?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Due to the misuse of antibiotics, the multidrug-resistant Staphylococcus aureus (MDRSA) has caused serious infections and become more difficult to deal with. Here we propose to synthesise copper oxide nanoparticles (CuO-NPs) using a cell-free filter of Streptomyces rochei to enhance antibiotics activity against (MDRSA) and kill them. Characterisation of CuO-NPs using ultraviolet, dynamic light scattering, zeta potential, transmission electron microscopic (TEM), and X-ray diffraction, were investigated. The antibacterial action of the CuO-NPs was tested against standard strain and clinical isolates using the agar well diffusion method and the microdilution assay. The results showed the monodispersed spherical shape CuO-NPs with a mean diameter of 10.7 nm and were found to be active against (MDRSA). By a combination of CuO-NPs with different antibiotics, the highest synergistic effect was observed with cefoxitin, the minimum inhibitory concentration (MIC) was reduced to 6.5 for CuO-NPs, and 19.5 for cefoxitin. Time-kill assay showed the highest reduction in log10 colony-forming unit (CFU)/ml of initial inoculum of MRSA after 24 h. The HFB-4 cells cultured in the presence of CuO-NPs showed normal morphology with 100% viability at 8 µg/ml. TEM showed that combination (1/4 MIC cefoxitin +1/16 MIC CuO-NPs) highly damages bacterial cells’ shape. The biosynthesis CuO-NPs showed antibacterial activity against S. aureus suggesting a promising alternative in clinical.
Introduction
Bacterial resistance major global health threat, which causes millions of deaths cases every year, although the broad development of antimicrobial including (antibiotics and antiviral) [Citation1]. Treatment of infections caused by multidrug-resistant bacteria (MDRB) through antibiotics options is often limited. In this context, multidrug-resistant Staphylococcus aureus (MDRSA) causes critical infections in hospitals, with a high death rate [Citation2,Citation3]. The World Health Organisation (WHO) recommendations decrease the misuse of antibiotics to avert the evolution and widespread bacterial resistance [Citation4,Citation5]. These clinical problems highlight the serious request for the development of novel approaches for the eradication of multidrug-resistant bacteria (MDRB) [Citation6,Citation7]. Due to declining antibiotic discovery and increased emerging bacterial resistance, treatment strategies based on nano-therapy are urgently needed. In this regard, the nanoparticles can help to overcome these problems and enhance drug efficacy [Citation8–10]. In addition, nanoparticles have the feature to be capable to target multiple biomolecules and microbes compromising the development of resistant strains, as well as being effective as antimicrobial agents through different mechanisms with respect to antibiotics [Citation11–13]. Metal nanoparticles can penetrate the cell wall and cell membrane of pathogenic bacteria and interfere with essential molecular pathways, formulating unique antimicrobial mechanisms. Enhancement of antibiotics activity through combinations with metal nanoparticles has been one of the successful approaches to overcoming emerging bacterial resistance [Citation14]. Copper nanoparticles (CuO-NPs) are bio-suitable and can be synthesised with eco-friendly methods. Biosynthesis of CuO-NPs using a cell-free filter of actinomycetes exhibits highly antibacterial activity against several bacteria species, Proteus mirabilis, Escherichia coli, Klebsiella pneumoniae, methicillin-resistant Staphylococcus aureus (MRSA), and Salmonella typhimurium comparable with gentamicin [Citation15,Citation16]. CuO-NPs have found several uses such as additives in skin products, plastic for food packaging, metal coatings (antibacterial coatings in dentistry), and virus disinfection in amalgam [Citation17,Citation18]. Copper-based nanoparticles establish several crucial features contrary to other metallic nanoparticles [Citation19,Citation20] and dissolve high than other noble metals by liberation ions in the surroundings [Citation11]. Regarding their antibacterial activity, CuO-NPs generate oxidative stress, leading to the disjoint of bacterial membranes [Citation21]. In addition, copper ions highly generate compounds that are toxic to bacteria. Moreover, copper is a necessary element for human and other mammalian life, and it is included in several pathways that are vital for living. Thus, copper ion liberation can activate and support some of these pathways, such as the renewal of tissues [Citation22–24]. Therefore, the aim of this study, biosynthesis of copper oxide nanoparticles (CuO-NPs), develops a new combination of CuO-NPs and antibiotics to combat MDRSA.
Materials and methods
Identification and antibiotic profiling of clinical isolates
Two clinical isolates coded as AS-185 and AS 325 were kindly obtained from the microbiology department at the Tripoli University Hospital, Tripoli, Libya. These isolates were identified using an automated VITEK2 system, Version 05.04 (BioMérieux Inc). The antibiotic profile of these isolates as well as Staphylococcus aureus ATCC 25923 was evaluated using the disc diffusion method on Mueller-Hinton Agar (MHA). MHA was inoculated with each bacterium using sterile cotton swabs. Antibiotic discs representing different classes of antibiotics (antibiotics panel were ciprofloxacin 5 μg/ml, nalidixic acid 30 μg/ml, rifampin 5 μg/ml, nitrofurantoin 300 U, gentamycin 10 μg/ml, amikacin 30 μg/ml, neomycin 30 μg/ml, clindamycin 2 μg/ml, erythromycin 15 μg/ml, tetracycline 30 μg/ml, chloramphenicol 30 μg/ml, fusidic acid 10 μg/ml, penicillin10U, amoxicillin 25 μg/ml, oxacillin1μg/ml, methicillin 25 μg/ml, amoxicillin/clavulanic acid 20/10 μg/ml, imipenem10μg/ml, cefoxitin 30 μg/ml, cefuroxime 30 μg/ml, vancomycin 30 μg/ml, polymyxin B 300 U and trimethoprim/sulfamethoxazole 25 μg/ml) were gently loaded on the prepared plates using sterile forceps. The prepared plates were incubated at 35 °C for 18 h. The diameter of the inhibition zone was measured in millimetres (mm), compared with the standard zone diameter given in the protocol chart. It can be determined whether the bacterial isolate is resistant, intermediate, or susceptible to the tested antibiotics [Citation25].
Biosynthesis of copper nanoparticles
Streptomyces rochei (MH577306) used for the biosynthesis of the CuO-NPs was previously characterised [Citation26]. CuO-NPs were synthesised as follows: S. rochei was cultivated on starch nitrate broth medium and incubated on an orbital shaker (150 rpm) for 7 days at 28 °C. After the incubation period, the cell-free supernatant was obtained by filtering through cotton and then centrifugation at 5000 rpm for 30 min to remove cell debris [Citation27]. The copper sulphate solution (1 mM) was mixed with obtained filtrate at an equal ratio (vol./vol.). The synthesis of CuO-NPs was detected by observing the change in colour of the reaction mixture. The copper ions reduction was indicated via visual observation of colour and by monitoring the UV-Vis spectra [Citation28]. The obtained of CuO-NPs were purified by centrifuged and washed with deionised water, and the CuO-NPs were dried using a hot air oven at 60 °C until a constant weight was obtained [Citation29].
Characterisation of biosynthesized CuO-NPs
The optoelectronic properties of the biosynthesized CuO-NPs were measured by ultraviolet-visible absorption spectra (UV-Vis, Hitachi U-2800) in the range of 200–900 nm. The Fourier transform infra-red spectroscopy (FTIR) spectrum of the sample was recorded on an Agilent system Cary 630 FTIR model – Chemistry Department, Faculty of Science, Al-Azhar University, Cairo, Egypt, in the range 400–4000 cm−1 in FTIR spectroscopy at a resolution of 1 cm−1. The spectral data obtained were compared with the reference chart to identify the functional groups present in the sample. The size and shape of the products were observed by High-resolution transmission electron microscopic (HR-TEM) (JEOL 2100 Japan, at National Research Centre (NRC), Giza, Egypt). The crystalline structure of the biosynthesized CuO-NPs was characterised by X-Ray diffraction that was carried out using the Shimadzu apparatus with nickel filter and Cu-Ka target, Shimadzu Scientific Instruments (SSI), Kyoto, Japan. The particle size distribution of CuO-NPs was evaluated using dynamic light scattering (DLS) measurement conducted with a Malvern Zetazier Instrument. Measurements were taken in the range between 0.1 and 1000 μm. Data obtained were analysed using Zetasizer software. The X-ray diffraction (XRD) and DLS were measured at the National Centre for Radiation Research and Technology (NCRRT), Cairo, Egypt. Zetasizer Nano ZS, Malvern, UK was used to characterise the zeta potential of the nanoparticles in the solution. Generally, the Characterisation of CuO-NPs was carried out according to Radha and Gayathri [Citation30].
Antibacterial activity of biosynthesized CuO-NPs
A Stock solution of CuO-NPs (5 mg/ml) was prepared then the antibacterial activity was determined against MDRSA clinical isolates AS-185, AS 325, and S. aureus ATCC 25923 standard strains. This assay was performed using the agar well diffusion technique, in which, wells (8 mm) were cut in Mueller Hinton agar (Merck Kga A, Germany) plates inoculated with tested bacteria. Fifty microliters of CuO-NPs solution were pipetted into a well. After incubation at 37 °C for 24 h, the inhibition zone diameter around each well was measured in mm. Antibiotic paper discs of cefoxitin were used for comparison in this assay that was carried out according to the Kirby–Bauer method [Citation3]. The experiment was performed in three replicates.
Assessment of antibacterial activity for CuO-NPs in combination with antibiotics
To determine the synergistic effect of CuO-NPs in combination with different common antibiotics: amoxicillin, amoxicillin-clavulanate, cefoxitin, cefuroxime, ciprofloxacin, erythromycin, gentamicin, amikacin, methicillin, nalidixic acid, neomycin, oxacillin, penicillin, polymyxin B, and tetracycline were used. A disc diffusion method was performed to screen the synergistic activity of CuO-NPs and antibiotics. All antibiotics were used in the form of standard antibiotic discs 6 mm in diameter (HiMedia Laboratories Pvt. Ltd, Mumbai, Maharashtra, India). Bacterial suspension of MDRSA AS-185 and SA-325 were spread on MHA plates then, the standard antibiotic disc was loaded with 25 µl of prepared CuO-NPs (0.25 mg/ml) and gently placed on the MHA plates as well as a standard antibiotic disc without CuO-NPs and another paper disc loaded with 25 µl of prepared CuO-NPs (0.25 mg/ml) only. After 24 h of incubation at 37 °C, the inhibition zone (mm) was measured. This assay was performed in triplicate and the increase in fold area was estimated by measuring the mean of the inhibition zone produced by an antibiotic alone and in combination with CuO-NPs, according to the following equation:
Where A is ZOI for the antibiotic and B is ZOI for the antibiotic + NPs [Citation31].
Determination of minimum inhibitory concentrations (MIC) of cefoxitin and CuO-NPs
The MIC values of cefoxitin (HiMedia Laboratories Pvt. Ltd. India.) and CuO-NPs were determined using a microdilution assay in a 96-well plate. Mueller Hinton broth medium was inoculated with a cell suspension of S. aureus SA-325 isolate and S. aureus ATCC 25923 ∼ (106 CFU/ml) and 100 µl of the inoculated medium was distributed in each well. Tested compounds (cefoxitin and CuO-NPs) were tested in a twofold serial dilution. Cefoxitin was tested at concentrations ranging from 10,000 to 4.8 µg ml−1 and CuO-NPs started with 833–0.4 µg ml−1. The experiment was performed according to the criteria of M7-A728. Wells containing negative control (medium + cefoxitin or CuO-NPs at the tested concentrations) were performed to determine the differences in optical density. The viability of bacterial cells was evaluated by adding 30 µl of (0.18%) sterile resazurin solution (Hi-media) to all wells and re-incubated at 35 °C for 18–24 h. By visual examination, If the colour in wells of the microtiter plate does not change and stayed blue or violet, this indicates that the bacterial isolate cannot grow at that concentration, while the colour change to pink, red, or purple, this indicated the growth of bacteria at that concentration. Also, the growth was assayed using a microtiter enzyme-linked immunosorbent assay (ELISA) reader (Mindray MR-96A, China) by monitoring absorbance at 600 nm. The MICs of CuO-NPs or cefoxitin were determined as the lowest concentrations that inhibited the visible growth of the bacteria [Citation32].
Evaluation CuO-NPs, and combinations with cefoxitin using checkerboard method
The stock solution of CuO-NPs was freshly prepared at 100 mg/ml also, cefoxitin was prepared with a concentration of 10 mg/ml. Checkerboard technique was performed using 96 well microplates containing Mueller–Hinton Broth (Difco) with cefoxitin concentrations which initiated from MIC to 1/128 MIC in the rows and CuO-NPs concentrations which initiated from MIC to 1/128 MIC along columns. Multidrug resistance Staphylococcus aureus isolate was inoculated at a density of ∼106 (CFU)/ml/well. Checkerboard microdilution technique was performed in duplicate and estimated after 24 h of incubation at 37 °C. Wells with no cefoxitin - CuO-NPs combinations (only medium with MDRSA) were used as growth control. Wells with a medium containing the used combinations only (without MDRSA) were used as a negative control [Citation33]. The variation in the growth of MDRSA was calculated by measuring the optical density at the start and the end of the experiment (after 24 h) using an ELISA plate reader at 630 nm (Mindray MR-96A, China) [Citation34]. The fractional inhibitory concentration index (FICi) explains the interaction between cefoxitin, and CuO-NPs is calculated with the following equation:
Therefore, The FIC index (FICI), calculated as the sum of each FIC
The results were interpreted as follows: FICI ≤ 0.5 is defined as synergy, 0.5 < FICI < 1, partial synergy, 2 ≥ FICI ≥ 1, additive, 2 < FICI < 4, indifferent, while antagonism is considered when FICI ≥ 4 indicates antagonism [Citation35].
Time-kill assay
Time-kill assay against MDRSA SA-325 was conducted to evaluate the killing potencies of cefoxitin and CuO-NPs combinations that showed synergistic action in the checkerboard test. Mueller Hinton broth medium with cefoxitin and CuO-NPs combinations were inoculated with cells of MDRSA isolate, at a count of ∼106 CFU/ml, and incubated at 37 °C. Aliquots of each treatment, as well as a positive control (medium without combinations inoculated with the same cell count of S. aureus SA-325), were withdrawn at periods of 0,1, 2, 3, 4, 5, 6, 12, 18, and 24 h hands-on with serially diluted in sterile saline solution. A hundred microliters of each dilution were inoculated onto nutrient agar plates in triplicate. These plates were incubated at 37 °C for 18–24 h and CFU/ml was counted [Citation33,Citation36,Citation37]. The kill measurement and the rate of bacterial death were determined by plotting the viable colony counts as a log10 (CFU/ml) against the time. The interaction was classified as bacteriostatic or bactericidal. Bacteriostatic action was defined as a decrease of <3 log10 CFU/ml and the bactericidal effect was defined as a decrease of ≥3 log10 CFU/ml after 24 h of incubation compared with the initial inoculum [Citation38].
In vitro cytotoxicity of CuO-NPs against normal cells
The cytotoxic activity of CuO-NPs was evaluated in vitro, using the HFB-4 (normal human melanocytes) cell lines according to Abu-Serie and El-Fakharany [Citation39] in triplicate. The cell viability and proliferative potential based on their metabolic activity were determined with MTT (3-(4,5-dimethyl thiazolyl-2)-2,5-diphenyltetrazolium bromide) assay. The adherent culture medium was replaced with a medium containing different concentrations of 0.0, 1, 2, 4, 8, 16, 32, 64, and 128, µg/ml of CuO-NPs and incubated for 24 h. After incubation for 24 h, the cells were washed three times with fresh media or cold PBS and incubated with 0.5 mg/ml MTT (Sigma-Aldrich) for 2–5 h. Then MTT solution was removed and 200 μl of DMSO was added to each well. The optical density (OD) of each dose was read at 570 nm using a microplate reader (BMG LabTech, Germany). The % cell viability and % cell death were calculated using the following formulas:
Electron microscopy study
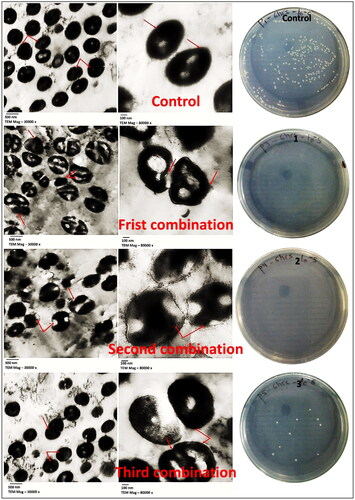
To determine the effect of CuO-NPs and antibiotics combination on MDRSA-325 cells, a transmission electron microscope was used to investigate the morphological changes of the exposed cells. The MDRSA-325 were inoculated Muller Hinton broth medium and treated with the three most potent combinations (cefoxitin and CuO-NPs) obtained from checkerboard assay (1/4MIC + 1/16MIC 625 + 13), (1/4MIC + 1/32MIC 625 + 6.5) and (1/8MIC + 1/16MIC 312.5 + 13) (µg/µg)/ml separately and used bacterial suspension without any combination (as control). After 6 h of incubation at 37 °C, the inoculation reaches to log phase and then is adjusted to 0.5 McFarland standard to obtain the final inoculation doses of approximately 105 CFU/ml [Citation40]. The specimens were prepared for transmission electron microscopic (TEM) procedures described by Bozzola and Russell [Citation41], using a JEOL-JEM 1010 Transmission Electron Microscope at 80 KV at the Regional Centre for Mycology and Biotechnology (RCMB), Al-Azhar University, Cairo, Egypt.
Statistical analysis
The data were expressed as the mean ± SE value, which was calculated by using Minitab 18 software extended with a statistical package and Microsoft Excel 365.
Results and discussion
Isolation and identification of multidrug resistance S. aureus (MDRSA)
The clinical isolates were identified using the vitek2 automated system as Staphylococcus aureus with a very good probability of 95% for both S. aureus − 185, and S. aureus − 325. The antibiotic susceptibility of these isolates and reference strain S. aureus ATCC 25923 was determined using 23 antibiotics representing different classes of antibiotics. Generally, the results indicated the widespread emergence of multidrug resistance among the tested isolates, where S. aureus − 325 was insensitive to most tested antibiotics except nitrofurantoin, fusidic acid vancomycin, and trimethoprim-sulfamethoxazole, While S. aureus − 185 was found sensitive to rifampin, amikacin, clindamycin, chloramphenicol, imipenem, and vancomycin comparable with reference strain S. aureus – ATCC 25923 which was found sensitive to most tested antibiotics. The results of this assay were shown in and . According to the results of this assay, both S. aureus − 185 and S. aureus − 325 are classified as methicillin and multidrug-resistant S. aureus. Methicillin and multidrug-resistant S. aureus were isolated from clinical samples and communities [Citation2,Citation3,Citation42].
Figure 1. Antibiotics sensitivity of S. aureus strains (A) S. aureus ATCC-25923, (B) S. aureus SA-325, and (C) S. aureus -SA-185.
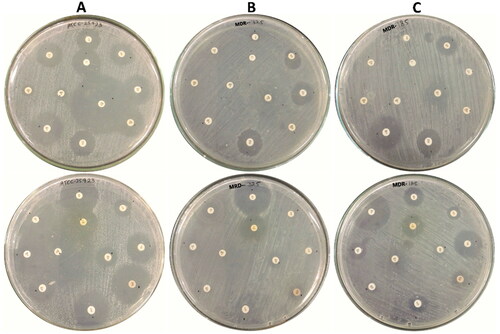
Table 1. Antibiotics profile of S. aureus SA-185, S. aureus SA-325, and S. aureus ATCC-25923.
Extracellular biosynthesis of CuO-NPs
The cell-free filtrate of S. rochei (MH577306) was used as a reducing agent for the synthesis of CuO-NPs. Sarah and his team [Citation43] used cell-free filtrate of Streptomyces species for the biosynthesis of CuO-NPs. The synthesis of CuO-NPs was detected by observing the change in colour of the reaction mixture produced from the mixing of the precursor (CuSO4 solution – with light blue) with filtrate of S. rochei to turbid green after the incubation. The green colour is an indicator of CuO-NPs formation where nanoparticles of copper seem green colour in aqueous solutions and the formation of these colours depends on the surface vibration of plasmon [Citation15,Citation16].
Characterisation of biosynthesized CuO-NPs
UV-Visible spectrophotometric examination of CuO-NPs
Investigation of the reaction mixture using Uv-visible spectroscopy is one of the important techniques that are widely used to emphasise the formation of nanoparticles because the shape and position of the peak are sensitive to a particle size that indicates the formation of nanoparticles. The results of UV-Visible absorption spectra of CuO-NPs in the reaction mixture indicated that two peaks in different regions of the UV-Vis spectrum were observed: one broad peak in the visible range at 550–650 nm and the other appeared as a sharp peak in the ultraviolet range at 300 nm as shown in . Our results agree with Sarah et al. [Citation43], who mentioned that when cell-free supernatant of Streptomyces species was added to CuSO4 solution and incubated for 48 h the colour of the reaction mixtures changed from blue to green. Depending on individual particle characteristics, such as shape, size, and capping agents, the exact location of the surface plasmon resonance band can vary. Brause et al. [Citation44] reported that surface plasmon resonance is dominant in the optical absorption of metal nanoparticles, and particle size is linked to the absorption peak. The surface plasmon resonance of CuO-NPs in an aqueous solution increase to longer wavelengths, with particle size increasing. The position and form of copper nanocluster absorption of plasmon are strongly dependent on particle size, stabilising molecules or surface adsorbed particles, and the media’s bioelectricity [Citation45].
Dynamic light scattering (DLS) and zeta potential measurement of CuO-NPs
DLS analysis is used to determine the size of the nanoparticles in colloid solutions. The main average size of the biosynthesized CuO-NPs was about 10.7 nm (SD: ±0.8 nm), as shown in . Our results agree with Ponnusamy et al. [Citation46], who reported a varied size of CuO-NPs ranging from 5 and 50 nm. in diameter. The zeta potential value of the dispersed CuO-NPs in distilled water was recorded at −1.3 mV . The stability of biosynthesized nanoparticles was depending on particle surface charge which determines by zeta potential analysis. The high positive or negative zeta potential values remain particles far from each other and hence prevent aggregations [Citation47].
X-ray diffraction (XRD) of CuO-NPs
The crystalline nature of biosynthesized CuO-NPs was analysed using a powder X-Ray diffractometer. CuO-NPs exhibit XRD pattern at 2θ values of 32.42°, 35.54°, 38.73°, 48.74°, 53.7°, 58.2°, 61.3°, 66°, 67.82°, 72.34° and 75.2° which were observed in . These peaks are assigned to the 110, 002, 111, 20–2, 020, 202, 11–3, 310, 22–1, 311 and 004 reflection planes of face-centered cubic (fcc) metallic copper, respectively. Our results are in good agreement with those of powder CuO obtained from the international centre of diffraction data card (JCPDS-98-004-8581) confirming the formation of crystalline structure. These results also agree with Sarah et al. [Citation42], who mentioned that the XRD pattern of the CuO-NPs synthesised using Streptomyces sp. showed 2θ values of 35°, 38°, 48°, 53°, 58°, 65°, 67°, and 72°, corresponding to XRD planes (111), (202), (020), (202). Additionally, no extra diffraction bands of other phases are found referring to the purity of the biosynthesized CuO-NPs.
Fourier transform infra-red spectroscopy (FTIR)
The FTIR analysis was used for the characterisation of the biosynthesized nanoparticles. showed that biosynthesized CuO-NPs exhibit peaks ranging between 500 and 4000 cm1. The sharp peaks at 3902.73 cm−1 were indicative of the hydroxyl group (–OH) in the carboxyl group (–COOH), the peak at 1635.64 cm−1 was attributed to the N–H bending of amines, the peak at 1396.46 cm−1 was ascribed to the NO2 asymmetric stretching of nitro compounds. FTIR analysis may be confirmed the biosynthesis of CuO-NPs according to the presence of a broad band under 1000 cm−1, specifically at 671 cm−1. The FTIR analysis was used to characterise and identify the functional groups of the metabolites which share in the biosynthesis of nanoparticles [Citation46]. Moreover, the FTIR analysis suggested that CuO-NPs were surrounded by different organic matter such as protein or enzymes and confirmed the copper-amino acid complex formation [Citation48].
High-resolution transmission electron microscopy
HR-TEM of the CuO-NPs exhibited a particle size ranging from 1.52 to 8.49 nm and most particles were spherical with an average size of 5 nm. Well-dispersed nanoparticles dissolved in glycerol (nanofluid form) were scattered and didn’t observe any aggregated nanoparticles and the size of nanoparticles didn’t change, while the aggregation of nanoparticles was observed when the nanoparticles dispersed in water as shown in . This means that the dispersion of CuO-NPs in glycerol (nanofluid) leads to preventing the growth and aggregation of nanoparticles and enhances their stability. Both the products of nanoparticles were spherical in their morphology. In earlier work, TEM analysis of CuO-NPs was biosynthesized by Streptomyces gresius showed that the average particle size ranges between 5 and 50 nm with quasi-spherical shaped nanoparticles [Citation46]. Generally, nanoparticle sizes are affected by metallic core, coating, and stabiliser substances which accumulate on nanoparticle surfaces [Citation49].
Antibacterial activity of CuO-NPs
Antibacterial activity of CuO-NPs (dispersed in distilled.H2O) against multidrug-resistant S. aureus strains SA-325, SA-185, and S. aureus ATCC 29523 were evaluated using the agar well diffusion method as shown in . The results showed that CuO-NPs exhibits antibacterial activity against MDRSA with inhibition zone ranging from 14 to 24 mm. These results showed that the CuO-NPs had high activity against Gram-positive bacteria either standard or clinical strains, comparable with some antibiotics [Citation50]. CuO-NPs are currently attracting attention owing to their antibacterial activity and their low price. Several studies indicated that CuO-NPs have antibacterial activity against a wide spectrum of pathogenic bacteria [Citation9,Citation51–53]. Chatterjee et al. found that the inhibition zone ranged from 5 mm (P. aeruginosa) to 26 mm (E. coli), while it was 21 mm in S. aureus [Citation54]. Some reports indicate that the antibacterial activity of CuO-NPs exceeds silver nanoparticles (AgNPs), and this is an advantage added to these particles, being cheap and easy to obtain in addition to the ease of their synthesis [Citation55–57]. Copper used for decades as a strong antimicrobial agent capable of reducing microorganisms by 99.9%. Copper has been registered with the US Environmental Protection Agency [EPA] as an antimicrobial agent that can reduce pathogenic bacteria associated with microbial infection. Several studies have confirmed that CuO-NPs have antibacterial activity against a wide spectrum of different strains of gram-positive and Gram-negative bacteria, such as S. aureus, P. aeruginosa, E. coli, V. cholera, and B. subtilis [Citation58]. CuO-NPs have peculiar behaviours that result in effective antibacterial activity associated with the stimulation of essential body functions. [Citation59].
Table 2. Antibacterial activity of biosynthesized CuO-NPs against S. aureus strains.
Assessment of synergistic activity between CuO-NPs and antibiotics
With MDRSA on the rise, new approaches are required for its combating. Biologics and non-antibiotic adjuvants offer this opportunity for expansion. In the current study, the efficacy of biosynthesized CuO-NPs alone and in combination with different antibiotics was studied against multi-drug resistant Staphylococcus isolates. All of these combinations showed effectiveness against the tested bacteria but unevenly. The highest synergistic effect of CuO-NPs was observed in the presence of cefoxitin and polymyxin B where fold-area increases reached 8.0 and 7.03 respectively, while was 0.19 with tetracycline, which represented the weakest synergy with CuO-NPs. The CuO-NPs did not give any synergism with nalidixic acid while it gave an antagonistic effect with amoxicillin when tested against S. aureus SA-185 as shown in , and . Generally, the results of the antibiotic-CuO-NPs combination studies reported that CuO-NPs could enhance the antimicrobial activity of most tested antibiotics. It is believed that the synergistic mechanisms between CuO-NPs and antibiotics are through the release of copper which interacts with surface microbial structures, followed by their penetration through the cell membrane, and the generation of reactive oxygen species (ROS) [Citation60]. The synergistic interaction between copper and erythromycin against a variety of Gram-positive and Gram-negative bacteria has been studied by Sultana et al. [Citation61]. They found that the erythromycin-copper complex was more effective against Enterococcus faecalis, Escherichia coli, Proteus vulgaris, Salmonella typhi, and Shigella dysentery at 0.5–1 µg/ml concentrations, Klebsiella pneumonia, S. epidermidis, and S. aureus were less affected at 128 µg/ml concentrations. Wozniak-Budych et al. [Citation62] also investigated the antimicrobial activity of CuO-NPs-rifampicin complex against Gram-positive and Gram-negative bacteria. They found that this complex was able to damage the cell membranes of S. aureus and facilitate the disintegration of the biofilm and DNA damage. Kiranmai et al. [Citation63] found that CuO-NPs were able to improve the enhancement of ciprofloxacin, gentamycin, and amoxicillin against Gram-positive and Gram-negative bacteria, and the greatest enhancement was with ampicillin. Zhang et al. [Citation60] tested the action of nanoparticles with 22 types of antibiotics against E. coli. They found the best synergy with cephalexin that made the cell membrane more permeable. Also, Kaur et al. [Citation64] reported synergistic activity between CuO-NPs with azithromycin, erythromycin, and norfloxacin against Gram-positive and Gram-negative bacteria. Synergistic activity increased, especially against Klebsiella, Staphylococcus, and Pseudomonas bacteria. Cefoxitin has some active groups, such as the carboxyl and amine groups that interact with the metallic ions which enhance their antibacterial activity [Citation65]. Combination approaches should extend beyond biologically active molecules to include smart controlled delivery strategies. Infection control must integrate antimicrobial stewardship, new antibiotic molecules, biologics, and delivery strategies into effective combination therapies designed to: fight the infection, avoid resistance, and protect the natural microbiome [Citation66]. Because cefoxitin exhibits the highest synergistic effect when combined with CuO-NPs in our study, we choose this combination for further study.
Figure 3. Synergistic activity y of CuO-NP combinations with standard antibiotics against S. aureus (A) S. aureus SA-185 and (B) S. aureus SA-325.
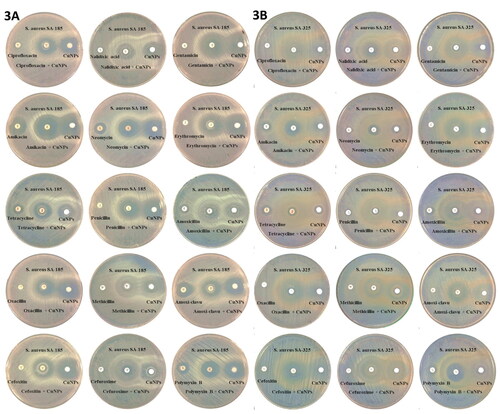
Table 3. Screening of synergistic effect biosynthesized CuO-NPs and antibiotics against (MDRSA).
Determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) for CuO-NPs and cefoxitin
The estimation of MIC using microdilution method indicated that the tested strains were susceptible to the CuO-NPs at a concentration of 208 µg/ml, while the MBC was 416 µg/ml as shown in . The MIC and MBC of metal nanoparticles depend on several factors, including the size and shape of the nanoparticles, as well as the tested microorganism and the inoculum size [Citation56]. Ashaiyoth et al. [Citation67] found that the MIC of CuO-NPs ranges from 2 to 128 µg/ml, it was calculated against Gram-negative and Gram-positive pathogenic bacteria. Also, Ruparelia et al. [Citation56] found that the MIC for E. coli was 140–280 µg/ml, B. subtilis was 20 µg/ml, and for S. aureus was 140 µg/ml; while the MBC for E. coli was 160–300 µg/ml, Bacillus subtilis was 40 µg/ml, and S. aureus was 160 µg/ml. In the same context, Das et al. [Citation68] indicated that the CuO-NPs showed MIC values ranging from 250 to 500 µg/ml, including S. aureus, which was the value of 500 µg/ml. Also, Raiiygandhi et al. [Citation69] found the antibacterial activity of CuO-NPs against K. pneumoniae and P. aeruginosa with a MIC of 100 µg/ml, while the MBC effect was at 150 µg/ml. Prabhu et al. and Maruthupandy et al. [Citation70,Citation71] reported that the MIC values of 100 and 150 µg/ml of CuO-NPs were effective against E. coli and S. aureus, respectively. Ramachandran et al. [Citation72] confirmed that the MIC was 250 µg/ml, which is completely inhibiting MDR-resistant infectious pathogens. Some reports indicated that the MIC and MBC values of CuO-NPs range from 100 to 5000 µg/ml. The reason may be that these particles are highly amenable to the oxidation that forms the insoluble metallic copper; thus, the inhibition of microbial contact diminishes, and more nanoparticles are needed to kill bacteria [Citation73]. This could explain the relatively high MIC values for CuO-NPs compared to AgNPs. Also, Sultana et al. [Citation61] noted S. aureus resists copper ions compared to other ions (such as cobalt, nickel, and chromium). In our study, the MIC values of cefoxitin were 9.7 and 2500 µg/ml against reference strain S. aureus ATCC 29523 and clinical strain S. aureus SA-325 respectively. Also, the MBC values were ≥19.5 and ≥5000 µg/ml for reference strain S. aureus ATCC 29523 and clinical strain S. aureus SA-325 respectively. Some studies indicate that bacteria can resist antibiotics at concentrations greater than 10–1000 times the concentrations needed to kill sensitive or wild strains [Citation74]. This resistance may be due to the various mechanisms obtained, such as the formation of biofilms or the production of some hydrolytic enzymes for this antibiotic [Citation75]. A study revealed that S. aureus’s resistance to several antibiotics, including cefoxitin was closely related to the production of β-lactamase [Citation76]. Some strains of multidrug-resistant K. pneumoniae were reported to have MIC of the cefoxitin up to 1000 µg/ml [Citation77].
Table 4. Determination of minimum inhibitory concentrations of biosynthesized CuO-NPs and cefoxitin.
Checkerboard assay
Checkerboard interactions between cefoxitin and CuO-NPs were determined against the tested bacteria by measuring the FIC index. The twenty-four of the fifty-one combinations that prevented growth showed a synergistic action. The real synergy does not appear until the MICs of both agents are reduced to a quarter or less. Through these interactions, the MIC of cefoxitin decreased from 2500 µg/ml to less than 625 (4-fold), it reached 19.5 (128-fold) in some combinations. Also, the MIC for CuO-NPs decreased from 208 µg/ml to less than 52 (4-fold), it reached 6.5 (32-fold) in some combinations as shown in Table S1 and . Similar results had been reported by Selvaraj et al. [Citation78] who used checkerboard and time-kill assays against tested bacteria to confirm the synergistic action between CuO-NPs and amoxiclav. The amoxiclav incubated concentration decreased to 32-fold against S. aureus and 16-fold against P. mirabilis. Zhang et al. [Citation60] observed a strong synergistic effect of CuO-NPs and cephalexin against E. coli, which made the cell wall more fragile. They concluded from the study that the CuO-NPs were the source of cell damage and penetration. Besides, the presence of antibiotics promoted the release of copper ions (Cu2+) and increased the uptake of these ions, which led to the generation of reactive oxygen species (ROS). Also, the presence of Cephalexin greatly enhanced cellular permeability compared to other antibiotics. Also, the synergistic action of the ceftriaxone with metallic nanoparticles was reported by Shanmuganathan et al. [Citation79] where they stated the antimicrobial activities of the unconjugated and the conjugated AgNPs (50 µg/ml) were 10 and 20 mm against K. pneumoniae, and 18 and 23 mm against S. aureus, respectively.
Figure 4. (A) Checkerboard assay to test the synergistic interaction of combined cefoxitin and CuO-NPs against S. aureus SA-325. (Pink colour indicates to growth of the bacteria and blue means the bacteria was inhibited by the combination). (B) Killing curves for cefoxitin and CuO-NPs at different treatments 1, 2, 3 and control.
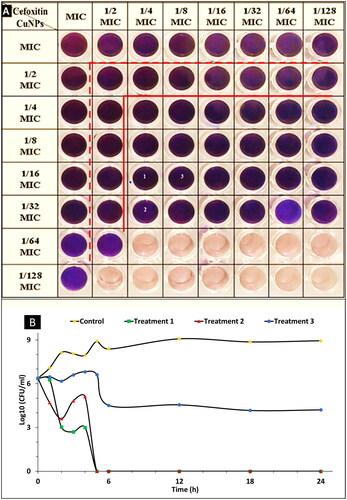
Time-kill assay
The results in represented the growth curve of S. aureus SA-325 treated with the selected fractions as well as the untreated bacteria (control, inoculum without any antimicrobial agent neither cefoxitin nor CuO-NPs). In the treatment1 1/4 MIC cefoxitin +1/16 MIC CuO-NPs) (625 + 13 µg ml−1), the synergistic effect appeared in the second hour of incubation showing a bactericidal activity when the log10 (CFU/ml) decreased to 3.34, and the complete eradication of the tested bacteria occurred after 24 h. In the case of treatment 2 (1/4 MIC cefoxitin +1/32 MIC CuO-NPs) (625 + 6.5 µg ml−1) the synergistic effect appeared also at the second hour of incubation, which showed a bacteriostatic activity when the log10 (CFU/ml) decreased to 2.76 while bactericidal activity appeared after five hours that reached to 6.37 decreases in log10 (CFU/ml), and the complete eradication for the tested bacteria occurred after 24 h. In treatment 3 (1/8 MIC cefoxitin + 1/16 MIC CuO-NPs (312.5 + 13 µg ml−1), the synergistic effect appeared after six hours of incubation, showing a bacteriostatic activity with a decrease in log10 (CFU/ml) to 2.56 as represented in Table S2. These findings indicate that cefoxitin was reactive against the investigated bacteria when used with CuO-NPs. This might be due to the production of copper ions, which denature -lactamase and decrease its activity when they interact with it. The permeability of the metal ions through the cell wall and membrane increased concurrently with the disarming of the bacteria and the antibiotic’s reactivation, resulting in large amounts of reactive oxygen species (ROS) that hasten the breakdown of the cellular system. Selvaraj et al. [Citation78] observed similar results after using of CuO-NPs and amoxiclav combination against S. aureus. They noticed a decrease to 2.5 log10 CFU/ml within 4 h and more than 3.5 log10 CFU/ml after 8 h and the complete killing of the cell count occurred within 20 h. They concluded that the mixture could reduce the biofilm formation by S. aureus by 93%, and they said that CuO-NPs could reactivate amoxiclav. On contrary, Hall et al. [Citation80] treated MRSA with ceftriaxone at a concentration of (4, 40, 400 µg/ml) alone and observed a decline in the number of cells reached −2 log after 5 h.
In-vitro cytotoxicity of biosynthesized CuO-NPs
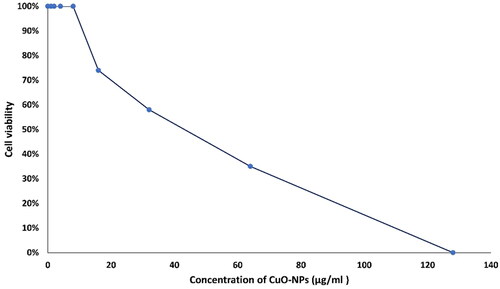
As observed in CuO-NPs did not exert toxicity on normal cells at low concertation, so they could have the potential to act as non-toxic drug delivery at concertation of 8 µg/ml or less. The effect of CuO-NPs on normal cells showed that they induced a cytotoxic effect at higher concentrations (IC50 41.60 µg/ml). This could be due to the fact that copper is an essential element involved in most metabolic pathways, but at higher concentrations, copper may interfere with a different cell function including structural, catalytic, and regulatory activities, resulting in inhibition of cell proliferation. Studies by De Jong et al. [Citation81] reported that CuO-NPs were safe in rats at concertation up to 32 mg/kg. The fibroblast cells cultured with CuO-NPs revealed normal morphology with 99.47% viability at concertation 30 µg/ml [Citation78]
Electron microscopy study
Examination of treated bacterial cells using transmission electron microscopy showed damages caused by those combinations 1, 2, and 3, compared with non-treated control. Combination (1/8 MIC cefoxitin + 1/16 MIC CuO-NPs (312.5 + 13 µg ml−1) showed greatest damages on bacterial cell from other combinations. Based on the micrographs, the untreated bacterial cells appeared with intact cell walls and did not show any changes in bacterial morphology (such as appearance, shape, or size) as shown in . The morphology change indicates a disorder in the cell wall because the cell wall is the one that represents the cytoskeleton in bacterial cells. Also, the increase in the size of cells indicates a defect in the permeability of the cell membrane because it is the one that controls osmosis. Often, the variance in the distribution of components compared to the control group, as well as the presence of transparent places, indicates a disruption in the structure of the cell wall, resulting in a fragmented and fragile wall formation. This is what is observed when cells are treated with the CuO-NPs-cefoxitin combination. Besides the typical morphology of untreated cells, they also appear normal in cell division, unlike the treated cells that appear large and unable to divide. After their treatment with combinations 1, Many abnormalities developed, such as the abnormal form of the cells that appear with areas of different densities in the cytoplasm, as well as the fact that the cell walls appear to be shattered in substantial parts of them. Treatment No. 2 had a considerable effect on bacterial cells, as evidenced by the cells exhibiting the same abnormalities as in treatment No. 1, but the cells looked to be bigger and the cytoplasmic area more dense. In addition, some small black dots appeared at increased magnification, possibly indicating the accumulation of CuO-NPs on the peripheral and inside of these cells. The cells treated with treatment No. 3 appeared more similar to the control cells (untreated), as the cells appeared to have an undifferentiated density and the cell walls were incompletely intact, except that the size of the cells was slightly larger than the control cells and the number of cells in the stages of division appeared to be less. Finally, treatment of S. aureus strain SA-325 with these combinations revealed that combinations 1 and 2 exhibited bactericidal effects while combination 3 reduced the bacterial growth showing bacteriostatic effect compared with control as shown in . This reinforces the statement that nanoparticles inhibited the antibiotic resistance mechanism, which made the antibiotic enhance the permeability of metal ions and nanoparticles [Citation78]. Some dark spots on treated cells can be seen, especially on treatments 2 and 3, which may indicate the accumulation of nanoparticles on the external structure of the bacterial cell. On the other hand, some authors indicate CuO-NPs and Cu ions penetrated the bacterial cell and caused further damage by possibly interacting with sulfur- and phosphorus-containing compounds DNA, and violating cell divisions, which leaded largening of the bacterial cells [Citation82].
Conclusion
The failure of S. aureus treatment has occurred due to antibiotic resistance. So, there is an urgent necessity to combat the limitations of traditional antibiotics. Biosynthesis of CuO-NPs using S. rochei is an emerging trend in bio-nanotechnology and thus has a broad range of implementation in the biomedical field due to its eco-friendly nature and biocompatibility. The biosynthesized CuO-NPs showed potent antibacterial activity at different concentrations against different clinical isolates of MDRSA and reference strains. The results described herein provide significant enhancement of cefoxitin activity against clinical isolates MDRSA if it is combined with CuO-NPs under the conditions implemented in the current study. Besides, results obtained in this work, CuO-NPs offer promising treatments for infectious illnesses by targeting specific and hard-to-reach sites where pathogens are harboured. Moreover, they enhance the effect of drugs, even in the case of resistant bacteria.
Author contributions
G.M.E.-S. conceived the presented idea, co-wrote the paper, and supervised the research. M.H.K. and M.A .A. performed time-kill assay, electron microscopic studies, and characterisation of nanoparticles and co-wrote the paper. M. A. A. and M.H.S. maintained, identified the clinical isolate, and performed its antibiotic profiling. S. A.M. and M. A. A. performed the minimum bactericidal concentration and interpreted its results. M.K. A.I., M.A .A. and A.S.H. characterisation of nanoparticles and performed determination of minimum inhibitory concentrations (MIC) and checkerboard of cefoxitin and CuO-NPs. A.A.A. and A.A.R performed a time-kill assay, cytotoxicity interpreted its results. Finally, all authors discussed the results, commented, and revised the manuscript.
Supplemental Material
Download MS Word (23.7 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Fisher RA, Gollan B, Helaine S. Persistent bacterial infections and persisted cells. Nat Rev Microbiol. 2017;15(8):453–464.
- Akhavan O, Ghaderi E. Cu and CuO nanoparticles immobilized by silica thin films as antibacterial materials and photocatalysts. Surf Coat Technol. 2010;205(1):219–223.
- Sharaf MH, El-Sherbiny GM, Moghannem SA, et al. New combination approaches to combat methicillin-resistant Staphylococcus aureus (MRSA). Sci Rep. 2021;11(1):4240.
- Labruere R, Sona AJ, Turos E. Anti-methicillin-resistant Staphylococcus aureus nanoantibiotics. Front Pharmacol. 2019;10:1121.
- Merlin C. Reducing the consumption of antibiotics: would that be enough to slow down the dissemination of resistances in the downstream environment? Front Microbiol. 2020;11:33.
- Zaman SB, Hussain MA, Nye R, et al. Review on antibiotic resistance: alarm bells are ringing. Cureus. 2017;9(6):e1403.
- Chatterjee A, Modarai M, Naylor NR, et al. Quantifying drivers of antibiotic resistance in humans: a systematic review. Lancet Infect Dis. 2018;18(12):e368–e378.
- Kaufmann S, Dorhoi A, Hotchkiss R, et al. Host directed therapies for bacterial and viral infections. Nat Rev Drug Discov. 2018;17(1):35–56.
- Zazo H, Colino CI, Lanao JM. Current applications of nanoparticles in infectious diseases. J Control Release. 2016;224:86–102.
- El-Sherbiny GM, Lila MK, Shetaia YM, et al. Antimicrobial activity of biosynthesized silver nanoparticles against multidrug-resistant microbes isolated from cancer patients with bacteremia and candidemia. Indian J Med Microbiol. 2020;38(3&4):371–378.
- Sánchez-López E, Gomes D, Esteruelas G, et al. Metal-based nanoparticles as antimicrobial agents: an overview. Nanomaterials. 2020;10(2):292.
- Lemire JA, Harrison JJ, Turner RJ. Antimicrobial activity of metals: mechanisms, molecular targets, and applications. Nat Rev Microbiol. 2013;11(6):371–384.
- Wang W, Li B, Yang H, et al. Efficient elimination of multidrug-resistant bacteria using copper sulfide nanozymes anchored to graphene oxide nanosheets. Nano Res. 2020;13(8):2156–2164.
- Lee NY, Ko WC, Hsueh PR. Nanoparticles in the treatment of infections caused by multidrug-resistant organisms. Front Pharmacol. 2019;10:1153.
- Roy K, Sarkar CK, Ghosh CK. Antibacterial mechanism of biogenic copper nanoparticles synthesized using Heliconia psittacorum leaf extract. Nanotechnol Rev. 2016;5(6):529–536.
- Rasool U, Hemalatha S. Marine endophytic actinomycetes assisted synthesis of copper nanoparticles (CuO-NPs): characterization and antibacterial efficacy against human pathogens. Mater Lett. 2017;194:176–180.
- Ramteke L, Gawali P, Jadhav BL, et al. Comparative study on antibacterial activity of metal ions, monometallic and alloy noble metal nanoparticles against nosocomial pathogens. BioNanoSci. 2020;10(4):1018–1036.
- Poggio C, Colombo M, Arciola CR, et al. Copper-alloy surfaces and cleaning regimens against the spread of SARS-CoV-2 in dentistry and orthopedics. From fomites to anti-infective nanocoatings. Materials. 2020;13(15):3244.
- Gawande MB, Goswami A, Felpin FOX, et al. Cu, and Cu-based nanoparticles: synthesis and applications in catalysis. Chem Rev. 2016;116(6):3722–3811.
- Nikolova MP, Chavali MS. Metal oxide nanoparticles as biomedical materials. Biomimetics. 2020;5(2):27.
- Broglie JJ, Alston B, Yang C, et al. Antiviral activity of gold/copper sulfide core/shell nanoparticles against human norovirus virus-like particles. PLoS One. 2015;10(10):e0141050.
- Kornblatt AP, Nicoletti VG, Travaglia A. The neglected role of copper ions in wound healing. J Inorg Biochem. 2016;161:1–8.
- Yamada M, Foote M, Prow TW. Therapeutic gold, silver, and platinum nanoparticles. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7(3):428–445.
- Rubilar O, Rai M, Tortella G, et al. Biogenic nanoparticles: copper, copper oxides, copper sulphides, complex copper nanostructures, and their applications. Biotechnol Lett. 2013;35(9):1365–1375.
- Patel J. M100 performance standards for antimicrobial susceptibility testing 240. Wayne: Clinical and Laboratory Standards Institute; 2017.
- Abushiba M, El-Sherbiny G, Moghannem S, et al. Enhancement of antibiotics activity by microbially synthesized silver nanoparticles. Afr J Biol Sci. 2019;15(1):137–153.
- El-Naggar NEA, Mohamedin A, Hamza SS, et al. Extracellular biofabrication, characterization, and antimicrobial efficacy of silver nanoparticles loaded on cotton fabrics using newly isolated streptomyces sp. SSHH-1E. J Nanomater. 2016;2016:1–17.
- Abd-Elnaby HM, Abo-Elala GM, Abdel-Raouf UM, et al. Antibacterial and anticancer activity of extracellular synthesized silver nanoparticles from marine Streptomyces rochei MHM13. Egypt J Aquat Res. 2016;42(3):301–312.
- Singh M, Kumar M, Kalaivani P, et al. Metallic silver nanoparticle: a therapeutic agent in combination with antifungal drug against human fungal pathogen. Bioprocess Biosyst Eng. 2013a;36(4):407–415.
- Radha KV, Gayathri K. Synthesis, characterization, and application of copper nano particles. Rev Int J Eng Res Technol. 2019;08(03):412–421.
- Birla SS, Tiwari VV, Gade AK, et al. Fabrication of silver nanoparticles by phoma glomerata and its combined effect against Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus. Lett Appl Microbiol. 2009;48(2):173–179.
- Bagherzade G, Tavakoli MM, Namaei MH. Green synthesis of silver nanoparticles using aqueous extract of saffron (Crocus sativus L.) wastages and its antibacterial activity against six bacteria. Asian Pac J Trop Biomed. 2017;7(3):227–233.
- Sopirala MM, Mangino JE, Gebreyes WA, et al. Synergy testing by etest, microdilution checkerboard, and time-kill methods for pan-drug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2010;54(11):4678–4683.
- Isaei E, Mansouri S, Mohammadi F, et al. Novel combinations of synthesized ZnO NPs and cefazidime: evaluation of their activity against standards and new clinically isolated Pseudomonas aeruginosa. Avicenna J Med Biotechnol. 2016;8(4):169.
- Joung D-K, Choi S-H, Kang O-H, et al. Synergistic effects of oxyresveratrol in conjunction with antibiotics against methicillin-resistant Staphylococcus aureus. Mol Med Rep. 2015;12(1):663–667.
- Barry AL. Methods for determining bactericidal activity of antimicrobial agents: Approved guideline. Wayne: National Committee for Clinical Laboratory Standards; 1999.
- Konaté K, Hilou A, Mavoungou JF, et al. Antimicrobial activity of polyphenol-rich fractions from Sida alba L. (malvaceae) against cotrimoxazole-resistant bacteria strains. Ann Clin Microbiol Antimicrob. 2012;11(1):5.
- Lorian V. Antibiotics in laboratory medicine. Philadelphia: Lippincott Williams and Wilkins; 2005.
- Abu-Serie MM, El-Fakharany EM. Efficiency of novel nanocombinations of bovine milk proteins (lactoperoxidase and lactoferrin) for combating different human cancer cell lines. Sci Rep. 2017;7(1):16769.
- Payne JN, Waghwani HK, Connor MG, et al. Novel synthesis of kanamycin conjugated gold nanoparticles with potent antibacterial activity. Front Microbiol. 2016;7:607.
- Bozzola JJ, Russell LD. Electron microscopy: principles and techniques for biologists. 2nd ed. Boston: Jones & Bartlett Learning; 1999.
- Hiramatsu K, Katayama Y, Matsuo M, et al. Multi-drug-resistant Staphylococcus aureus and future chemotherapy. J Infect Chemother. 2014;20(10):593–601.
- Sarah B, Moaz IMH, Mohamed HA, et al. Biosynthesis of copper oxide nanoparticles using streptomyces MHM38 and its biological applications. J Nanomater. 2021;2021:1–16.
- Brause R, Möltgen H, Kleinermanns K. Characterization of laser-ablated and chemically reduced silver colloids in aqueous solution by UV/VIS spectroscopy and STM/SEM microscopy. Appl Phys B. 2002;75(6-7):711–716.
- Krishnaraj C, Jagan E, Rajasekar S, et al. Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against waterborne pathogens. Colloids Surf B Biointerfaces. 2010;76(1):50–56.
- Ponnusamy P, Kolandasamy M, Viswanathan E, et al. Antifungal activity of biosynthesized copper nanoparticles evaluated against red root-rot disease in tea plants. J Exp Nanosci. 2016;11(13):1019–1031.
- Dougherty GM, Rose KA, Tok JBH, et al. The zeta potential of surface‐functionalized metallic nanorod particles in aqueous solution. Electrophoresis. 2008;29(5):1131–1139.
- Cuevas A, Viera I, Torre MH, et al. Infrared spectra of the copper(II) complexes of amino acids with hydrophobic residues. Acta Farm Bonaerense. 1998;17(3):213–218.
- Tomaszewska E, Soliwoda K, Kadziola K, et al. Detection limits of DLS and UV-Vis spectroscopy in characterization of polydisperse nanoparticles colloids. J Nanomater. 2013;2013:1–10.
- Kruk T, Szczepanowicz K, Stefańska JP, et al. Synthesis and antimicrobial activity of monodisperse copper nanoparticles. Colloids Surf B Biointerfaces. 2015;128(1):17–22.
- Chatterjee AK, Chakraborty R, Basu T. Mechanism of antibacterial activity of copper nanoparticles. Nanotechnology. 2014;25(13):135101.
- Zakharova OV, Godymchuk AY, Gusev AA, et al. Considerable variation of antibacterial activity of Cu nanoparticles suspensions depending on the storage time, dispersive medium, and particle sizes. Biomed Res Int. 2015;2015:412530.
- Rajendran A, Siva E, Dhanraj C, et al. A green and facile approach for the synthesis copper oxide nanoparticles using Hibiscus rosa-sinensis flower extracts and it’s antibacterial activities. J Bioprocess Biotech. 2018;8(3):324.
- Chatterjee AK, Sarkar RK, Chattopadhyay AP, et al. A simple robust method for synthesis of metallic copper nanoparticles of high antibacterial potency against E. coli. Nanotechnology. 2012;23(8):085103.
- Yoon KY, Byeon JH, Park JH, et al. Susceptibility constants of Escherichia coli and Bacillus subtilis to silver and copper nanoparticles. Sci Total Environ. 2007;373(2–3):572–575.
- Ruparelia JP, Chatterjee AK, Duttagupta SP, et al. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008;4(3):707–716.
- Gutiérrez MF, Malaquias P, Hass V, et al. The role of copper nanoparticles in an etch-and-rinse adhesive on antimicrobial activity, mechanical properties and the durability of resin-dentine interfaces. J Dent. 2017;61:12–20.
- Shobha G, Moses V, Ananda S. Biological synthesis of copper nanoparticles and its impact. Int J Pharm Sci Invent. 2014;3(8):6–28.
- Ermini ML, Volian V. Antimicrobial nano-agents: the copper age. ACS Nano. 2021;15(4):6008–6029.
- Zhang Y, Wang L, Xu X, et al. Combined systems of different antibiotics with nano-CuO against Escherichia coli and the mechanisms involved. Nanomedicine (Lond). 2018;13(3):339–351.
- Sultana N, Arayne MS, Sabri R. Erythromycin synergism with essential and trace elements. Pak J Pharm Sci. 2005;18(2):35–39. 65
- Woźniak-Budych MJ, Przysiecka L, Langer K, et al. Green synthesis of rifampicin-loaded copper nanoparticles with enhanced antimicrobial activity. J Mater Sci Mater Med. 2017;28(3):42.
- Kiranmai M, Kadimcharla K, Keesara NR, et al. Green synthesis of stable copper nanoparticles and synergistic activity with antibiotics. pharmaceutical-sciences. 2017;79(5):695–700.
- Kaur P, Ajinkya GN, Diksha S, et al. Synergistic effect of copper nanoparticles and antibiotics to enhance antibacterial potential. Biomater Technol. 2019;1/:133–147.
- Anacona JR, Rodriguez A. Synthesis and antibacterial activity of ceftriaxone metal complexes. Transition Met Chem. 2005;30(7):897–901.
- Benjamin DB, Amanda EB. Therapeutic strategies to combat antibiotic resistance. Adv Drug Deliv Rev. 2014;30(78):14–27.
- R KC, C A, Oli A, et al. Potential bactericidal effect of silver nanoparticles synthesized from enterococcus species. Orient J Chem. 2014;30(3):1253–1262.
- Das PE, Abu-Yousef IA, Majdalawieh AF, et al. Green synthesis of encapsulated copper nanoparticles using a hydroalcoholic extract of Moringa oleifera leaves and assessment of their antioxidant and antimicrobial activities. Molecules. 2020;25(3):555.
- Rajivgandhi G, Maruthupandy M, Muneeswaran T, et al. Biologically synthesized copper oxide nanoparticles enhanced intracellular damage in ciprofloxacin-resistant ESBL producing bacteria. Microb Pathog. 2019;127:267–276.
- Prabhu YT, Rao KV, Sai VS, et al. A facile biosynthesis of copper nanoparticles: a micro-structural and antibacterial activity investigation. J Saudi Chem Soc. 2017;21(2):180–185.
- Maruthupandy M, Rajivgandhi G, Muneeswaran T, et al. Biologically synthesized zinc oxide nanoparticles as nanoantibiotics against ESBLs producing gram-negative bacteria. Microb Pathog. 2018;121:224–231.
- Ramachandran G, Rajivgandhi G, Maruthupandy M, et al. Isolation and identification of antibacterial compound from marine endophytic actinomycetes against multi drug-resistant bacteria. Ann Microbiol Immunol. 2018;1(1):1003.
- Bagchi B, Dey S, Bhandary S, et al. Antimicrobial efficacy and biocompatibility study of copper nanoparticle adsorbed mullite aggregates. Mater Sci Eng C Mater Biol Appl. 2012;32(7):1897–1905.
- Mah TF, O'Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;9(1):34–39.
- Kant S, Asthana S, Missiakas D, et al. A novel STK1-targeted small-molecule as an “antibiotic resistance breaker” against multidrug-resistant Staphylococcus aureus. Sci Rep. 2017;7(1):1–19.
- Torimiro N, Moshood AA, Eyiolawi SA. Analysis of beta-lactamase production and antibiotics resistance in Staphylococcus aureus strains. J Infect Dis Immun. 2013;5(3):24–28.
- Fantin B, Carbon C. In vivo antibiotic synergism: contribution of animal models. Antimicrob Agents Chemother. 1992;36(5):907–912.
- Arul Selvaraj RC, Rajendran M, Nagaiah HP. Re-potentiation of β-lactam antibiotic by synergistic combination with biogenic copper oxide nanocubes against biofilm forming multidrug-resistant bacteria. Molecules. 2019;24(17):3055.
- Shanmuganathan R, MubarakAli D, Prabakar D, et al. An enhancement of antimicrobial efficacy of biogenic and ceftriaxone-conjugated silver nanoparticles: green approach. Environ Sci Pollut Res Int. 2018;25(11):10362–10370.
- Hall MJ, Westmacott D, Wong-Kai IP. Comparative in-vitro activity and mode of action of ceftriaxone (Ro 13-9904), a new highly potent cephalosporin. J Antimicrob Chemother. 1981;8(3):193–203.
- De Jong WH, De Rijk E, Bonetto A, et al. Toxicity of copper oxide and basic copper carbonate nanoparticles after short-term oral exposure in rats. Nanotoxicology. 2019;13(1):50–72.
- Bondarenko O, Ivask A, Käkinen A, et al. Sub-toxic effects of CuO nanoparticles on bacteria: kinetics, role of Cu ions and possible mechanisms of action. Environ Pollut. 2012;169:81–89.

![Figure 2. Characterisation of CuO-NPs, (A) Uv-visible, (B) DLS pattern, (C) Zeta potential, (D) X-ray diffraction pattern (XRD), (E) FTIR spectrum and (F) transmission electron microscopic (TEM) [(F1) dispersed in distilled water, (F2) dispersed in glycerol].](/cms/asset/666ce33e-4c54-4ae8-8297-d773836a038c/ianb_a_2126492_f0002_c.jpg)