compatible properties.
nHA is also finding applications in targeted and controlled drug delivery systems (DDSs) with improved adsorption across biological barriers. Porous nHA can be simply infused with drug and then used as targeted delivery system to the bones as well as to strengthen the architecture of newly formed bone tissue. Such systems can be utilised in developing the latest DDSs for the treatment and prophylaxis of bone related disorders, such as tumours, metastases, and osteoporosis. Moreover, the nHA based DDSs loaded with antibiotics can deliver drugs directly to the infected bone tissue [Citation3].
Besides strengthening the bone skeleton, nHA based DDSs are being used as carriers for biopharmaceuticals such as vitamins, hormones, or anti-inflammatory substances. Several studies have investigated nHA for protein delivery and gene therapy applications [Citation4–6] driven by their high loading capacity, biodegradability, and non-toxicity. nHA-based DDSs can be synthesised through chemical cross-linking and adsorption to attain various mechanical and physical aspects [Citation7]. However, before clinical implementation, the pharmaceutical dosage form is required to achieve a desirable drug release and absorption profile to initiate the pharmacological effect [Citation8]. Native nHA is not suitable for oral dosage formulation as nHA is rapidly deteriorated by acidic medium in gastric tract. This issue is resolved by forming an intestinal system of pH 8–9 with a targeted and controlled liberation [Citation9,Citation10]. In order to design bone targeting DDS, it is desirable to make a biocompatible and biodegradable drug carrier system that can improve the mechanical strength of bone tissue and deliver drug payload. Hence, calcium phosphates, mainly nHA, as carriers in bone disorders seem to be reasonable and apatite is considered as an osteoinductive as well as biocompatible carrier of pharmacological material. Moreover, apatite re-forms the dead tissue and enable remodelling. The morphology of nHA depends on the synthesis mechanism and impacts the physical properties. Controlling physical properties of HA plays a crucial role in elucidating the pathogenesis of diseases caused by exogenous and endogenous particles.
This review summarises the literature to establish a structure-application relationship of nHA as a versatile platform for delivery of active pharmaceutical ingredients, specifically for anti-resorptive, anti-cancer, and antibiotics, proteins, genetic material, and radionuclides.
nHA as a drug carrier
nHA as a carrier for anti-resorptive drugs
The introduction of anti-resorptive medicines in medical practice should be acknowledged as a treatment for bone damage. The treatment is based on the individual recommendations for patient and treatment’s aetiology. Disordered bone metabolism often results in osteoporosis and fibrous dysplasia while disorders related to bone metastases include osteomalacia, and Paget disease [Citation11].
Bisphosphonates (BPs)
It is the most significant class of antiresorptive drugs. BP is often linked with zoledronate, calcium phosphates (CaPs), and alendronate. In bone metastases, BPs are first-line therapy. Moreover, it is also employed in Paget’s disease and osteoporosis [Citation12].
Alendronate (ALN)
CaP-alendronate (CaP-ALN) system has a beneficial effect on in vitro bone cell development. Incorporation of Cap-ALN in the cellular growth medium hinders osteoclasts’ development and proliferation but support osteoblasts. The types of CaP, which are modified by ALN, also affect osteoblasts. According to an observation, octacalcium phosphate enhances osteoblasts’ differentiation to a greater degree as compared to the pure nHA [Citation13]. The impact of different types of CaP on the osteoclasts has recently been explored [Citation14]. For example, different inflammatory reactions are observed in vitro tissue cultures with these composites. This is due to the titanium discs covered with nHA presents a high quantity of ALN that subsequently lower the tumour necrosis factor (TNF-α) and interleukin 6 (IL-6) production and therefore nHA-ALN play a role in controlling the system’s inflammatory reaction [Citation15]. Titanium covered with nHA-ALN was implanted into the rabbit’s medullary cavities of the left tibiae and it demonstrated excellent stability and merger with the bone, without showing any migration. nHA-ALN composites are prepared in powder form by utilising the standard wet precipitation technique. Alternatively, such implants could also be manufactured by utilising the mechano-chemical technique such as milling with high-frequency vibration (the reactive milling technique) [Citation16,Citation17]. Such composites can be made in micro-sized systems having improved drug releasing behaviour, known as microspheres. To accomplish this target, analysts manufactured water-in-oil emulsions by utilising Span 85 as emulgent. ALN modified nHA spheres can be manufactured either in their pure form or as a compound with the polymer phase via reliable oil/water emulsion with poly (β-hydroxybutyrate-co-β-hydroxy valerate) (PHBV) copolymer [Citation18].
There is also a possibility of covalent bonding between ALN and nHA. The interaction depends upon the synchronisation between negatively charged oxygen atoms (anion form) of the bisphosphonate and Ca2+ ions of the mineral phase [Citation19]. Analytical techniques such as CP/MAS NMR, XRD, and FT-IR can be used to study the chemical bonding of nHA with risedronate or etidronate [Citation20]. ALN modified nHA can covalently bind with the drug that makes the mineral phase operational. Li et al. exploited this bonding and employed nHA functionalised with ALN as an ibuprofen carrier. They examined FT-IR spectra and noticed a linkage between the amino group of nHA-ALN and the carboxyl group of the ibuprofen [Citation21].
Zoledronate (ZOL)
Various in vitro studies on bone cells explored the alteration of nHA with ZOL. The outcomes are like modifications with ALN, i.e. promoting osteoblast differentiation and hindering osteoclasts activation. Boanini et al. conducted a close examination of the modification of nHA with ALN and ZOL [Citation22]. They observed that the pro-apoptosis effect on osteoclasts is more dominant in the case of nHA-ZOL. The latest in vitro studies examine ZOL-nHA composites with strontium (Sr). A suitable concentration of strontium salts has an anabolic effect on the bone tissue. A comparative study was conducted on the effects of nHA-Sr and nHA-ZOL on bone cells. It was observed that the hindering effect on osteoclasts is dominant by nHA-ZOL, whereas the stimulating effect on osteoblasts is better with nHA-Sr. The nHA-Sr-ZOL solution is suitable for achieving therapeutic targets [Citation23]. Slowly coating the titanium implants by adding an increasing concentration of nHA-Sr and reducing nHA-ZOL quantity has been considered the substitute of nHA-Sr-ZOL. Boanini et al. synthesised nHA-Sr-ZOL coating by utilising the modern combinatorial matrix-assisted pulsed laser evaporation method [Citation24].
nHA as a carrier for anti-cancer drugs
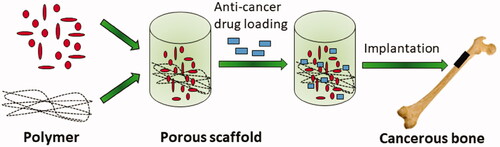
Cancer is one of the most common causes of mortality despite advancements in prevention, diagnosis, and novel treatment techniques. Bone marrow damage is one of the severe off-target undesired effect of anti-cancer drugs. Teniposide and 5-fluorouracil directly damages the bone marrow and iphosphamide and cyclophosphamide result in thrombopenia and leukopoenia [Citation25,Citation26]. Two major factors that are considered when devising new anti-cancer treatments are; designing medicine that can work against cancerous cells and upgrading the actions of currently available medicines to decrease their side effects. Targeted delivery of the anti-cancer drugs, to reduce the off-target effect, is emerging application of nHA materials (). Apatites-dependent substances and their derivatives have been utilised to meet the targets [Citation25]. HA mechanical strength permits the partial removal of bone, and then the removed tissue is filled in.
Several techniques are reported to synthesise cisplatin and nHA composites, which provide sustained drug release profile [Citation28]. These techniques include enclosing the drug into a block of ceramic nHA and sub-pressuring the drug in nHA suspension via centrifugation. This system resulted in extended release (up to eight weeks) of the cisplatin when administered in the thigh muscles of the mouse. Also, negligible distribution of cisplatin was observed to the blood and other organs. When mice with solid osteosarcoma were treated with this system, a high concentration of drugs in the affected part and low drug concentration in other organs was observed. After 30 days of administration, the growth of the tumour was remarkably slow and similar results were observed in the case of soft tissue and breast cancer [Citation29].
Further research shows the slow release of an active substance up to 12 weeks, followed by its implantation in the tibial muscles of the mice [Citation27]. When precisely the same drug delivery system was implanted in the mice experimental tumour, even slower release of the drug (more than three months) was observed. The tumour growth was inhibited more effectively after local administration of nHA-cisplatin system as compared to the conventional drug administration. In vitro examination of nHA microspheres infused with cisplatin presented 50% suppression of prostate, bone, and breast cancer cells [Citation30].
Cisplatin adsorption and release from the nHA suspension depend upon the ionic constitution of the solution during synthesis or the environment during release. In the case of phosphate buffer having 0.9% NaCl, low drug adsorption was noted. It shows that chloride ions hinder drug adsorption. However, the drug desorption enhances with the increase in the level of chloride ions in the solution. The drug desorption rate was low in the case of both Tris and phosphate buffer solutions. The cisplatin desorption from nHA is slow even in the presence of chloride ions in the solution [Citation31].
Other than cisplatin, extended-release HA systems are also synthesised with adriamycin (doxorubicin). When HA spheres with 0.8 mg of adriamycin were administered intravenously into the central region of chondrosarcoma, excellent therapeutic outcomes were noted. In rats, survivability enhanced by 90% and no diarrhoea or leukopoenia was observed. When nHA and adriamycin were incorporated subcutaneously in the mice, dose-dependent anti-cancer activity was observed. Around 6 mg of nHA granule had significant therapeutic efficacy, which resulted about 98% inhibition in tumour growth and improved the mortality in mice models. The tumour growth in mice was suppressed by inserting the block of nHA and adriamycin in the chondrosarcoma. Following the insertion in mice muscle, in vitro, the drug was continuously release over a period of 66 days, whereas it was released over 28 days in vivo [Citation32]. Xiong et al. reported the improved pharmacological action of doxorubicin via mitochondria and nuclei dual-targeting with nHA [Citation33].
Figure 2. Schematic design of the DOX/HAP-HA nanoparticles. (A) The main components of DOX/HAP-HA nanoparticles: DOX, HAP nanoparticles and HA shell. (B) The antitumor mechanism of DOX/HAP-HA nanoparticles, i.e. drug delivery into the cell nuclei and mitochondria. Tumor target ability of HAP-HA nanoparticles. (C) In vivo fluorescence imaging of the Heps tumor-bearing mice at 1,4,6, 10 and 24 h after intravenous injection of (I) free DiR, (II) DiR/HAP-HA nanoparticles and (III) DiR/HAP-HA nanoparticles with pre-injection of free HA. Arrows indicate the sites of tumors. (D) Ex vivo fluorescence imaging of the tumor and normal tissues harvested from the euthanized Heps tumor-bearing mice at 24 h post injection. The numeric label for each organ is as follows: 1, heart; 2, liver; 3, spleen; 4, lung; 5, kidney; 6, tumor. (E) Region-of-interest analysis of fluorescent signals from the tumors and normal tissues. Error bars indicated s.d. (n = 3). **P < 0.01. Reprinted with permission from Elsevier [Citation33].
![Figure 2. Schematic design of the DOX/HAP-HA nanoparticles. (A) The main components of DOX/HAP-HA nanoparticles: DOX, HAP nanoparticles and HA shell. (B) The antitumor mechanism of DOX/HAP-HA nanoparticles, i.e. drug delivery into the cell nuclei and mitochondria. Tumor target ability of HAP-HA nanoparticles. (C) In vivo fluorescence imaging of the Heps tumor-bearing mice at 1,4,6, 10 and 24 h after intravenous injection of (I) free DiR, (II) DiR/HAP-HA nanoparticles and (III) DiR/HAP-HA nanoparticles with pre-injection of free HA. Arrows indicate the sites of tumors. (D) Ex vivo fluorescence imaging of the tumor and normal tissues harvested from the euthanized Heps tumor-bearing mice at 24 h post injection. The numeric label for each organ is as follows: 1, heart; 2, liver; 3, spleen; 4, lung; 5, kidney; 6, tumor. (E) Region-of-interest analysis of fluorescent signals from the tumors and normal tissues. Error bars indicated s.d. (n = 3). **P < 0.01. Reprinted with permission from Elsevier [Citation33].](/cms/asset/7108fa21-cd3e-4283-931e-58c873ab029e/ianb_a_2016785_f0002_c.jpg)
Along with adriamycin, different other drugs are also explored by loading into nHA based anti-cancer drug delivery systems. These systems provided insight into the physicochemical characterisation on the nHA particles such as the dependence of drug release rate of Methotrexate (MTX) and Doxorubicin on the porosity of nHA particles. Incorporating these ceramic blocks lowered the therapeutic dose and, therefore, the toxicity was decreased as compared to the conventional dosing [Citation34]. The drug delivery system having nHA of 35–48% porosity appeared to be most promising where MTX was incorporated into the blocks of HA that resulted sustained release of MTX. 1.22–2.38 mg of MTX was originally infused in nHA that resulted in 0.22–0.32 μg of MTX released at the site of tumour, sufficient enough for therapeutic response without eliciting toxicity [Citation35].
nHA as carriers for antimicrobial therapies
Antibiotic therapy is preferred for the treatment of bacterial infections in bone. It is mostly utilised in stomatology, orthopaedic surgery, and prosthetics. The secondary bacterial infections create various complications; therefore, the success of bone fracture therapy, prosthesis insertion, and transplant surgeries depends on restraining secondary infections. Uncontrolled secondary infections may result in the degradation or separation of prosthesis. presents the schematics of anti-bacterial applications of HA biomaterials [Citation36].
Figure 3. (A) Sketch of the possible applications of the biomaterials developed. (B) Synthesis of N-thio-substituted β-lactams, reaction yields in parenthesis. Reprinted with permission from Nature [Citation36].
![Figure 3. (A) Sketch of the possible applications of the biomaterials developed. (B) Synthesis of N-thio-substituted β-lactams, reaction yields in parenthesis. Reprinted with permission from Nature [Citation36].](/cms/asset/5f5b5c9c-2219-4fcc-8325-c5d531a07a64/ianb_a_2016785_f0003_c.jpg)
The conventional techniques included local insertion of drug loaded polymethylmethacrylate (PMMA) microspheres together with IV administration of antibiotics, or coating of metal implants with polymeric-drug matrix [Citation37]. However, these techniques had some disadvantages such as XXX [Citation38]. In the case of PMMA microspheres, direct insertion was possible into the infected area, however, second surgery was required to remove the empty PMMA spheres as they are not completely biodegradable [Citation39]. Moreover, PMMA suffer from exothermic depolymerisation at the site of application, therefore, some drug molecule could fuse with PMMA during depolymerisation, consequently, increasing the possibility of inflammatory reactions. The incomplete antibiotic dispersal was another drawback of this system. Efforts were made to switch biodegradable PMMA with anhydrous calcium sulphate, however, it presented high cytotoxicity [Citation40]. Furthermore, when the implant’s covering was saturated with the antibiotic, it inhibited the release of drug and uniform dose distribution. Some effective anti-bacterial drugs for treating bone infections include glycopeptides, fluoroquinolones, cephalosporins, aminoglycosides, and glycopeptides [Citation41,Citation42].
Aminoglycoside antibiotics, including amikacin, and gentamycin, are beneficial for preventing and treating bone tissue infections. These drugs have limited oral bioavailability and high nephrotoxicity. Several studies are conducted for the synthesis of apatite-based carriers for the aminoglycosides and carbonated nHA appeared to be suitable due to its analogy with biological apatites. The research introduced a technique for synthesising mesoporous microspheres from gentamycin-filled carbonated nHA. These mesoporous microspheres exhibited good anti-bacterial efficacy and biocompatibility [Citation43]. Moreover, they prevented Staphylococcus epidermidis adhesion. According to studies, microspheres showed three folds greater adsorption gentamycin than the standard nHA. The mesoporous microstructure of the microspheres controlled the drug release at the site of infection [Citation44]. Coralline-derived nHA has also been utilised to synthesise the carriers of gentamycin [Citation45].
nHA composite is often utilised for synthesising efficacious systems to deliver sustained release of the antibiotics to the bones. For instance, research presented a system in which the very fine crystalline nHA was covered with polyethylene glycol (PEG) that increased the release time of gentamycin [Citation46]. Similarly, nHA/beta-glucan composites had been explored to form an aminoglycoside carrier. Amikacin and gentamycin were utilised to treat bacterial infections during the surgery as there were released swiftly and entirely in about 48 h.
Vancomycin belongs to the class of glycopeptide antibiotics that can be administered orally as well as intravenously. It is utilised against post-surgical infections and osteomyelitis. When used in combination with aminoglycosides, it presented ototoxicity and nephrotoxicity. The several carriers for vancomycin bone-delivery were explored and some were prepared from nHA [Citation47]. An example of such drug delivery systems involves nHA-poly lactic acid (PLA) microcapsules where pellets of vancomycin and nanocrystalline nHA were used to treat bone inflammation [Citation48]. The vancomycin released from the pellets was within therapeutic concentration for about 12 weeks. Porous nHA blocks, treated with heat and then saturated with vancomycin were incorporated into patients with chronic osteomyelitis from methicillin-resistant Staphylococcus aureus (MRSA). The design of HA based composite was further expanded with synthetic polymers (poly-L-lactide) and biopolymers (gelatin, collagen, or chitosan) [Citation49].
Along with aminoglycosides and glycopeptides, fluoroquinolones and β-lactam antibiotics were also explored for HA based delivery system against bone infections. Ciprofloxacin loaded HA presented size dependent release of ciprofloxacin from HA microspheres [Citation9]. nHA were used to make ampicillin loaded granules that were surface coated with several organic compounds such as phosphatidylcholine, chitosan, PLA, and collagen [Citation50]. It was observed that the most efficacious drug release (for 15 days) was obtained from the nHA granules covered with PLA. However, Queiroz et al. obtained favourable outcomes when utilising glass strengthened nHA as an ampicillin matrix [Citation51].
Cephalosporins, such as cefalotin, loaded nHA were coated on titanium implants for antimicrobial effect [Citation52]. Forsgren et al. utilised standard soaking and effectively applied cefalotin and bisphosphonate, i.e. clodronate, coating on titanium implants. It provided sustained release kinetics of clodronate and cefalotin [Citation53]. Ethylcellulose-enclosed ceftazidime was incorporated in the polyurethane and HA composite foam [Citation54]. The drug was released in a sustained and steady pattern and ceftazidime mechanically strengthened the nHA-polyurethane composite [Citation55].
nHA as a carrier for miscellaneous drugs
HA can also be utilised as a carrier for non-steroidal anti-inflammatory drugs (NSAIDs) such as indomethacin [Citation56], aspirin [Citation57], and naproxen [Citation58]. Ibuprofen was used as a model drug due to its suitable molecular size, valuable pharmacological characteristics, and short half-life [Citation44]. Ibuprofen was loaded into nHA nanospheres or porous tablets drug released was studied. The porosity of spheres controlled the drug release where initial delay in drug release was induced by accumulated body fluids on the spheres, i.e. corona formation, and subsequently by porosity of spheres. The problem of indomethacin lipophilicity was resolved by forming a liposomal layer around nHA [Citation59]. The liposomal nHA system sustained the drug release for additional XX hours and resulted an osteoconductive effect on the cells.
HA is also explored as nanomaterials for vitamins delivery to the bones. Combination of HA and poly lactide-co-glycolic acid (PLGA) was loaded with vitamin D3 (cholecalciferol) [Citation60] that resulted in sustained release of vitamin D3 due to slow deterioration of the composites. Such slow deteriorating systems can be used for prolonged therapy of osteoporosis. On the other hand, release of the drug from bone substitute materials can result in osteoblasts activation and bone regeneration. HA materials also demonstrated successful application for delivery and fast transport of vitamin K2 [Citation61]. Vitamin K2 was loaded in the apatite/collagen composite to enrich and balance the osteoclast/osteoblast environment, via quick release. It is observed that the vitamin K2 release occurred quickly in acidic pH relative to basic pH because of more high solubility of the carrier in acidic medium [Citation62].
nHA as a carrier for gene delivery
Genetic materials, i.e. DNA and RNA, are unstable and need protection against deterioration, making it difficult to transfer genetic materials into target cells [Citation63]. The most commonly utilised virus-like gene delivery systems face immunogenicity and pathogenicity. Thus, it is necessary to look for other immune-compatible and non-toxic carriers. Besides dendrimers, polymers (PLA), liposomes, and biopolymers (chitosan), synthetic nHA have also been utilised as carriers for gene therapy [Citation64]. Research presented the gene incorporation into the cells to initiate the synthesis of a particular protein and silencing gene as well as RNA silencing. The initial outcomes are incredibly favourable. Current research is carried on the effect of HA particles’ size and structure on the gene transporting system. It was observed that the nHA crystals of 20 nm quickly undergo endocytosis relative to 40 nm or 80 nm crystals. Zhao et al. noted that nHA crystals of 100–200 nm size could also be utilised as carriers in gene therapy.
The positive charged HA and negatively charged gene backbones interact with each other to form HA-gene complexes, inducing gene condensation and packing of the genetic material [Citation65]. Furthermore, it possesses an exceptionally high binding efficiency, thereby allowing for the integration of broader gene regulatory sequence to facilitate regulation of expression and enhance control of gene expression throughout cell membranes [Citation66]. This also enhances mobility of HA–gene complexes across cell membranes [Citation67]. Since HA is found to break under acidic conditions depending on its crystalline nature. In addition, gene detachment from nHA is observed at pH 5 (endosomal pH), which is similar to pH 4 [Citation68]. An increase in the concentration of hydroxyapatite allows endosomal breakup through osmotic imbalance and the gene-vector dissociation, which is needed before nucleic entry can occur. Nuclear pore complexes go through conformational changes when Ca2+ is present in order to allow transport of genes into the nucleus. Due to the various structures of tissue and cell, these nanoparticles are suitable for a wide variety of tissue types, and they can be targeted to specific cells through surface functionalization with the appropriate ligands [Citation69]. This means that, in addition, these particles can be prepared using simple and inexpensive methods.
There are many controversial records regarding the effect of crystals structure on the carrier efficacy. Another main factor is surface charge. It is noted that nHA particles having a positive charge can diffuse conveniently in the cell relative to the negatively charged particles [Citation60]. The charge can be altered when the surface is functionalised by utilising polymers, carboxylic acids, amino acids, and fatty acids. shows the gene delivery in calcium phosphate nanocarriers to increase stability and transfection [Citation70].
Figure 4. (A) Schematic illustration of the formation of folic acid-polyethylene glycol-pamidronate/calcium phosphate/NLS/pDNA (FA-PEG-Pam/CaP/NDs) nanoparticles and the extracellular and intracellular trafficking for the systemic delivery of plasmid DNA to tumors. Nanoparticles would be internalized into tumor cells via the Folate receptors (FR) mediated endocytosis. After being internalized (i), in the acidic endosomes, FA-PEG-Pam/CaP/NDs nanoparticles would dissolve and the embedded pDNA could escape the endosome by endosome rupture owing to the increase of osmotic pressure (ii). Eventually, pDNA is transported to the nucleus with the aid of NLS due to its nuclear locating ability (iii). (B) In vivo image of RFP gene expression delivered by FA-PEG-Pam/CaP/NDs and its reference formulations injected into bearing 4T1 tumor xenografts BALB/c mice monitored by an NIR fluorescence imaging system. In vivo antitumor activity. The mean (C) tumor volume, (D) body weight, (E) inhibition rate and tumor graph. Scale bar indicates 1 cm. (F) survival rate analysis of BALB/c mice bearing 4T1 tumor xenografts, after intravenous administration of Saline, CaP/NDs, Lipofectamine/NDs and FA-PEG-Pam/CaP/NDs. (G) and (H) Western blotting analysis of p53 protein expression in tumor xenografts transfected with different formulations. (I) H&E staining of tumors of different groups. Blue and pink represent nucleic and cytoplasm, respectively. Red circle indicated apoptosis/necrosis regions and scale bars represent 100 μm. * p < 0.05, ** p < 0.01, and *** p < 0.001. Reprinted with permission from MDPI [Citation69].
![Figure 4. (A) Schematic illustration of the formation of folic acid-polyethylene glycol-pamidronate/calcium phosphate/NLS/pDNA (FA-PEG-Pam/CaP/NDs) nanoparticles and the extracellular and intracellular trafficking for the systemic delivery of plasmid DNA to tumors. Nanoparticles would be internalized into tumor cells via the Folate receptors (FR) mediated endocytosis. After being internalized (i), in the acidic endosomes, FA-PEG-Pam/CaP/NDs nanoparticles would dissolve and the embedded pDNA could escape the endosome by endosome rupture owing to the increase of osmotic pressure (ii). Eventually, pDNA is transported to the nucleus with the aid of NLS due to its nuclear locating ability (iii). (B) In vivo image of RFP gene expression delivered by FA-PEG-Pam/CaP/NDs and its reference formulations injected into bearing 4T1 tumor xenografts BALB/c mice monitored by an NIR fluorescence imaging system. In vivo antitumor activity. The mean (C) tumor volume, (D) body weight, (E) inhibition rate and tumor graph. Scale bar indicates 1 cm. (F) survival rate analysis of BALB/c mice bearing 4T1 tumor xenografts, after intravenous administration of Saline, CaP/NDs, Lipofectamine/NDs and FA-PEG-Pam/CaP/NDs. (G) and (H) Western blotting analysis of p53 protein expression in tumor xenografts transfected with different formulations. (I) H&E staining of tumors of different groups. Blue and pink represent nucleic and cytoplasm, respectively. Red circle indicated apoptosis/necrosis regions and scale bars represent 100 μm. * p < 0.05, ** p < 0.01, and *** p < 0.001. Reprinted with permission from MDPI [Citation69].](/cms/asset/299f03b3-e876-45f7-8a00-91fd62676922/ianb_a_2016785_f0004_c.jpg)
nHA as a carrier for proteins
The potential of proteins to adsorb on the nHA surface has been utilised to synthesise nHA-based drug delivery systems. The substances utilised were mainly porous granules, porous blocks, porous nHA, and forms like protein incorporated in hollow nHA spheres. The microsphere showed suitable porosity, and thus slow drug liberation resulted [Citation71]. These spheres for transporting protein can be acquired in various ways. For example, an interaction was regulated between borane glass spheres and the alkaline solution of KH2PO4 [Citation72]. Resultantly, the glass firstly changed into calcium phosphate and, ultimately, into nHA. nHA can be used for synthesising a matrix for the controlled liberation of several proteins such as bone morphogenetic proteins (BMPs), haemoglobin, cytochrome C, lysozyme, albumins, insulin, and growth factors [Citation73].
Several studies are available on nHA-BMP systems [Citation74]. Bone morphogenetic proteins can be utilised in the post-surgical application as they play a role in controlling the development and differentiation of chondroblasts and osteoblasts. Their responsibility is to mimic the mending and reviving of destructed tissues. BMP-2 morphogenetic protein is utilised only on the collagen matrix. As BMP-2 has less affinity with collagen, its more significant dose is necessary; thus, the treatment expenses elevate. Research presented nanocrystalline nHA as a carrier for BMP-2 morphogenetic protein. High adsorption and extended liberation of BMP-2, i.e. about 15 days, was displayed by the material. The utilisation of polyelectrolyte multilayer film modified the liberation kinetics. illustrates the study of the fibrinogen (Fgn) interaction on the nHA films, while (B) and (C) represents Au surfaces with phosphate ion concentrations and temperatures, respectively [Citation75].
Figure 5. (A) Illustration of the investigation of the fibrinogen (Fgn) interactive states on the HAp NP films of this study. Fng adsorption amount changes on the 15-2-HAp NP film and Au surfaces with the different (B) phosphate ion concentrations and (C) temperatures. Reprinted with permission from Elsevier [Citation74].
![Figure 5. (A) Illustration of the investigation of the fibrinogen (Fgn) interactive states on the HAp NP films of this study. Fng adsorption amount changes on the 15-2-HAp NP film and Au surfaces with the different (B) phosphate ion concentrations and (C) temperatures. Reprinted with permission from Elsevier [Citation74].](/cms/asset/9d3d618c-2157-4ba4-8606-8fd530ac5ffc/ianb_a_2016785_f0005_c.jpg)
nHA as a carrier for radionuclide
Inorganic nanocarriers could be employed in the nuclear medicine for several purposes, for instance, as carriers for therapeutic or diagnostics radionuclides. Currently, many inorganic nanocarriers are under investigation, for instance, nanoparticles of hydroxyapatite (HA) [Citation76], Ag [Citation77], TiO2 [Citation78], and BaSO4 [Citation79]. The main benefit of nanocarriers is their radiation stability and high surface area that permits them to reabsorb ions followed by retaining recoil radionuclides.
There are many studies where the researchers labelled the nHA with diagnostic radionuclides e.g. 99mTc and 18 F. Albernaz and co-workers’ radio-labelled nHA with 99mTc for imaging of bone cancer [Citation76]. Sandhöfer et al. prepared the citrate modified nHA for 18 F radio-labelling [Citation80]. The researchers also investigated on therapeutic radionuclides like 223Ra and 177Lu for nHA radio-labelling. 177Lu-HAp is studied for the treatment and therapy of rheumatoid arthritis [Citation81] and hepatocellular carcinoma [Citation82]. They performed in-vitro and in-vivo primary tests and the outcomes were promising. Furthermore, NPs and nHA granules are radio-labelled with Radium-223 [Citation83]. Initial studies with nHA were also done with radionuclides (short-lived) of Cu and Zn [Citation84]. In a dosimetry experiment, labelling of nHA with 90Y and 153Sm is compared in radio-synoviorthesis [Citation85], while nHA labelled with 169Er and 177Lu was used in radiation synovectomy [Citation86]. In addition to all these, nHA has been considered for removal of radionuclide from radio-active waste [Citation87].
Suchánková and co-workers described the application of nHA as a theranostic carrier for 99mTc and 223Ra [Citation88]. They used two strategies for nHA radio-labelling and carried out by 223Ra and 99mTc for therapy and diagnostic applications, respectively. 99mTc is adsorbed on ready-made nanocarriers, while 223Ra is incorporated into nanocarriers. Labelling achievement is good in all cases, and it was greater than 94%. Subsequently, in-vitro stabilisation experiment was performed in different solutions, including bovine blood serum, physiological saline, 1% human albumin solution, bovine blood plasma and 5% human albumin solution. In-vitro work was conducted in two different studies: a short-term (31 h for 99mTc and 59 h for 223Ra) test and a long-term test (approx. 55 days). Because of the identical levels of radioactivity emitted by the three samples, there is no difference in the activity of the solutions. In terms of long-term stability, best finding was gained for 223Ra, with average published activities ranging from 6% for 59 h in all matrices to 3% for 55 days in a long-term context.
Severin et al. worked on the sorption of the actinium (III) ions as 225, 228Ac isoptopes on HA with various textures [Citation89]. A “reverse” generator using an extraction chromatographic sorbent based on diglycolamide derivative (DGA Resin) was proposed for 228Ac production. They found the product chemical yield ≥90%. The optimal acidity of the solution during sorption (pH 6–7) and the ratio of solid and liquid phases (20 mg of the sorbent per mL of the solution) were determined in preliminary experiments. The process kinetics is adequately described by pseudo-second-order model. The stationary state is reached rapidly (in 10 min) when a HAP suspension is used, whereas time (20–30 min) is needed for textured samples.
Summary
Creating efficacious drug carriers is a big challenge in material engineering, chemistry, and ceramic technology. Several beneficial treatments of bone metastases, osteomyelitis, and tumours become questionable because of the low vascularity of bone tissue disorders. Hence, studies and research on nHA as a multifunctional substance for implants and controlled drug liberation systems appear justified and practically utilised. nHA-based systems can also be utilised as a carrier for the drugs, proteins, radionuclides and genetic material.
Acknowledgements
We thank Dr. Ayesha Ihsan (National Institute for Biotechnology and Genetic Engineering, Pakistan), Dr. Mubashar Rehman (Quaid-i-Azam University, Pakistan) and Dr. Ikram Ullah Khan (Government College University Faisalabad, Pakistan) for thier formatting-related writing assistance and scholarly discussions.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data are included in the submission/manuscript file.
References
- Dorozhkin SV. Calcium orthophosphate-based bioceramics. Materials. 2013;6(9):3840–3942.
- Liu Q, Huang S, Matinlinna JP, et al. Insight into biological apatite: physiochemical properties and preparation approaches. Biomed Res Int. 2013;2013:929748.
- Ginebra M-P, Canal C, Espanol M, et al. Calcium phosphate cements as drug delivery materials. Adv Drug Deliv Rev. 2012;64(12):1090–1110.
- Zhang S, Xing M, Li B. Recent advances in musculoskeletal local drug delivery. Acta Biomater. 2019;93:135–151.
- Tobin EJ. Recent coating developments for combination devices in orthopedic and dental applications: a literature review. Adv Drug Deliv Rev. 2017;112:88–100.
- El-Khouri RJ, Szamocki R, Sergeeva Y, et al. Multifunctional layer-by-layer architectures for biological applications. Funct Polym Film. 2011;2:11–58.
- Munir MU, Ihsan A, Sarwar Y, et al. Hollow mesoporous hydroxyapatite nanostructures; smart nanocarriers with high drug loading and controlled releasing features. Int J Pharm. 2018;544(1):112–120..
- He Q, Pan L, Wang Y, et al. Bioinspired synthesis of large-pore, mesoporous hydroxyapatite nanocrystals for the controlled release of large pharmaceutics. Cryst Growth Des. 2015;15(2):723–731.
- Munir MU, Ihsan A, Javed I, et al. Controllably biodegradable hydroxyapatite nanostructures for cefazolin delivery against antibacterial resistance. ACS Omega. 2019;4(4):7524–7532..
- Jain AK, Panchagnula R. Skeletal drug delivery systems. Int J Pharm. 2000;206(1–2):1–12.
- Chen JS, Sambrook PN. Antiresorptive therapies for osteoporosis: a clinical overview. Nat Rev Endocrinol. 2011;8(2):81–91.
- Cremers S, Drake MT, Ebetino FH, et al. Pharmacology of bisphosphonates. Br J Clin Pharmacol. 2019;85(6):1052–1062.
- van Houdt CIA, Gabbai-Armelin PR, Lopez-Perez PM, et al. Alendronate release from calcium phosphate cement for bone regeneration in osteoporotic conditions. Sci Rep. 2018;8:1–13.
- Bose S, Vu AA, Emshadi K, et al. Effects of polycaprolactone on alendronate drug release from Mg-doped hydroxyapatite coating on titanium. Mater Sci Eng C Mater Biol Appl. 2018;88:166–171.
- Bigi A, Boanini E, Capuccini C, et al. Biofunctional alendronate-hydroxyapatite thin films deposited by matrix assisted pulsed laser evaporation. Biomaterials. 2009;30(31):6168–6177.
- Bigi A, Boanini E. Functionalized biomimetic calcium phosphates for bone tissue repair. J Appl Biomater Funct Mater. 2017;15(4):e313–e325.
- Vargas-Becerril N, Patiño-Carachure C, Rodriguez-Lorenzo LM, et al. Synthesis of hybrid compounds apatite–alendronate by reactive milling and effects on the structure and morphology of the apatite phase. Ceram Int. 2013;39(4):3921–3929.
- Chen S, Guo R, Xie C, et al. Biomimetic mineralization of nanocrystalline hydroxyapatites on aminated modified polylactic acid microspheres to develop a novel drug delivery system for alendronate. Mater Sci Eng C Mater Biol Appl. 2020;110:110655.
- Kim CW, Yun Y-P, Lee HJ, et al. In situ fabrication of alendronate-loaded calcium phosphate microspheres: controlled release for inhibition of osteoclastogenesis. J Control Release. 2010;147(1):45–53.
- Errassifi F, Sarda S, Barroug A, et al. Infrared, Raman and NMR investigations of risedronate adsorption on nanocrystalline apatites. J Colloid Interface Sci. 2014;420:101–111.
- Li D, Zhu Y, Liang Z. Alendronate functionalized mesoporous hydroxyapatite nanoparticles for drug delivery. Mater Res Bull. 2013;48(6):2201–2204.
- Boanini E, Torricelli P, Gazzano M, et al. The effect of zoledronate-hydroxyapatite nanocomposites on osteoclasts and osteoblast-like cells in vitro. Biomaterials. 2012;33(2):722–730.
- Boanini E, Torricelli P, Gazzano M, et al. Combined effect of strontium and zoledronate on hydroxyapatite structure and bone cell responses. Biomaterials. 2014;35(21):5619–5626.
- Boanini E, Torricelli P, Sima F, et al. Strontium and zoledronate hydroxyapatites graded composite coatings for bone prostheses. J Colloid Interface Sci. 2015;448:1–7.
- Emoto M, Naganuma Y, Choijamts B, et al. Novel chemoembolization using calcium-phosphate ceramic microsphere incorporating TNP-470, an anti-angiogenic agent . Cancer Sci. 2010;101(4):984–990.
- Quintanilha JCF, de Sousa VM, Visacri MB, et al. Involvement of cytochrome P450 in cisplatin treatment: implications for toxicity. Cancer Chemother Pharmacol. 2017;80(2):223–233.
- Marques C, Ferreira JMF, Andronescu E, et al. Multifunctional materials for bone cancer treatment. Int J Nanomedicine. 2014;9:2713–2725.
- Sumathra M, Sadasivuni KK, Kumar SS, et al. Cisplatin-loaded graphene oxide/chitosan/hydroxyapatite composite as a promising tool for osteosarcoma-affected bone regeneration. ACS Omega. 2018;3(11):14620–14633.
- Nadar RA, Asokan N, Degli Esposti L, et al. Preclinical evaluation of platinum-loaded hydroxyapatite nanoparticles in an embryonic zebrafish xenograft model. Nanoscale. 2020;12(25):13582–13594.
- Abdel-Bary AS, Tolan DA, Nassar MY, et al. Chitosan, magnetite, silicon dioxide, and graphene oxide nanocomposites: Synthesis, characterization, efficiency as cisplatin drug delivery, and DFT calculations. Int J Biol Macromol. 2020;154:621–633.
- Benedetti M, De Castro F, Romano A, et al. Adsorption of the cis-[Pt(NH3)2(P2O7)](2-) (phosphaplatin) on hydroxyapatite nanocrystals as a smart way to selectively release activated cis-[Pt(NH3)2Cl2] (cisplatin) in tumor tissues. J Inorg Biochem. 2016;157:73–79.
- Kim H, Mondal S, Bharathiraja S, et al. Optimized Zn-doped hydroxyapatite/doxorubicin bioceramics system for efficient drug delivery and tissue engineering application. Ceram Int. 2018;44(6):6062–6071.
- Xiong H, Du S, Ni J, et al. Mitochondria and nuclei dual-targeted heterogeneous hydroxyapatite nanoparticles for enhancing therapeutic efficacy of doxorubicin. Biomaterials. 2016;94:70–83.
- Meshkini A, Oveisi H. Methotrexate-F127 conjugated mesoporous zinc hydroxyapatite as an efficient drug delivery system for overcoming chemotherapy resistance in osteosarcoma cells. Colloids Surf B Biointerfaces. 2017;158:319–330.
- Sun H, Liu S, Zeng X, et al. Morphology effect of nano-hydroxyapatite as a drug carrier of methotrexate. J Mater Sci Mater Med. 2017;28(10):158.
- Giacomini D, Torricelli P, Gentilomi GA, et al. Monocyclic β-lactams loaded on hydroxyapatite: new biomaterials with enhanced antibacterial activity against resistant strains. Sci Rep. 2017;7:1–12.
- ter Boo G-JA, Grijpma DW, Moriarty TF, et al. Antimicrobial delivery systems for local infection prophylaxis in orthopedic- and trauma surgery . Biomaterials. 2015;52:113–125.
- Uskokovic V. Nanostructured platforms for the sustained and local delivery of antibiotics in the treatment of osteomyelitis. Crit Rev Ther Drug Carrier Syst. 2015;32(1):1–59.
- Dash A, Cudworth G. II, Therapeutic applications of implantable drug delivery systems. J Pharmacol Toxicol Methods. 1998;40(1):1–12.
- Rauschmann MA, Wichelhaus TA, Stirnal V, et al. Nanocrystalline hydroxyapatite and calcium sulphate as biodegradable composite carrier material for local delivery of antibiotics in bone infections. Biomaterials. 2005;26(15):2677–2684.
- Makarov C, Cohen V, Raz-Pasteur A, et al. In vitro elution of vancomycin from biodegradable osteoconductive calcium phosphate-polycaprolactone composite beads for treatment of osteomyelitis. Eur J Pharm Sci. 2014;62:49–56.
- Butini ME, Cabric S, Trampuz A, et al. In vitro anti-biofilm activity of a biphasic gentamicin-loaded calcium sulfate/hydroxyapatite bone graft substitute. Colloids Surf B Biointerfaces. 2018;161:252–260.
- Munir MU, Ahmed A, Usman M, et al. Recent advances in nanotechnology-aided materials in combating microbial resistance and functioning as antibiotics substitutes. Int J Nanomedicine. 2020;15:7329–7358.
- Yu M, Zhou K, Li Z, et al. Preparation, characterization and in vitro gentamicin release of porous HA microspheres. Mater Sci Eng C Mater Biol Appl. 2014;45:306–312.
- Madhumathi K, Rubaiya Y, Doble M, et al. Antibacterial, anti-inflammatory, and bone-regenerative dual-drug-loaded calcium phosphate nanocarriers-in vitro and in vivo studies. Drug Deliv Transl Res. 2018;8(5):1066–1077.
- Schnieders J, Gbureck U, Thull R, et al. Controlled release of gentamicin from calcium phosphate-poly(lactic acid-co-glycolic acid) composite bone cement. Biomaterials. 2006;27(23):4239–4249.
- Martínez-Vázquez FJ, Cabañas MV, Paris JL, et al. Fabrication of novel Si-doped hydroxyapatite/gelatine scaffolds by rapid prototyping for drug delivery and bone regeneration. Acta Biomater. 2015;15:200–209.
- Jiang J-L, Li Y-F, Fang T-L, et al. Vancomycin-loaded nano-hydroxyapatite pellets to treat MRSA-induced chronic osteomyelitis with bone defect in rabbits. Inflamm Res. 2012;61(3):207–215.
- Yu J, Chu X, Cai Y, et al. Preparation and characterization of antimicrobial nano-hydroxyapatite composites. Mater Sci Eng C Mater Biol Appl. 2014;37:54–59.
- Labay C, Buxadera-Palomero J, Avilés M, et al. Modulation of release kinetics by plasma polymerization of ampicillin-loaded β-TCP ceramics. J Phys D: Appl Phys. 2016;49(30):304004.
- Queiroz AC, Santos JD, Monteiro FJ, et al. Adsorption and release studies of sodium ampicillin from hydroxyapatite and glass-reinforced hydroxyapatite composites. Biomaterials. 2001;22(11):1393–1400.
- Kang M-K, Lee S-B, Moon S-K, et al. The biomimetic apatite-cefalotin coatings on modified titanium. Dent Mater J. 2012;31(1):98–105.
- Forsgren J, Brohede U, Strømme M, et al. Co-loading of bisphosphonates and antibiotics to a biomimetic hydroxyapatite coating. Biotechnol Lett. 2011;33(6):1265–1268.
- Liu H, Zhang L, Shi P, et al. Hydroxyapatite/polyurethane scaffold incorporated with drug-loaded ethyl cellulose microspheres for bone regeneration. J Biomed Mater Res B Appl Biomater. 2010;95(1):36–46.
- Sultan M. Hydroxyapatite/polyurethane composites as promising biomaterials. Chem Pap. 2018;72(10):2375–2395.
- Trofimov AD, Ivanova AA, Zyuzin MV, et al. Porous inorganic carriers based on silica, calcium carbonate and calcium phosphate for controlled/modulated drug delivery: fresh outlook and future perspectives. Pharmaceutics. 2018;10(4):167.
- Li S, Wang K, Chang K-CA, et al. Preparation and evaluation of nano-hydroxyapatite/poly (styrene-divinylbenzene) porous microsphere for aspirin carrier. Sci China Chem. 2012;55(6):1134–1139.
- Čalija B, Milić J, Cekić N, et al. Chitosan oligosaccharide as prospective cross-linking agent for naproxen-loaded Ca-alginate microparticles with improved pH sensitivity. Drug Dev Ind Pharm. 2013;39(1):77–88.
- Xu Q, Tanaka Y, Czernuszka JT. Encapsulation and release of a hydrophobic drug from hydroxyapatite coated liposomes. Biomaterials. 2007;28(16):2687–2694.
- Sumathra M, Munusamy MA, Alarfaj AA, et al. Osteoblast response to vitamin D3 loaded cellulose enriched hydroxyapatite mesoporous silica nanoparticles composite. Biomed Pharmacother. 2018;103:858–868.
- Otsuka M, Hirano R. Bone cell activity responsive drug release from biodegradable apatite/collagen nano-composite cements-in vitro dissolution medium responsive vitamin K2 release. Colloids Surf B Biointerfaces. 2011;85(2):338–342.
- Cosijns A, Vervaet C, Luyten J, et al. Porous hydroxyapatite tablets as carriers for low-dosed drugs. Eur J Pharm Biopharm. 2007;67(2):498–506.
- Chen J, Gao P, Yuan S, et al. Oncolytic adenovirus complexes coated with lipids and calcium phosphate for cancer gene therapy. ACS Nano. 2016;10(12):11548–11560.
- Zhao X-Y, Zhu Y-J, Chen F, et al. Nanosheet-assembled hierarchical nanostructures of hydroxyapatite: surfactant-free microwave-hydrothermal rapid synthesis, protein/DNA adsorption and pH-controlled release. CrystEngComm. 2013;15(1):206–212.
- Liu T, Tang A, Zhang G, et al. Calcium phosphate nanoparticles as a novel nonviral vector for efficient transfection of DNA in cancer gene therapy. Cancer Biother Radiopharm. 2005;20(2):141–149.
- Maitra A. Calcium phosphate nanoparticles: second-generation nonviral vectors in gene therapy. Expert Rev Mol Diagn. 2005;5(6):893–905.
- Truong-Le VL, Walsh SM, Schweibert E, et al. Gene transfer by DNA-gelatin nanospheres. Arch Biochem Biophys. 1999;361(1):47–56.
- Bisht S, Bhakta G, Mitra S, et al. pDNA loaded calcium phosphate nanoparticles: highly efficient non-viral vector for gene delivery. Int J Pharm. 2005;288(1):157–168.
- Khosravi DK, Mozafari MR, Rashidi L, et al. Calcium based non-viral gene delivery: an overview of methodology and applications. Acta Med Iran. 2010;48(3):133–141.
- Zhao M, Li J, Chen D, et al. A valid bisphosphonate modified calcium phosphate-based gene delivery system: increased stability and enhanced transfection efficiency in vitro and in vivo. Pharmaceutics. 2019;11:468.
- Chen Y, Chen S, Kawazoe N, et al. Promoted angiogenesis and osteogenesis by dexamethasone-loaded calcium phosphate nanoparticles/collagen composite scaffolds with microgroove networks. Sci Rep. 2018;8:1–12.
- Fischer J, Kolk A, Pautke C, et al. Future of local bone regeneration – protein versus gene therapy. J Craniomaxillofac Surg. 2011;39(1):54–64.
- Frede A, Neuhaus B, Klopfleisch R, et al. Colonic gene silencing using siRNA-loaded calcium phosphate/PLGA nanoparticles ameliorates intestinal inflammation in vivo. J Control Release. 2016;222:86–96.
- Uddin MH, Matsumoto T, Ishihara S, et al. Apatite containing aspartic acid for selective protein loading. J Dent Res. 2010;89(5):488–492.
- Galindo TGP, Yamada I, Yamada S, et al. Studies on preparation of surfactant-assisted elliptical hydroxyapatite nanoparticles and their protein-interactive ability. Mater Chem Phys. 2019;221:367–376.
- Albernaz M. D S, Ospina CA, Rossi AM, et al. Radiolabelled nanohydroxyapatite with 99mTc: perspectives to nanoradiopharmaceuticals construction. Artif Cells Nanomed Biotechnol. 2014;42(2):88–91.
- Kucka J, Hrubý M, Konák C, et al. Astatination of nanoparticles containing silver as possible carriers of 211At. Appl Radiat Isot. 2006;64(2):201–206.
- Cędrowska E, Pruszynski M, Majkowska-Pilip A, et al. Functionalized TiO2 nanoparticles labelled with 225 Ac for targeted alpha radionuclide therapy. J Nanoparticle Res. 2018;20:1–10.
- Reissig F, Hübner R, Steinbach J, et al. Facile preparation of radium-doped, functionalized nanoparticles as carriers for targeted alpha therapy. Inorg Chem Front. 2019;6(6):1341–1349.
- Sandhöfer B, Meckel M, Delgado-López JM, et al. Synthesis and preliminary in vivo evaluation of well-dispersed biomimetic nanocrystalline apatites labeled with positron emission tomographic imaging agents. ACS Appl Mater Interfaces. 2015;7(19):10623–10633.
- Chakraborty S, Vimalnath KV, Rajeswari A, et al. Preparation, evaluation, and first clinical use of 177Lu‐labeled hydroxyapatite (HA) particles in the treatment of rheumatoid arthritis: utility of cold kits for convenient dose formulation at hospital radiopharmacy. J Label Compd Radiopharm. 2014;57(7):453–462.
- Das T, Banerjee S. Theranostic applications of lutetium-177 in radionuclide therapy. Curr Radiopharm. 2016;9(1):94–101.
- Severin AV, Vasiliev AN, Gopin AV, et al. Dynamics of sorption—desorption of 223 Ra therapeutic α-Emitter on granulated hydroxyapatite. Radiochemistry. 2019;61(3):339–346.
- Ma O, Al N, Ap O, et al. Hydroxyapatite and porphyrin-fullerene nanoparticles for diagnostic and therapeutic delivery of paramagnetic ions and radionuclides. Bull Russ State Med Univ. 2018; 7(6):86–93.
- Berdeguez MBT, Thomas S, Medeiros S, et al. Dosimetry in radiosynoviorthesis: 90Y VS. 153Sm. Health Phys. 2018;114(1):1–6.
- Chakraborty S, Das T, Chirayil V, et al. Erbium-169 labeled hydroxyapatite particulates for use in radiation synovectomy of digital joints–a preliminary investigation. Radiochim Acta. 2014;102(5):443–450.
- Rigali MJ, Brady PV, Moore RC. Radionuclide removal by apatite. Am Mineral. 2016;101(12):2611–2619.
- Suchánková P, Kukleva E, Nykl E, et al. Hydroxyapatite and titanium dioxide nanoparticles: Radiolabelling and in vitro stability of prospective theranostic nanocarriers for 223Ra and 99mTc. Nanomaterials. 2020;10(9):1632.
- Severin AV, Vasiliev AN, Gopin AV, et al. Sorption and diffusion behavior of actinium (iii) ions in contact with hydroxyapatite as a transporter of medical radionuclides. Russ Chem Bull. 2020;69(12):2286–2293.