ABSTRACT
A lepidocrocite-like potassium titanate, (Kx(LixTi1-x)O2; Lss) with a layered structure can provide a reactive interlayer space for soft-chemical reaction such as ion-exchange or intercalation and the hydrated sodium derivative (Lss-Na) was obtained by an ion-exchange reaction. The crystal structures of the hydrated and dehydrated Lss-Na were refined by using synchrotron powder X-ray diffraction data. Two steps of dehydrated processes for the Lss-Na were observed by in-situ synchrotron powder X-ray diffraction. The ion-exchange property for Lss-Na was superior to those for Lss and Lss-H and was corelated with the effective basal space which was expanded by formation of two layers of water molecules.
1. Introduction
The layered inorganic compounds have a characteristic crystal structure with reactive interlayer spaces; for example, KCa2Nb3O10, K2Ti4O9, KTiNbO5, α-Ti(HPO4)2·H2O, etc [Citation1]. Among them some alkaline metal titanates can produce protonated derivatives by ion-exchange reaction with acid solution, which form inorganic-organic complexes by intercalation [Citation2] or nano-sheets by exfoliation [Citation3]. A lepidocrocite-like titanate is one of alkaline metal titanates with a layered structure and has been paid attention to ion-exchange reaction, intercalation, exfoliation and fabrication of nano-architecture using nano-sheets [Citation4]. General formula of a lepidocrocite-like titanate may be represented as AxTi2-yMyO4, (A: K, Rb, Cs; M: vacancy, Li, Mg, Co, Ni) and the relation of the values of x and y depend on the valence of M; x = y for M = vacancy, x = 2y for M = monovalent cation, x = 2y for M = divalent cation, x = 3y for M = trivalent cation [Citation5]. The crystal structure of a lepidocrocite-like titanates has the corrugated layers formed by edge- and corner-sharing (Ti2-yMy)O6 octahedra [Citation5]. The large alkaline metal ions located in the interlayers are exchangeable with various metal ions and organic compounds [Citation6], and the protonated derivative having a relatively weak solid acid is often used as a precursor for ion-exchange reaction [Citation7–11]. The Na-derivative with a lepidocrocite-like titanate had been reported by Shirpour et al., however, the detail of the crystal structure and ion-exchange property for the Na-derivative have not been clarified yet [Citation12]. The dehydrated Na-derivative exhibited high performance for a sodium ion secondary battery [Citation12–15], and the Na-derivative was expected to have good property of ion-exchange because of high degree of hydration. To our best knowledge, there has been no report for the crystal structure of the hydrated Na-derivative which was reported to have the P-lattice and recover to the C-lattice by dehydration [Citation12,Citation14]. In this paper, we will report the crystal structure and ion-exchange reaction of the Na-derivative (Lss-Na) which was obtained from a lepidocrocite-like potassium titanate, (Kx(Ti1-x,Lix)O2; Lss).
1.1. Experimental
The Na derivative (Lss-Na) was prepared by ion-exchange reaction using a lepidocrocite-like layered potassium titanate (K0.75Li0.25Ti1.75O4: hereafter; Lss) served by Otsuka Chemical Co., Ltd. Lss (10 g) was stirred in 500 mL of NaCl (Kanto Chemical Co., Inc, 99.5%) solution with the molar ratio of Na/K = 10/1 at room temperature for 6 days. The protonated derivative (Lss-H) was prepared by stirring Lss (20 g) in HCl solution (12 M HCl 20 mL + H2O 380 mL) at room temperature for 2 days. The product was filtrated, washed with a distilled water and dried at room temperature for 2 days. Hereafter the Na and H derivatives as termed as Lss-Na (Na0.75(Li0.25Ti1.75) O4·2.0H2O) and Lss-H (Ha1.00(Ti1.75□0.25)O4 · 0.7H2O), respectively. The Li+ ions in the intralayer of Lss were leached to form vacancies during H+ ion-exchange reaction. The products were identified from their X-ray powder diffraction (XRD) patterns. Thermal behavior was investigated by Thermogravimetric analysis (TGA, Rigaku TG8120 Thermo plus EVO, Rigaku Co.) at a heating rate of 10°C/min. The crystal structures of Lss and Lss-Na were refined by synchrotron X-ray diffraction (SXRD) data by using multiple MYTHEN detectors in Debye-Sherrer camera at BL02B2 beam line in SPring-8 [Citation16]. High temperature in situ X-ray diffraction data was also obtained in the same beamline. RIETAN-FP [Citation17] was used for crystal structure refinement and the structures were drawn using VESTA [Citation18]. The specimen was crushed in an agate mortar. The crushed specimen was dispersed in ethanol and we dropped it onto a microgrid. TEM observation was carried out using FEI’s Tecnai Osiris with an acceleration voltage of 200 kV.
The basal spacing for ion-exchanged compounds at constant humidity was investigated by putting a sample in a desiccator in which a constant relative humidity of 0, 10, 43, 58, or 87% was established by P2O5, ZnCl2·0.5H2O, K2CO3·2H2O, NaBr·2H2O, or Na2CO3·5H2O, respectively [Citation19]
The ion-exchange properties of Lss, Lss-H and Lss-Na were carried out as follows. Lss, Lss-H or Lss-Na (2 g) was stirred in alkaline metal, alkaline-earth metal and transition-metal chloride solutions (300 mL) with the molar ratio of counter metal ion /Na = 4 at room temperature for 4 days. The products were filtrated, washed with a distilled water and dried at room temperature for 2 days. The ion-exchanged amount of metal ions was determined by the following leaching process. The dried sample (0.4 g) was put in an autoclave with 1 M HNO3 solution (30 mL) and it was heated at 180°C for 24 h. The solid product was identified as a rutile-type TiO2 and the amount of metal ions in the solution was analyzed by an inductively coupled plasma atomic emission spectroscopy (ICP-AES, SPS3520UV-DD, Hitachi High-Thech Science Co.)
2. Results and discussion
2.1. Crystal structures of Lss and Lss-Na
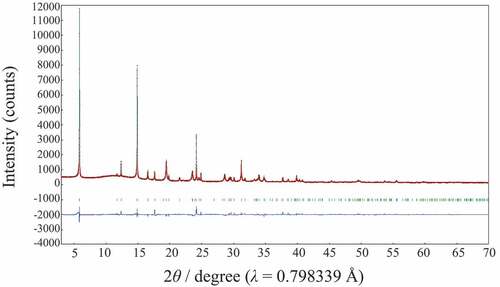
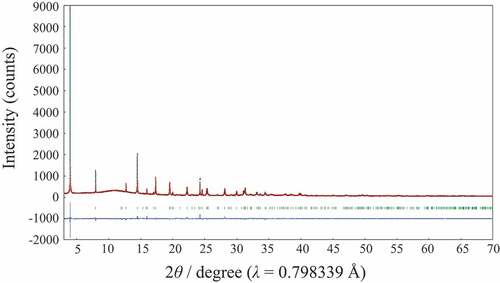
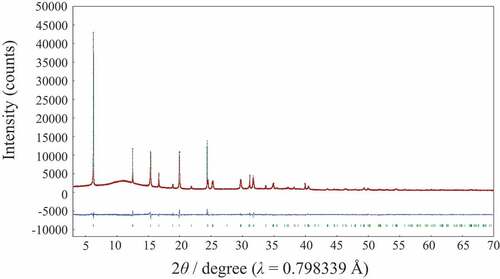
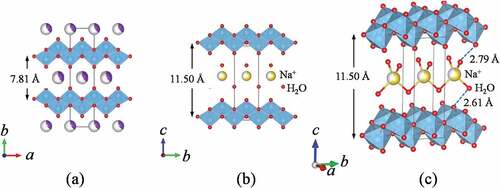
The mother compound, Lss has a lepidocrocite-like structure and the crystal structure refinement on the basis with the previous data [Citation20,Citation21] was successful (Rwp = 5.79% and Rp = 3.77%) by using SXRD data. shows Rietveld refinement profile for Lss. The lattice parameters with the orthorhombic cell (Cmcm (#63)) were a = 3.8141(3), b = 15.617(6) and c = 2.9664(6) Å, and these values were good agreement well with the previous data (a = 3.821, b = 15.591, c = 2.973 Å [Citation20] and a = 3.8237(2), b = 15.532(1), c = 2.9727(3) Å [Citation21]). Although the crystal structure of Lss-Na was proposed by Shirpour M et al. [Citation12], the detailed crystal structure has not been reported yet and then the crystal structure determination was attempted by using the X-ray powder diffraction data. The X-ray powder diffraction pattern of Lss-Na could be indexed with the orthorhombic cell of a = 2.98, b = 3.80, c = 11.50 Å. Since no systematic absence was observed, there are three possible space group; P222 (#16), Pmm2 (#25), and Pmmm (#47). This assignment is supported by the TEM observation as shown in . The selected area electron diffraction pattern taken by the TEM suggests the P lattice with a ~ 2.9 and b ~ 3.8 Å. The crystal structure refinement of Lss-Na using SXRD data was carried out on the assumption that the layered structure was kept during the ion-exchange reaction. The reasonable R-values (Rwp = 5.19%, Rp = 3.86%) were led for the structure model with the space group of Pmm2. Among them the space group, Pmmm proposed by Shirpour M et al. and the other, P222 led no satisfactory crystal structure. shows Rietveld refinement profile for Lss-Na. The details of structure refinement and the structure parameters are summarized in , respectively. The selected interatomic distances for Lss and Lss-Na are listed in . The crystal structures of Lss-Na and the mother compound, Lss are shown in . The interlayer of the hydrated Lss-Na is expanded by incorporation of H2O molecules, simultaneously gliding of the opposed layers to transform from the C to P lattices. The interlayer distance increased from 7.81 to 11.50 Å by hydration. Although in Lss the convex in the opposite (Ti,Li)O2 layers is facing, in Lss-Na the convex and concave are facing by gliding of the opposed layers. This transformation during the ion-exchange make it possible to accommodate large amount of H2O molecules in the interlayer occupied by K+ ion. The Na+ ions are surrounded tetrahedrally by four H2O molecules and the Na+ layer is sandwiched by two H2O layers in the interlayer space. The distances of Na+ ion and O atom of H2O are 2.69 and 2.12 Å and the distances of oxygen atoms between H2O and (Ti,Li)O2 layer are 2.61 and 2.79 Å and these are reasonable values for the oxygen distances via a hydrogen bonding. In the layered-type HTiNbO5 the oxygen distances via a hydrogen bonding in the interlayer were found to be from 2.50 to 2.58 Å [Citation22]. The mean (Ti,Li)-O distances of two types of (Ti,Li)O octahedra in Lss-Na are 2.023 and 1.97 Å and do not change from that (1.989 Å) in Lss.
Table 1. Crystal data for Lss, Lss-Na and Lss-Na at 200°C.
Table 2. Structural parameters for Lss, Lss-Na and Lss-Na at 200°C.
Table 3. Selected interatomic distances (Å) for Lss, Lss-Na and Lss-Na at 200°C.
Figure 2. A selected area electron diffraction pattern taken by the TEM for Lss-Na. The incidental beam direction is considered to be [0 0 Citation1]. The indexed spots are considered to be 100 and 010, respectively.
![Figure 2. A selected area electron diffraction pattern taken by the TEM for Lss-Na. The incidental beam direction is considered to be [0 0 Citation1]. The indexed spots are considered to be 100 and 010, respectively.](/cms/asset/cda106a0-cc3b-4caa-9728-1b53be8f6008/tace_a_2173849_f0002_b.gif)
Figure 4. Crystal structures for Lss (a) and Lss-Na (b). The coordination environment (c) of Na+ ion in the interlayer for Lss-Na. The solid lines denote the unit cell.
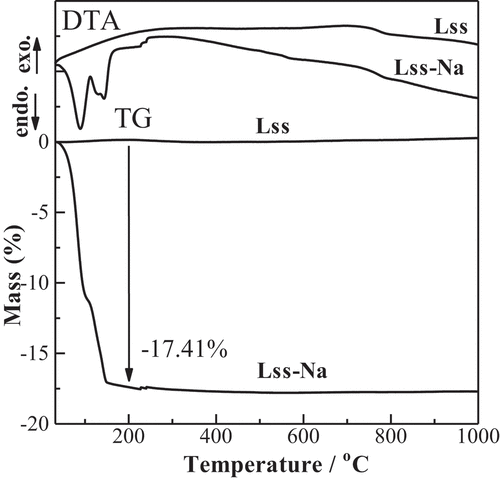
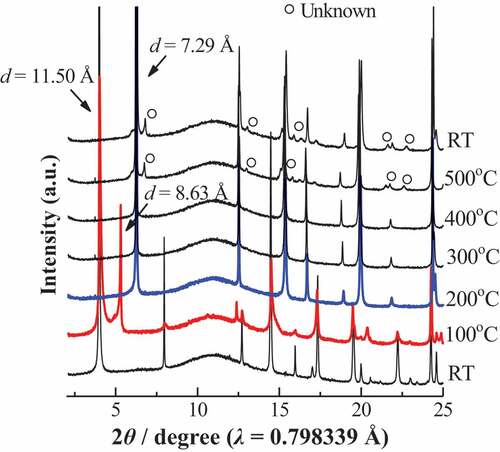
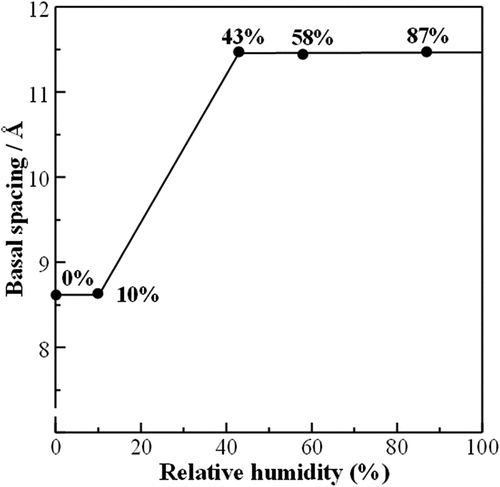
The TG-DTA curves of Lss and Lss-Na are shown in . Although no mass loss is observed in Lss, two steps of mass losses are observed up to 200°C in Lss-Na. The amount of water molecules in the interlayer was calculated to n = 2.0. This value is good agreement with that form the crystal structure analysis. The corresponding dehydrated behavior is observed in the in situ high temperature SXRD patterns as shown in . The interlayer water was released up to 200°C and the intermediate phase was observed at 100°C. The crystal structure of the dehydrated Lss-Na was refined successfully with the assumption of the same space group Cmcm (#63) as the mother compound, Lss. The final factors were Rwp = 5.06% and Rp = 3.76% and the lattice parameters were a = 3.7830(3), b = 14.578(5) and c = 2.9723(3)Å. From this result the gliding of the opposite layers caused by hydration recovered to form the C lattice from the P one by dehydration of the interlayer. The basal spacing of for Lss-Na at 200°C is shrunk to 7.29 Å which is shorter than that of Lss and this corresponds to ionic size of ions in the interlayer. This result was good agreement with the previous data by Katogi et al. [Citation14] shows Rietveld refinement profile for Lss-Na at 200°C. The details of structure refinement and the structure parameters are summarized in , respectively. The selected interatomic distances for Lss-Na at 200°C are listed in . At 500°C the product with shorted interlayer space of d ~ 7.2 Å is observed and this phase remains when the sample cools down to room temperature. This result suggests that above 500°C the Lss-Na decomposed partially to form the product with d ~ 7.2 Å. The amount of the interlayer water in Lss-Na is changed depending on the relative humidity as shown in . When the relative humidity is low (≤ 10 %), the interlayer space was d ~ 8.6 Å and the amount of the water was n = 1.0. This corresponds to the first step of the thermal dehydration. Under the high humidity (≥ 43%) the interlayer space is expanded to d ~ 11.5 Å and the amount of the water increased to n = 2.0.
2.2. Ion-exchange properties of Lss-Na, Lss and Lss-H
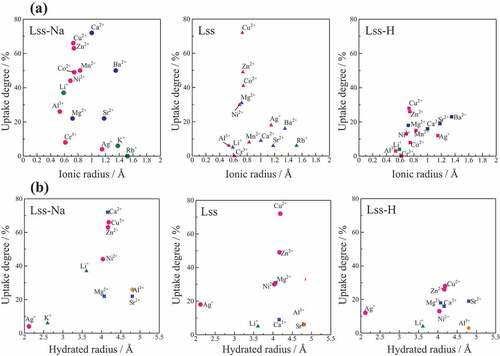
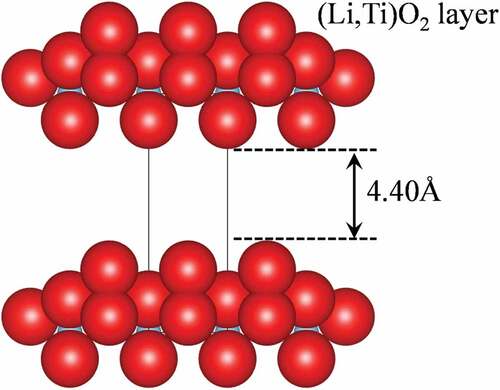
shows ionic radius and hydrated radius dependences of uptake degree of various metal ions for Lss-Na, Lss and Lss-H. As a general tendency, Lss-Na exhibited high uptake degree when compared with those of Lss and Lss-H, and Lss-H showed the lowest uptake degree among them. It was reported that Lss-H had high uptake degree for Li+ and Na+ ions, depending on the values of pH because Lss-H is a solid acid [Citation2]. In this work Lss-H exhibited low performance for ion-exchange reaction because of low pH condition. In the case of Lss divalent cations with the ionic radius of ~ 0.8 Å were high uptake degree. When compared with uptake degree between Lss-Na and Lss, the difference was observed for the uptake degree of Ca2+ and Mn2+ ions. A high uptake degrees of Ca2 + and Mn2 + ions were observed in Lss-Na, but a low uptake degrees for both cations in Lss. This difference is considered to be caused by the hydration of the layers. When the uptake degree is plotted against the hydrated radius [Citation23], the hydrated radius of a high uptake degree ions in Lss-Na is observed in the range of 3.5 to 4.5 Å. This criteria is corresponding to the effective interlayer distance (4.40 Å) between the hydrated layers of Lss-Na as shown in . Consequently, it was considered that the interlayer hydration of Lss-Na caused selectivity of the exchanged ions in aqueous solution.
3. Conclusion
The crystal structure of Lss-Na (Na0.75(Li0.25Ti1.75)O4·2.0H2O) with the lepidocrocite-like structure was refined for the first time by synchrotron powder X-ray diffraction data. The water content in the interlayer and the basal spacing were dependent on a relative humidity. The best property of ion-exchange reaction for Lss-Na among Lss-Na, Lss and Lss-H was corelated with the effective basal space which was expanded by formation of two layers of water molecules.
Acknowledgments
The experiments at SPring-8 were performed with the approval of the Japan Synchrotron Radiation Research Institute (JASRI) (Proposal No. 2018A1189).
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Schaak RE, Mallouk TE. Perovskites by Design: a toolbox of solid-state reactions. Chem Mater. 2002;14(4):1455–1471.
- Sasaki T, Watanabe M, Michiue Y, et al. Preparation and acid-base properties of a protonated titanate with the lepidocrovite-like layer structure. Chem Mater. 1995;7(5):1001–1007.
- Sasaki T, Ebina Y, Tanaka T, et al. Layer-by-layer assembly of Titania Nanosheet/Polycation composite film. Chem Mater. 2001;13(12):4661–4667.
- Tanaka T, Ebina Y, Takada K, et al. Oversized Titania Nanosheet crystallites derived from flux-grown layered single crystals. Chem Mater. 2003;15(18):3564–3568.
- Reid AF, Mumme W, Wadsley AD. A new class of compound M x+A x 3+ Ti 2− x O 4 (0.60 < x < 0.80) typified by Rb x Mn x Ti 2− x O 4. Acta Crystallogr B. 1968;24(9):1228–1233.
- England WA, Birkett JE, Goodenough JB, et al. Ion exchange in the Csx[Ti2-x/2Mgx/2]O4 structure. J Solid State Chem. 1983;49(3):300–308.
- Kumada N, Horiuchi O, Muto F, et al. A new layered type compound, HTaWO6*nH2O (n=0.5-1.5) Prepared from LiTaWO6 by Ion exchange reaction. Mater Res Bull. 1988;23(2):208–216.
- Kumada N, Takeshita M, Muto F, et al. Ion exchange of HTaWO6*nH2O (n=0.5-1.5) with a layered structure. Mater Res Bull. 1988;23(7):1053–1060.
- Kinomura N, Amano S, Kumada N. Intercalation of n-Alkylamines and n-Alkyldiamines into HTaWO6*nH2O. Solid State Ion. 1990;37(4):317–321.
- Kinomura N, Kumada N. Intercalation of weak Lewis bases into HTaWO6*nH2O. Solid State Ion. 1992;51(1–2):1–5.
- Kumada N, Fukasawa Y, Yoneskai Y. Ion-exchange reaction of protonated layer compounds with Bi3+ Ion. Clay Sci. 2006;12:331–335.
- Shirpour M, Cabana J, Doeff M. Lepidocrocite-like layered titanate structures: new Lithium and sodium Ion intercalation anode materials. Chem Mater. 2014;26(8):2502–2512.
- Markus IM, Engelke S, Shirpour M, et al. Experimental and Computational investigation of lepidocrocite Anodes for Sodium-Ion Batteries. Chem Mater. 2016;28(12):4284–4291.
- Katogi A, Kubota K, Chihara K, et al. Synthesis and electrochemical performance of C-Base-Centered Lepidocrocite-like Titanates for Na-Ion batteries. ACS Appl. Energy Mater. 2018;1(8):3630–3635.
- Reeves KG, Ma J, Fukunishi M, et al. Insights into Li +, Na +, and K +Intercalation in Lepidocrocite-Type Layered TiO 2 Structures. ACS Appl. Energy Mater. 2018;1(5):2078–2086.
- Kawaguchi S, Takemoto M, Osaka K, et al. High-throughput powder diffraction measurement system consisting of multiple MYTHEN detectors at beamline BL02B2 of SPring-8. Rev Sci Instrum. 2017;88(8):085111.
- Izumi F. Three-Dimensional MK Visualization in Powder Diffraction. Solid State Phenom. 2007;130:5–20.
- Momma K, Izumi F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J Appl Crystallogr. 2011 Dec;44(6):1272–1276.
- Nassau K, Shiever JW, Bernstein JL. Crystal growth and properties of mica‐like potassium niobates. J Electrochem Soc. 1969;116(3):348–352.
- Roth RS, Parker HS, Brower WS. Crystal chemistry of lithium in octahedrally coordinated structures III. A new structure-type in the system K2O:Li2O:TiO2 (KxLixTi4-x/2O8). Mater Res Bull. 1973;8(3):327–332.
- Sasaki T, Kooli F, Iida M, et al. A mixed alkali metal titanate with the lepidocrocite-like layered structure. preparation, crystal structure, protonic form, and acid-base intercalation properties. Chem Mater. 1998;10(12):4123–4128.
- Rebbah H, Pannetier J, Raveau B. Localization of hydrogen in the layer oxide HTiNbO5. J Solid State Chem. 1982;41(1):57–62.
- Tansel B. Significance of thermodynamic and physical characteristics on permeation of ions during membrane separation: hydrated radius, hydration free energy and viscous effects. Sep Purif Technol. 2012;86:119–126.