?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Infections caused by drug-resistant bacteria are major health concerns worldwide. We successfully synthesized cephradine gold nanoparticles (Ceph-Au NPs) and cephradine silver nanoparticles (Ceph-Ag NPs) and compared their efficacy against resistant human pathogens. X-Ray diffraction (XRD), Atomic Force Microscopy (AFM) and Transmission Electron Microscopy (TEM) results showed that average particle size of Ceph-Au NPs and Ceph-Ag NPs were 7 and 12 nm, respectively. Fourier Transform Infra-red spectroscopy (FTIR) spectra revealed the conjugation of –NH2 and –OH functional moieties with the nanoparticle (NP) surfaces. These NPs significantly inhibited the biofilm of Streptococcus mutans (S. mutans) and methicillin-resistant Staphylococcus aureus (MRSA) in the range of 61.25–250 µg/mL. Ceph-Au NPs are more active than Ceph-Ag NPs and can be used to treat the diseases associated with MRSA and S. mutans.
Introduction
Nanotechnology involves nanoparticles (NPs) synthesis of different shapes and sizes with controlled dispersity, for their potential use for human benefits. Metallic NPs have wide applications in different fields, such as medical sciences, synthetic biology, and healthcare. The solubility and biocompatibility of NPs may be increased by controlling their size or surface properties at the nano scale [Citation1]. The numerous advantages of gold nanoparticles (AuNPs) in in vivo drug delivery as compared to conventional drugs are well known; they can carry an increased drug concentration to the infected site [Citation2,Citation3], they have a long circulation time [Citation4], and they can bind excess drug molecules to NP surfaces [Citation5]. The formulation of new methodologies to synthesize metallic NPs of unique sizes and shapes is an area of common interest, and researchers are making efforts to develop environmentally benign materials with minimum side effects. Different biological methods have been utilised for the synthesis of Pt, Ag and Au NPs [Citation6–9]. Silver nanoparticles (AgNPs) have gained much attention recently because of their unique properties, especially their morphology and geometry, and have been used in electronic devices, data packing, for the coating of materials, and in medical sciences for the diagnosis and cure of diseases [Citation10,Citation11]. AgNPs possess high toxicity against bacterial pathogens and affect Gram-positive and Gram-negative bacteria equally [Citation12]. Among the noble metals, AgNPs were proved to be effective in biomedical fields because of their unique physiochemical characteristics [Citation13]. The catalytic and antimicrobial activities and biological effectiveness of AgNPs are associated with their specific surface area. Nanotechnology plays a major role in the treatment of chronic diseases, such as cancer, AIDS, and brain disorders, via targeted drug delivery [Citation14]. Multifunctional nanomedicines are simultaneously capable of specificity, diagnosis, and targeted drug delivery by combining with active molecules and imaging agents [Citation15]. For targeted drug delivery, the NP surface is decorated with an antigen and an antibody that leads the drug to the selective target [Citation16]. Polymeric coated NPs are considered a powerful tool for targeted drug delivery in the brain, because of their penetrative capability of the blood–brain barrier and passive targeting [Citation17]. Subsequently, antibiotic-bound nanoparticles could be a compelling bactericidal material. Enhanced efficacy of AgNPs and AuNPs combined with antibacterial agents has previously been reported by various researchers. For example, conjugation of AgNP to doxycycline is more bactericidal against E. coli as compared to doxycycline alone. [Citation18]. The integration of nanomedicines with other techniques has emerged as a new paradigm for cancer treatment [Citation19]. The NP size, shape, and composition can be used to achieve drug accumulation in tissues, drug exposure, bioavailability, and prolonged circulation in systems [Citation20,Citation21]. Cephradine, a first-generation cephalosporin drug, is used in the treatment of delicate organs, such as the urinary tract, respiratory tract, and soft tissues. Cephradine was found to be active against both Gram-positive and Gram-negative bacteria by inhibiting or producing cross-links during bacterial cell wall synthesis [Citation22,Citation23]. Apart from its biological importance, cephradine possess low solubility in water, which limits its bioavailability and biomedical applications. In the current study, simple chemical methods were used to synthesise AgNPs and AuNPs, to increase their bioavailability in cells and tissues. Recently, the antibacterial activity of cephradine AgNPs have been reported [Citation22–24]. Here, we report the comparative antibacterial efficacy of cephradine conjugated with silver (Ceph-Ag NPs) and cephradine conjugated with gold (Ceph-Au NPs). Cephradine-loaded silver and gold nanoparticles were proved effective to drugs in a localised zone and in a prolonged way, could represent an appropriate tool to increase nanomaterials activity while reducing its cytotoxicity. Thus, in this paper cephradine supported silver and gold nanoparticles were prepared, characterised and evaluated for antimicrobial and antibiofilm activity.
Methodology
Cephradine drug was purchased from Abbott pharmaceutical company (Islamabad, Pakistan). Tetrachloroauric acid AuCl4.5H2O and AgNO3 were purchased from Merck, and sodium hydroxide (NaOH) and hydrochloric acid were from Sigma-Aldrich. High-performance liquid chromatography commercial grade methanol, and double distilled water were used throughout the experiments.
Synthesis of NPs
Two solutions of 1 mM of AuCl4.3H2O and AgNO3 was prepared by dissolving 193 mg and 85 mg in a small amount of water, respectively, which was further diluted to 500 ml of distilled water. 1 mM solution of cephradine was mixed with different concentrations of gold and silver solutions, and within a few minutes, changes in the colour of the solutions were observed with the naked eye. Later, NP synthesis was confirmed using a Beckman-DU 20 ultraviolet–visible (UV–Vis) spectrophotometer.
Optimization of NPs
NPs were optimized using different cephradine-to-metal ratios and kept at room temperature. The change in the solution colour indicated NP formation and was later confirmed by UV–Vis spectrophotometry. Different metal to drug ratio was used for nanoparticles synthesis and kept on stirring. Solution was kept under investigation while stirring. At first no change in solution colour was noticed. Later mild solution of NaBH4 was prepared in distilled water and added to the reaction mixture via drop wise. Soon after the addition of reducing agent, change in solution colour was noticed with naked eyes. For stability purpose, the synthesized nanoparticles were kept at room temperature for 24 h. After 24 h, their UV-Vis spectra were recorded, which further confirmed nanoparticles synthesis. The ratio with the best absorbance was selected for NP synthesis. NPs were synthesised within a few minutes after the mixing of cephradine with the metal salt. For complete reduction of the metal salt to metal ions, the reaction mixture was stirred for 4 h and then UV–Vis data were recorded.
Stability of NPs
The stability of NPs was assessed at different pH levels, temperature, and salt concentration. UV-Vis spectra were recorded after holding the NP at room temperature for 24 h. Increasing or decreasing the absorbance provided information on the stability of NP.
Characterization of NPs
AgNP and AuNP formation was confirmed using the Beckman-DU 20 UV–Vis spectrophotometer operated in the range of 200–700 nm. The NPs synthesized were centrifuged at 12,000 rpm, to remove excess ions and the drug. Under high centrifugation, nanoparticles were settled down and washed twice with double distilled water. Later nanoparticles were removed, dried and subject to towards further morphological analysis. Surface studies of NPs were recorded using transmission electron microscopy (TEM; JEOL, Model JFC-1200EX) operated at the voltage of 80 kV. Cephradine loading on silver and gold nano surfaces was confirmed using a Fourier transform infra-red (FTIR) spectrophotometer (IR-460 Shimadzu, Kyoto, Japan).t
Biological potential of NPs
The Ceph-Ag and Ceph-Au NPs synthesized were subjected to antimicrobial and antibiofilm activities. The activity was performed on Gram-positive Enterococcus faecalis (ATCC: 29212), Streptococcus mutans (ATCC: 25175), and methicillin-resistant Staphylococcus aureus (MRSA) (ATCC: 43300); and Gram-negative Enterobacter aerogenes (ATCC: 13048), Klebsiella pneumonia (ATCC: 13882), and Proteus mirabilis (ATCC: 12453) bacterial strains. All the strains were maintained and passaged using tryptic soy agar and broth (Oxoid, city, UK), except for S. mutans, which was maintained in brain heart infusion (Oxoid) agar and broth supplemented with 1% sucrose. These strains were incubated aerobically at 37 °C for 24 h.
The minimum inhibitory concentrations (MICs) of the AuNPs and AgNPs synthesised were evaluated using the broth dilution method as described previously [Citation25]. Briefly, the compounds were twofold serially diluted in their respective media, followed by the addition of inoculum (5 × 105 cells/mL) and incubated aerobically overnight at 37 °C. The eleventh and twelfth wells served as positive (media with bacterial cells only) and negative (only media) controls. Plates were incubated overnight at 37 °C. The next day, the MIC was recorded as the lowest concentration that inhibited bacterial growth.
The biofilm-inhibiting potential of the NPs synthesised against the above-mentioned strains were evaluated using the crystal violet staining assay as described earlier [Citation26]. The percentage of biofilm inhibition was calculated using the following formula:
The biofilm-eradicating potential of cephradine NPs against S. mutans, MRSA, and K. pneumoniae was analysed by the crystal violet staining method [Citation26]. Briefly, the static biofilm was grown overnight and then the biofilms were challenged with the NPs synthesised at concentrations of 0.1, 1, and 10 µg/mL and further incubated overnight. The next day, the plates were washed to remove planktonic cells, biofilms were stained with crystal violet, and the percentage of biofilm eradication was measured using the above mentioned formula.
Results and discussion
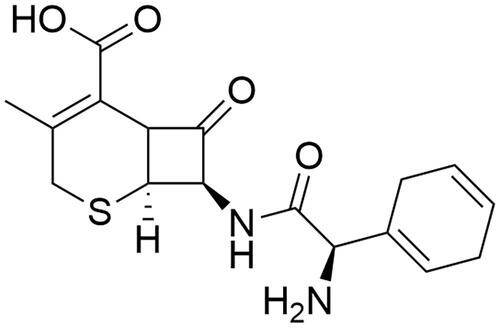
To synthesize the nanoparticles of silver and gold metals, cephradine was used as a capping and stabilising agent by chemical method. A great advantage using a chemical method is that a large-scale nanoparticle can be synthesized in a less time and stability of the size can also be maintained by using capping mediator. The main reason to select this drug is presence of amino moiety and carboxylic acid in its structure which have great potential to stabilise the silver and gold metals during the synthesis ().
Reaction optimization
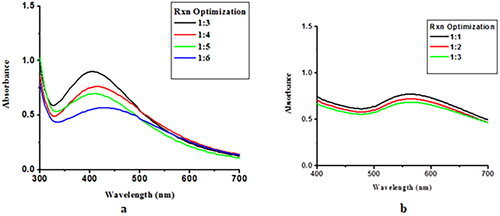
Cephradine-loaded silver and gold nanoparticles were synthesized using chemical method and kept at room temperature for 24 h. After 24 h, UV-Vis spectra was recorded, and the nanoparticles ration which gives best optimization peak will be selected for NPs synthesis at large scale. Cephradine-loaded AgNPs and AuNPs were optimized at 1:3 (ligand:metal) and 1:1 (ligand:metal) salt concentrations, respectively; the maximum absorption was measured at 430 and 550 nm, respectively. Hence, 1:3 (ligand:metal) and 1:1 (ligand:metal) of Cephradine-loaded AgNPs and AuNPs was used, respectively, for bulk NPs synthesis at large scale. Changing the drug concentration led to a decline in absorbance intensity, which shows the decomposition of AuNPs. Optimum conditions are necessary for the synthesis of NPs. Excess drug can cause instability, which leads to the agglomeration of AuNPs [Citation27–29]. The reaction optimisation of Ceph-Ag NPs and Ceph-Au NPs is shown in . The reduction of Au+3 to Au0 takes place within a few minutes. NPs were stirred constantly and UV–Vis spectra were recorded. An increase in absorbance intensity over time shows that the complete reduction of Au+3 to Au0 and Ag+1 to Ag0 took place in 4 h () [Citation30,Citation31].
Reaction optimization against pH
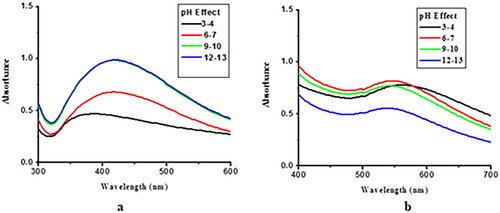
NP stability was checked at different pH values. Ceph-Au and Ceph-Ag NPs were found to be slightly acidic in nature, with a pH of 5–6 and 6–7, respectively. The effects of pH on the stability of Ceph-Au and Ceph-Ag NPs are shown in . NPs were found to be stable at all pH values.
Reaction optimization against temperature
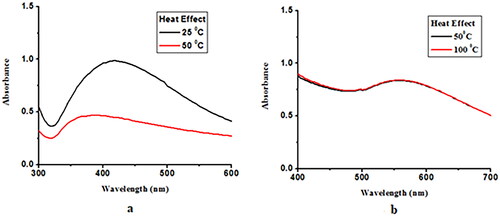
NPs were heated up to 100 °C and UV–Vis spectra were recorded at each range of temperature. Ceph-Ag NPs were unstable at high temperature, whereas for Ceph-Au NPs, the rate of reaction, that is, the conversion of metal to metal ions, remains constant with the increase in temperature and the reaction time was reduced [Citation32]. The increase in absorption at high temperature was associated with the uniform size distribution of gold particles [Citation33]. Reaction kinetics at high temperature increases and more Au+3 change into nuclei; hence, the optical density increases, which shows a linear correlation with the AuNP concentration [Citation34]. The effects of temperature on the rate of reaction is shown in .
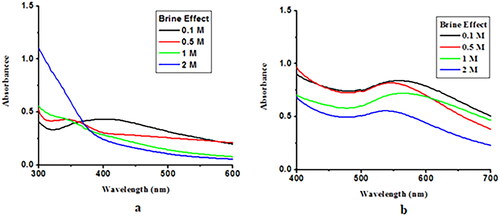
Effect of brine
The effects of high concentrations of saline (1 M) on capped AgNP and AuNP were also investigated. 5 ml of freshly prepared Ceph-Ag and Ceph-Au NPs were placed in 4 separate vials. Next, 0.1, 0.5, 1, and 2 M NaCl solutions were added to these vials. The resulting solutions are thoroughly stirred and stored at room temperature for 24 h. UV–Vis spectra were recorded for Ceph-Ag and Ceph-Au NPs. The results showed that high concentration of brine reduced λ max. This rapid decrease in absorbance of Ag/Au NPs containing NaCl may be attributed to the aggregation effect promoted by Cl−1 ions. From these observations, it was concluded that at a higher sodium chloride concentration, aggregation was dominant ().
FTIR analyses
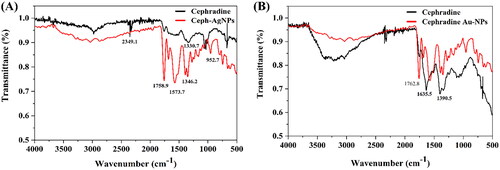
To establish the mode of interaction, FTIR analysis of cephradine (alone) was compared with those of Ceph-Ag and Ceph-Au NPs. Cephradine alone showed its characteristic functional groups and infra-red (IR) bands at 3600–2400 cm−1 (O–H/N–H stretching vibrations of alcohol and amine), 1743 cm−1 (O = C–NH2 stretching vibrations), 1625 cm−1 (–C = C–), and 1361 cm−1 (primary amide N–H-bending). The disappearance of hydrogen bonding among cephradine molecule showed the interaction between –OH and –NH2 functional moieties with AgNP and AuNP surfaces. In the case of Ceph-Au NPs, the disappearance of the peak at 1743 cm−1 showed the interaction between the amide functional group and the cephradine surface ( and ). The mechanism of interactions of cephradine with AgNPs and AuNPs are presented in [Citation35].
Figure 7. Mechanism of the interaction between AgNPs, AuNPs and cephradine [Citation35].
![Figure 7. Mechanism of the interaction between AgNPs, AuNPs and cephradine [Citation35].](/cms/asset/9f347413-d6a9-4004-b397-73c15cf76ad9/ianb_a_2144340_f0007_c.jpg)
Table 1. FTIR spectral assignment of free cephradine, Ceph-Ag NPs and Ceph-Au NPs.
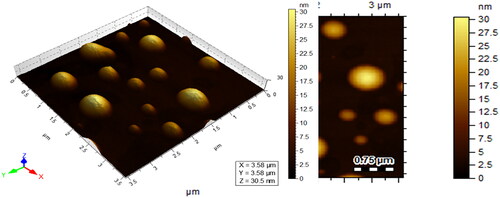
TEM analyses
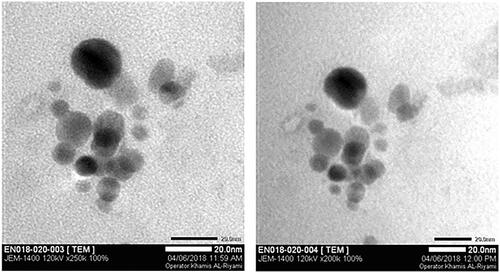
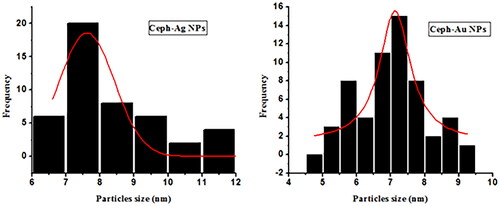
TEM images of the Ceph-Ag and Ceph-Au NPs synthesised can be seen in and . TEM images show heterogeneity among the particle sizes. The particle size was calculated using TEM images via Image J software. The NPs synthesised were round to oval in shape, with a size of 12.89 ± 2 nm (Ceph-Ag NPs) and 7 ± 1.82 nm (Ceph-Au NPs) (). The TEM results were in close agreement with X-ray diffraction (XRD).
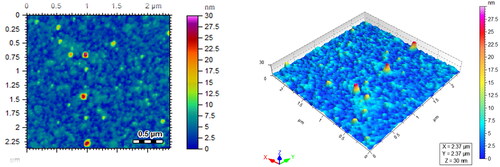
Atomic force microscopy characterisation
Surface studies of Ceph-Ag and Ceph-Au NPs were also made using atomic force microscopy (AFM). AFM results were in close agreement with those of TEM and X-ray diffraction (XRD) data. Ceph-Ag NPs ranged in size from 16 to 20 nm, whereas Ceph-Au NPs were of size 13–15 nm, as shown in and .
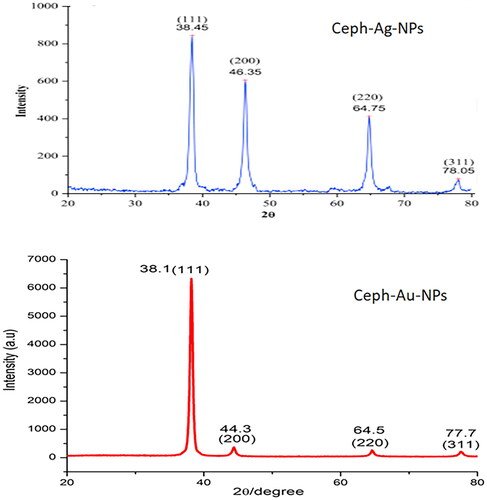
XRD characterization
The crystalline size of the Ceph-Ag and Ceph-Au NPs were carried out by XRD. The XRD analysis of Ceph based silver nanoparticles showed diffraction peaks at = 38.3°, 64.70°, and 77.78° respectively. When compared with the standard, the resulted XRD spectrum confirmed that the Ceph-Ag NPs were in nanocrystal form and crystalline in nature. The peaks are assigned to the planes (111), (220), and (311) facet of silver crystal, respectively (JSPDS file # 04-0783). The comparison of data with literature also confirmed that Ceph-Ag nanoparticles were face-centred, cubic, and crystalline in nature (correlated to JCPDS card: number 04-0783) [Citation36].
Similarly crystal structure of gold nanoparticles was also checked by XRD analysis. The diffraction peaks at 2 theta values of 38, 44.1, and 77.2, which may be attributed to the (111), (200), and (311) crystallographic planes of the face centres cubic lattice respectively (JSPDS file # 04-0783). The intense peak at 38.1 represents preferential growth in the (111) direction. The calculated sizes of Ceph-Ag and Ceph-Au NPs using the Debye Scherrer equation was 12 and 7 nm, respectively ().
Antimicrobial efficacy
The synthesized nanoparticles were tested against bacterial pathogens with dilution concentrations, 125 µg/mL, 250 µg/mL and 500 µg/mL. The NPs were found to be inactive against E. faecalis and P. mirabilis even at 500 µg/mL high concentration. However, Ceph-Ag NPs and Ceph-Au NPs were found to be active against E. aerogenes, K. pneumoniae, and MRSA at 500 µg/mL concentration. The Ceph-Au NPs were active against the oral pathogen S. mutans even at lower MIC concentration of 250 µg/mL as compared to Ceph-Ag NPs, which showed activity at 250 µg/mL. The qualitative data of synthesised nanoparticles revealed that MIC concentration of 125, 250 µg/mL are best concentrations for the inhibition of oral pathogen S. mutans as shown in .
Table 2. Antimicrobial and Biofilm Inhibition Activities of Ceph-Ag and Ceph-Au NPs.
The antibiofilm potential of the NPs varied with the strains used (); however, they exhibited strong antibiofilm activities against MRSA and S. mutans. Ceph-Ag NPs inhibited >50% biofilm of MRSA and S. mutans at 125 µg/mL concentration; Ceph-Au NPs inhibited >50% biofilm at 61.5 and 250 µg/mL for S. mutans and MRSA, respectively (). Both the compounds eradicated the preformed biofilm to some extent for MRSA and S. mutans at 500 µg/mL, but they were inactive against the preformed biofilm of K. pneumoniae (). These results indicated that Ceph-Au NPs were more active than Ceph-Ag NPs; therefore, cephradine-conjugated AuNPs can be used to treat the diseases associated with MRSA and S. mutans These findings suggest that synthetic nanoparticles and their conjugates can represent an effective and efficient strategy to combat epidemics and bacteria. The drug conjugated nanomaterial’s exposures were more toxic than cerpharidine alone for cell lines and bacterial biofilm. They fight bacterial biofilm and have a low effect on normal cells and these findings are consistent with previous research. [Citation37,Citation38].
Table 3. Preformed biofilm eradication capability of Ceph-Ag and Ceph-Au NPs.
The conjugated metallic nanoparticle with antibiotics tends to extend the antibacterial competences by reducing side effects and high doses requirement. It also reduces the likelihood that bacteria will develop resistance to this antibiotic-bound metal nanoparticle system [Citation39]. The functionalised composite has a lower MIC and a higher antibacterial effect compared to antibiotics alone [Citation40]. The mechanism for antibacterial activity of NPs is dependent on their sizes. Small NPs act by forming large irreversible pores as they cross the bacterial cell membrane [Citation41]. Larger NPs (size range = 80-100 nm) do not allow free movement of bacterial cell membranes, but many studies have also reported their ability to eradicate bacteria [Citation42,Citation43]. The mechanism for antibacterial efficacy involved direct contact of NPS with the bacterial cell membrane. The mechanism of action behind this nanoconjugate (Ceph-Ag NPs/Ceph-Au NPs) is due to the acting Ceph-Ag NPs and Ceph-Au NPs on the cell membranes of bacterial pathogens and causes them to rupture. In this mechanism, the adsorption of NPs in bacterial cells membrane caused tension and mechanical deformation leading cell rupture and death. The specificity of such nanoconjugates will pave the way for the development of new antibacterial therapies in which the healing effects of antibiotics do not compromise the health of the individual’s digestive system.
Conclusion
In this research work, a chemical reduction method was used for the synthesis of AgNPs and AuNPs conjugated with cephradine. Reaction was optimised using different metal to drug ratio and best optimum ratio was selected for nanoparticles synthesis. Stability of synthesized drugs conjugated nanomaterials were checked against extreme brine, pH and temperature and were found stable. Nanomaterials synthesis mechanism was designed using FTIR analysis and revealed that the NH2 and –OH functional moieties present in drugs is responsible for the capping and stabilization of prepared NPs. As per TEM photographs, the synthesized nanoconjugates were round or spherical in shape and exhibit heterogeneity in their size. Morphological studies of NPs were performed through XRD, AFM, and TEM analyses. Ceph-Ag and Ceph-Au NPs possess average particle sizes of 12 ± 2 and 7 ± 1.85 nm, respectively. The stability of the nanoconjugates synthesised was checked against various physiological parameters, that is, pH, temperature, and brine solution concentration. They also showed great antibiofilm potential against the tested strains. Biofilm eradication also estimated that AuNPs are more effective than AgNPs against the tested strains. Furthermore, Ag NPs and Au NPs could be an excellent drug delivery system for cephradine against biofilms of MDR Gram-negative bacteria. Overall, Ceph-Ag and Ceph-Au NPs are potential candidates towards biomedical application.
Future prospects
Cephradine is a β-lactam antibiotic, active against both Gram-positive and Gram-negative bacteria by inhibiting or producing cross-link during bacterial cell wall synthesis. Instead of biological importance, cephradine possess low solubility in water, which limits its bioavailability and biomedical applications. In the current study, simple chemical methods for AgNP and AuNP synthesis were used, to increase their bioavailability in cells and tissues. The newly synthesised Ceph-Ag and Ceph-Au NPs exhibited enhanced antibiofilm activities against E. aerogenes, K. pneumoniae, and the oral pathogen S. mutans, when compared to the original antibiotic. In comparative efficacy, Ceph-Au NPs are more active than Ceph-Ag NPs and can be used to treat the diseases associated with MRSA and S. mutans, in the future.
Summary points
In the current strategy, Ceph-Ag and Ceph-Au NPs were synthesised using chemical methods.
In this method, the β-lactam antibiotic cephradine was used as a capping agent.
The reaction was optimised using different metal-to-drug ratios and their stability was studied at different ranges of pH, temperature, and brine concentration.
Reaction kinetics decrease with an increase in temperature, and nanoparticles were found to be unstable in extremely acidic and basic environments.
FTIR spectral studies confirmed the interaction of –OH and –NH2 functionality with AgNP and AuNP surfaces.
The surface topology and particle size were investigated using XRD, AFM, and TEM. Ceph-Au and Ceph-Ag NPs synthesised were round to oval in shape, with average particle sizes of 7 and 12 nm, respectively.
The further potential of NPs synthesised was checked against drug-resistant bacteria E. faecalis, P. mirabilis, E. aerogenes, K. pneumoniae, MRSA, and the oral pathogen S. mutans.
Ceph-Au NPs are more active than Ceph-Ag NPs against MRSA and S. mutans.
Author contributions
Ajmal Khan, Saleha Suleman Khan and Ahmed Al-Harrasi conceived and designed the study. Ajmal Khan, Humera Jabeen, Touqeer Ahmad, Najeeb Ur Rehman, Huma Shareef, Rizwana Sarwar, Jalal Uddin and Javid Hussain performed most of the experiments, analysed and interpreted the data, contributed to the drafting of the manuscript. Saira Yahya and Nusrat Hussain performed antibacterial activities. Ajmal Khan, Humera Jabeen, Touqeer Ahmad Saleha Suleman Khan and Ahmed Al-Harrasi wrote the manuscript with inputs and comments from all co-authors. All authors approved the final version of the manuscript.
| Abbreviations | ||
| Ceph-Au NPs | = | Cephradine gold nanoparticles |
| Ceph-Ag NPs | = | Cephradine silver nanoparticles |
| XRD | = | X-Ray diffraction |
| AFM | = | Atomic Force Microscopy |
| TEM | = | Transmission Electron Microscopy |
| FTIR | = | Fourier Transform Infra-red spectroscopy |
| NP | = | nanoparticle |
| MRSA | = | methicillin-resistant Staphylococcus aureus |
| AgNPs | = | Silver nanoparticles |
| AuNPs | = | Gold nanoparticles |
| MIC | = | minimum inhibitory concentration |
| MBIC | = | minimum biofilm inhibitory concentration |
| AVG | = | average |
| SD | = | standard deviation |
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through Large Groups under grant number (RGP.2/64/43). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Leroux J-C, Allémann E, De Jaeghere F, et al. Biodegradable nanoparticles—from sustained release formulations to improved site specific drug delivery. J Controlled Release. 1996;39(2-3):339–350.
- Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003;55(3):329–347.
- Wagner V, Dullaart A, Bock A-K, et al. The emerging nanomedicine landscape. Nat Biotechnol. 2006;24(10):1211–1217.
- Sahoo S, Parveen S, Panda J. The present and future of nanotechnology in human health care. Nanomed Nanotechnol Biol Med. 2007;3(1):20–31.
- Han MY, Özyilmaz B, Zhang Y, et al. Energy band-gap engineering of graphene nanoribbons. Phys Rev Lett. 2007;98(20):206805.
- Zhou Y, Yu S, Cui X, et al. Formation of silver nanowires by a novel solid − liquid phase arc discharge method. Chem Mater. 1999;11(3):545–546.
- Husseiny M, El-Aziz MA, Badr Y, et al. Biosynthesis of gold nanoparticles using Pseudomonas aeruginosa. Spectrochim Acta A Mol Biomol Spectrosc. 2007;67(3-4):1003–1006.
- Sastry M, Ahmad A, Khan MI, et al. Biosynthesis of metal nanoparticles using fungi and actinomycete. Current Science. 2003;85(2):162–170.
- Sharma NC, Sahi SV, Nath S, et al. Synthesis of plant-mediated gold nanoparticles and catalytic role of biomatrix-embedded nanomaterials. Environ Sci Technol. 2007;41(14):5137–5142.
- Kemp MM, Kumar A, Mousa S, et al. Synthesis of gold and silver nanoparticles stabilized with glycosaminoglycans having distinctive biological activities. Biomacromolecules. 2009;10(3):589–595.
- Moldovan B, David L, Achim M, et al. A green approach to phytomediated synthesis of silver nanoparticles using Sambucus nigra L. fruits extract and their antioxidant activity. J Mol Liq. 2016;221:271–278.
- Lara HH, Ayala-Núñez NV, Turrent L, et al. Bactericidal effect of silver nanoparticles against multidrug-resistant bacteria. World J Microbiol Biotechnol. 2010;26(4):615–621.
- Stoimenov PK, Klinger RL, Marchin GL, et al. Metal oxide nanoparticles as bactericidal agents. Langmuir. 2002;18(17):6679–6686.
- Muthu MS, Singh S. Targeted nanomedicines: effective treatment modalities for cancer, AIDS and brain disorders. Nanomedicine (Lond). 2009;4(1):105–118.
- Koren E, Torchilin VP. Cell-penetrating peptides: breaking through to the other side. Trends Mol Med. 2012;18(7):385–393.
- Torchilin VP. Multifunctional nanocarriers. Adv Drug Deliv Rev. 2006;58(14):1532–1555.
- Chen Y, Dalwadi G, Benson H. Drug delivery across the blood-brain barrier. Curr Drug Deliv. 2004;1(4):361–376.
- Silva HF, Lima KM, Cardoso MB, et al. Doxycycline conjugated with polyvinylpyrrolidone-encapsulated silver nanoparticles: a polymer’s malevolent touch against Escherichia coli. RSC Adv. 2015;5(82):66886–66893.
- Kwon S, Ko H, You DG, et al. Nanomedicines for reactive oxygen species mediated approach: an emerging paradigm for cancer treatment. Acc Chem Res. 2019;52(7):1771–1782.
- Jain KK. Nanopharmaceuticals. In: The handbook of nanomedicine. Humana New York, NY: Springer; 2017. p. 201–271.
- Wilhelm S, Tavares AJ, Dai Q, et al. Analysis of nanoparticle delivery to tumours. Nat Rev Mater. 2016;1(5):16014.
- Fernandes R, Amador P, Prudêncio C. β-Lactams: chemical structure, mode of action and mechanisms of resistance. Reviews in Medical Microbiology. 2013;24(1):7–17.
- Masri A, Anwar A, Ahmed D, et al. Silver nanoparticle Conjugation-Enhanced antibacterial efficacy of clinically approved drugs cephradine and vildagliptin. Antibiotics. 2018;7(4):100.
- Ahmed D, Shah MR, Perveen S, et al. Cephradine coated silver nanoparticle their drug release mechanism, and antimicrobial potential against Gram-Positive and Gram-Negative bacterial strains through AFM. J Chem Soc Pak. 2018;40(2): 388–398.
- Khan AK, Ahmed A, Hussain M, et al. Antibiofilm potential of 16-oxo-cleroda-3, 13 (14) E-diene-15 oic acid and its five new γ-amino γ-lactone derivatives against methicillin resistant Staphylococcus aureus and Streptococcus mutans. Eur J Med Chem. 2017;138:480–490.
- Ahmed A, Khan AK, Anwar A, et al. Biofilm inhibitory effect of chlorhexidine conjugated gold nanoparticles against Klebsiella pneumoniae. Microb Pathog. 2016;98:50–56.
- Khalil MM, Ismail EH, El-Baghdady KZ, et al. Green synthesis of silver nanoparticles using olive leaf extract and its antibacterial activity. Arabian J Chem. 2014;7(6):1131–1139.
- Bromme R, Jucks R, Wagner T. How to Refer to ‘Diabetes’? Language in Online Health Advice. www.interscience.wiley.com
- Ghule K, Ghule AV, Liu J-Y, et al. Microscale size triangular gold prisms synthesized using bengal gram beans (cicer arietinum L.) extract and HAuCl4· 3H2O: a green biogenic approach. J Nanosci Nanotechnol. 2006;6(12):3746–3751.
- Lam SY, Shankar V, Erramilli MK, et al. Customer value, satisfaction, loyalty, and switching costs: an illustration from a business-to-business service context. j Acad Market Sci. 2004;32(3):293–311.
- Kim SC, Park KT, Hwang JW, et al. Comparative analysis of clinical outcomes for laparoscopic distal pancreatic resection and open distal pancreatic resection at a single institution. Surg Endosc. 2008;22(10):2261–2268.
- Jiang J, Oberdörster G, Biswas P. Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J Nanopart Res. 2009;11(1):77–89.
- Dong X, Ji X, Wu H, et al. Shape control of silver nanoparticles by stepwise citrate reduction. J Phys Chem C. 2009;113(16):6573–6576.
- Ojea-Jiménez I, Puntes V. Instability of cationic gold nanoparticle bioconjugates: the role of citrate ions. J Am Chem Soc. 2009;131(37):13320–13327.
- Ahmad T, Mahbood F, Sarwar R, et al. Synthesis of gemifloxacin conjugated silver nanoparticles, their amplified bacterial efficacy against human pathogen and their morphological study via TEM analysis. Artif Cells Nanomed Biotechnol. 2021;49(1):661–671.
- Roy K, Sarkar C, Ghosh C. Plant-mediated synthesis of silver nanoparticles using parsley (petroselinum crispum) leaf extract: spectral analysis of the particles and antibacterial study. Appl Nanosci. 2015;5(8):945–951.
- Abdel-Fattah WI, Ali GW. On the anti-cancer activities of silver nanoparticles. J Appl Biotechnol Bioeng. 2018;5(2):1–4.
- Khorrami S, Zarrabi A, Khaleghi M, et al. Selective cytotoxicity of green synthesized silver nanoparticles against the MCF-7 tumor cell line and their enhanced antioxidant and antimicrobial properties. Int J Nanomedicine. 2018;13:8013–8024.
- Zheng K, Setyawati MI, Lim T-P, et al. Antimicrobial cluster bombs: silver nanoclusters packed with daptomycin. ACS Nano. 2016;10(8):7934–7942.
- Mocan L, Tabaran FA, Mocan T, et al. Laser thermal ablation of multidrug-resistant bacteria using functionalized gold nanoparticles. Int J Nanomedicine. 2017;12:2255–2263.
- Ortiz-Benítez EA, Velázquez-Guadarrama N, Durán Figueroa NV, et al. Antibacterial mechanism of gold nanoparticles on Streptococcus pneumoniae. Metallomics. 2019;11(7):1265–1276.
- Zhou Y, Kong Y, Kundu S, et al. Antibacterial activities of gold and silver nanoparticles against Escherichia coli and bacillus Calmette-Guérin. J Nanobiotechnology. 2012;10(1):19–19.
- MubarakAli D, Thajuddin N, Jeganathan K, et al. Plant extract mediated synthesis of silver and gold nanoparticles and its antibacterial activity against clinically isolated pathogens. Colloids Surf B Biointerfaces. 2011;85(2):360–365.