ABSTRACT
The worldwide outbreak of the monkeypox virus (MPXV) has become a “Public Health Emergency of International Concern” (PHEIC). Severe monkeypox virus infection can be fatal, however, effective therapeutic methods are yet to be developed. Mice were immunized with A35R protein and A29L protein of MPXV, and the binding and neutralizing activities of the immune sera against poxvirus-associated antigens and viruses were identified. A29L protein and A35R protein-specific monoclonal antibodies (mAbs) were generated and their antiviral activities of these mAbs were characterized in vitro and in vivo. Immunization with the MPXV A29L protein and A35R protein induced neutralizing antibodies against the orthopoxvirus in mice. None of the mAbs screened in this study against A35R could effectively neutralize the vaccinia virus (VACV), while three mAbs against A29L protein, 9F8, 3A1 and 2D1 were confirmed to have strong broad binding and neutralizing activities against orthopoxvirus, among which 9F8 showed the best neutralizing activity. 9F8, 3A1, and 2D1 recognized different epitopes on MPXV A29L protein, showing synergistic antiviral activity in vitro against the VACV Tian Tan and WR strains; the best activity was observed when the three antibodies were combined. In the vivo antiviral prophylactic and therapeutic experiments, 9F8 showed complete protective activity, whereas 3A1 and 2D1 showed partial protective activity. Similarly, the three antibodies showed synergistic antiviral protective activity against the two VACVs. In conclusion, three mAbs recognized different epitopes on MPXV A29L protein were developed and showed synergistic effects against orthopoxvirus.
Background
Orthopoxviruses include smallpox, cowpox, vaccinia virus and MPXV. Severe orthopoxvirus epidemics, such as the smallpox pandemic many years ago, greatly harm human health; therefore, the MPXV outbreak of 2022 must be monitored. MPXV was first discovered in monkeys in 1958 and in humans in 1970 [Citation1], and since May 2022, cases of MPXV infection in humans have been reported globally. The World Health Organization (WHO) has declared the MPXV epidemic a Public Health Emergency of International Concern (PHEIC). As of May 09, 2023, 110 countries and regions have reported cases of MPXV, bringing the total number of confirmed cases to 87,314, with 129 total deaths [Citation2]. MPXV infection seriously damages multiple organ systems of the host, including the skin and mucosal barriers, lung, lymphatic and gastrointestinal tract, the skin of the host can be severely exfoliated, and airway inflammation and bronchopneumonia resulting from infection can limit air intake and impact the ability to ingest water and food [Citation3, Citation4]. Severe MPXV infections can result in death, with mortality rates of 10.6% and 3.6% for clade I and II viruses, respectively [Citation5].
Using effective therapeutic drugs can greatly reduce the severity of illness and mortality caused by viral infections, shorten the course of the disease, and relieve pain in patients [Citation6]. However, till date, few therapeutic drugs have been developed against MPXV infection; if the virus continues to spread worldwide, the human population will be at great risk. Therefore, effective drugs against MPXV infections must be urgently developed. Monoclonal antibody drugs can be employed for treating multiple viral infections [Citation7–9], during the novel coronavirus pneumonia pandemic, some monoclonal antibody drugs were approved for clinical use worldwide, saving lives. Immunization with multiple antigenic proteins of the orthopoxvirus can induce antiviral-neutralizing antibodies, such as the A33, A27, B5, H3, D7, and L1 proteins of VACV [Citation10–12]. Some neutralizing mAbs against orthopoxviruses have been developed, and representative mAbs have shown high antiviral effects in vivo and in vitro [Citation11, Citation13–16]. The generation of neutralizing mAbs is important for developing highly effective antiviral drugs against orthopoxviruses.
At present, most neutralizing mAbs against orthopoxviruses are induced by VACV-associated antigens; however, few studies have been conducted on neutralizing antibodies directly induced by the MPXV antigen. Whether the antigen proteins of MPXV can induce neutralizing antibodies and how the antiviral activity of neutralizing mAbs is directly induced by MPXV antigens warrant further study. A29L protein of MPXV is an intracellular mature virus (IMV) surface envelope protein that is homologous to VACV A27 protein and is widely conserved in the poxvirus family. The A27 protein plays various roles in the viral life cycle, including binding to cell surface heparan sulfate, regulating membrane fusion, and mediating IMV transport to form the extracellular enveloped virus (EEV) [Citation17, Citation18]. A35R protein of MPXV is the envelope component of EEV and homologous to VACV A33 protein, which plays a crucial role in transmitting viral particles between cells and is an important target for vaccine development [Citation19, Citation20]. Previously, our team developed a batch of mAbs induced by antigens of MPXV [Citation21]; in this study, we aimed to investigate the levels and activities of antiviral neutralizing antibodies induced by MPXV A29L and A35R proteins, to provide scientific clues for developing MPXV -specific drugs and vaccines.
Methods
This study aimed to develop mAbs that neutralize multiple orthopoxviruses induced by MPXV antigens. All in vivo studies were performed under the Institutional Animal Care and Use Committee guidelines and were approved by the Ethics Committee of the Southern Medical University Animal Centre.
Mice, cells and viruses
Six to eight-week-old female BALB/c mice were purchased from Shanghai Silaike Laboratory Animal Co., Ltd. and used for all experiments. The animals were maintained in individually ventilated cages and closely monitored for survival and signs of illness after the challenge. The guidelines for humane endpoints were strictly followed for all in vivo experiments; animals that lost more than 25% of their initial body weight were immediately euthanized by CO2 asphyxiation and recorded as non-survivors. All animals were randomly assigned to the treatment groups using a randomization tool in Microsoft Excel. BHK-21 cell lines were purchased from IMMOCELL (Xiamen, Fujian, China) and maintained in Dulbecco Modified Eagle’s Medium (DMEM) supplemented with 10% calf serum. VACV Tian Tan and VACV Western Reserve (VACV-WR; ATCC VR-119) were propagated and titrated in monolayer cultures of BHK-21 cells.
Expression of recombinant MPXV proteins A29L and A35R, Camelpox A27L and Taterapox A27L
The recombinant MPXV proteins A29L and A35R, Camelpox A27L and Taterapox A27L were produced using an Escherichia coli expression system. The protein sequences of MPXV A29L protein and A35R protein, Camelpox A27L protein and Taterapox A27L protein were downloaded from the Genebank and constructed on the PET-28a vector (MPXV A29L protein Genebank No. YP_010377135.1; MPXV A35R protein Genebank No. YP_010377142.1; Camelpox A27L protein Genebank No. ACV88141.1; Taterapox A27L protein Genebank No. YP_717458.1). BL21 receptor cells (Beijing Tsingke Biotech Co., Ltd; TSC-E01) containing the recombinant vector were transformed and plates were delineated, and single colonies were picked for expanded culture. The expression was induced by adding IPTG at a final concentration of 0.1-1 mM to the expanded culture broth and incubated in a shaker at 37°C and 220 rpm for 3-5 h. Subsequently, the supernatant was removed by centrifugation at 7830 rpm for 15 min, and the precipitate was solubilized by adding an appropriate amount of 1*PBS, mixed well and centrifuged at 7380 rpm for 10 min to remove the supernatant. Resuspend the precipitate with lysis buffer, and then use an ultrasonic cell disruptor to break the cells, and repeat this operation until the bacterial solution is slightly clarified and not sticky. The supernatant was removed and the protein was purified using His-tagged protein agarose high-speed purification resin (refer to its instructions). Transfer the purified protein to a dialysis bag in pre-cooled 1*PBS dialysate and stir gently at 4°C on a magnetic stirrer. The dialysate was replaced at 2-4 h, 6-8 h and 10-14 h, respectively. Finally, the dialyzed protein was concentrated to 1 mg/mL at 4°C with an ultrafiltration tube, samples were analyzed by SDS-PAGE and stored at −80°C until use.
Mouse immunization and monoclonal antibody production
The immunization regimen is shown in A. Six-week-old female BALB/c mice were immunized subcutaneously thrice, at 2-week intervals, with MPXV A29L protein or A35R protein. Two weeks after the final immunization, sera were collected and evaluated. Three days later, the spleens of immunized mice were taken in an ultra-clean table, ground in a cell sieve, followed by the addition of 5 ml RPMI-1640 medium, centrifuged at 1500 rpm for 5 min, continued to wash the cells with RPMI-1640 medium, centrifuged again, repeated this operation 3 times, and set aside. The collected SP2/0 cells were also washed 3 times according to the above operation and then set aside. Subsequently, mix the two, add about 25 mL of RPMI-1640 medium containing 10% serum, blow and mix well, centrifuge at 1500 rpm for 5 min, and remove the supernatant. Add 1 mL of pre-warmed PEG to the cells slowly, gently and repeatedly blow the cells for 1 min, then immediately add 20 mL of serum-free RPMI-1640 medium in a warm bath at 37°C and mix well, then centrifuge at 1500 rpm for 5 min. Place in a 37°C incubator. Positive cells were repeatedly blown into a cell suspension and added 20 uL to 200 uL of medium containing 15% serum as the initial concentration, and diluted via limiting dilution. The diluted cells were placed in a 37°C cell culture incubator; after 5 days, the cell growth was observed, and the monoclonal cell wells were picked out, and the positive cell line was screened in ELISA and continued to be cloned. After repeating the operation 3 times, the positive cell line was expanded and cultured to establish a stable hybridoma-positive cell line. The hybridoma cells prepared by the above method were made into cell suspension, and the cells were injected into the peritoneal cavity of mice (which had been injected intraperitoneally with 0.5 mL of Fever incomplete adjuvant one week in advance) with a syringe at 0.5 mL each, and the mice were observed from 6 days after injection until their abdomens were significantly enlarged, and the ascites was aspirated with a 5 mL syringe, centrifuged at 6000 rpm for 10 min, and the supernatant was collected and the precipitate was discarded. An equal volume of saturated ammonium sulfate solution was added to the ascites, and centrifuged at 12000rpm for 10 min, the supernatant was removed, the precipitate was dissolved with an appropriate amount of 1*PBS, centrifuged again, the supernatant was removed, and the impurities were filtered through a 0.22 µm membrane, and finally the corresponding antibody was purified by protein A agarose columns (Bio-Rad).
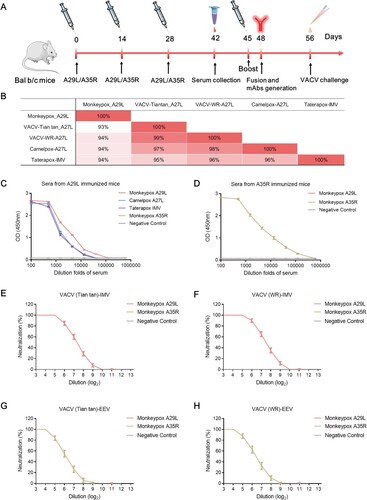
Figure 1. Immunization schedule and immunobiological analysis using the antigens of monkeypox A29L protein and A35R protein. (A), Schedule of A29L protein and A35R protein immunization, serum collection, mAbs generation, and VACV challenge. (B), Homology analysis of monkeypox A29L protein and homologous antigens from other orthopoxviruses. (C), Analysis of sera binding activity from A29L protein immunized mice. (D), Analysis of sera binding activity from A35R protein immunized mice. (E), Neutralization titre analysis of sera from A29L protein and A35R protein immunized mice against IMV form of VACV (Tian tan) strain. (F), Neutralization titre analysis of sera from A29L and A35R immunized mice against IMV form of VACV (WR) strain. (G), Neutralization titre analysis of sera from A29L protein and A35R protein immunized mice against EEV form of VACV (Tian tan) strain. (H), Neutralization titre analysis of sera from A29L protein and A35R protein immunized mice against EEV form of VACV (WR) strain.
Sequencing analysis
The variable gene regions of the antibodies were sequenced as follows. Total RNA was extracted from 107 hybridoma cells using the FastPure Cell/Tissue Total RNA Isolation Kit (Vazyme Biotech Co., Ltd; RC112). The extracted RNA was subjected to a reverse transcription reaction with the following primers: 5'-CCGTTTGKATYTCCAGCTTGGTSCC-3’ for reverse transcription of the light chain variable region gene and 5'-CGGTGACCGWGGTBCCTTGRCCCCA-3’ for reverse transcription of the heavy chain variable region gene using the Takara PrimeScript™ II 1st Strand cDNA Synthesis Kit. The coding regions of the H- and L-chains of 12G6 were amplified by PCR with the following primers: 5'-ATGGACTCCAGGCTCAATTTAGTTTTCCT-3’ (H-chain-forward) and 5'-CGGTGACCGWGGTBCCTTGRCCCCA-3’ (H-chain-reverse); and 5'-ATGAAGTTGCCTGTTAGGCTGTTGGTGCT-3’ (L-chain-forward) and 5'-CCGTTTGKATYTCCAGCTTGGTSCC-3’ (L-chain-reverse) using the Takara TaqTM Version 2.0 plus dye kit. The purified PCR products were then subcloned into pMD 18-T Vector (TaKaRa, Dalian, China) and sent to Shanghai Boya Company for sequencing. The sequences of the antibody variable regions were verified using BLAST alignment, and the corresponding amino acid sequences were determined.
Construction of chimeric antibodies
The chimeric versions of 9F8 (C9F8), 3A1 (C3A1), and 2D1 (C2D1) were constructed as follows. Briefly, The variable regions of each antibody were fused to the constant regions of human IgG1 HC or kappa LC by overlap extension PCR. Each antibody HC and LC was fused with signal peptides (METDTLLLWVLLLWVPGSTGD) and inserted into pTT5 at EcoR I/BamH I. Plasmid DNA was amplified in DH5α (Beijing Tsingke Biotech Co., Ltd; TSC-C01) and purified using a TIANQuick Midi Purification Kit (TIANGEN, China) The recombinant antibodies were expressed in Chinese hamster ovary (CHO) cells through transient transfection and purified from the culture media using MabSelect XtraTM affinity chromatography (Amersham GE Health).
ELISA
Microtiter plates (WANTAI BioPharm) were coated overnight at 4°C with 5 μg/mL purified proteins or viruses (50 μL per well). The plates were washed thrice with PBS containing 0.1% v/v Tween-20 (PBST) and blocked in PBS containing 2% w/v non-fat dry milk (blocking solution) for 2 h at 37°C. The plates were then washed with PBST. Serial dilutions of purified antibodies or sera were added to the wells and incubated at 37°C for 30 min. After three washes, 100 μL of horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG antibody solution was added to each well and incubated at 37°C for 30 min. After five washes, 100 μL of tetramethylbenzidine (TMB) substrate (WANTAI BioPharm) was added at room temperature in the dark. After 15 min, the reaction was stopped with a 2M H2SO4 solution, and the absorbance was measured at 450 nm. All samples were run in triplicate. The relative affinity of antibody binding to purified viruses or proteins was determined by measuring the concentration of antibody required to achieve the EC50. Competition ELISAs were carried out as described previously [Citation22], with an additional step involving preincubation of the antigen with an unlabelled competitor antibody at a 10-fold molar excess before adding the mAbs to the plate. The competition level was calculated as the percentage inhibition of the half-maximal binding concentration of the test antibody, relative to the absorbance without an added competitor.
Microneutralization assay
The neutralizing activity of the mAbs and sera was determined using the MV or EV forms of the VACV Tian tan or WR strain. Neutralization of VACV EV was performed using a 10% baby rabbit complement (Pelfreez). For EV neutralization, MV was depleted with blocking mAbs at 20 mg/mL. BHK-21 cells were maintained in DMEM supplemented with 10% calf serum at 37°C, and 5% CO2. On the day of the experiment, BHK-21 cells in a 96-well plate were washed twice with phosphate-buffered saline (PBS) and incubated in DMEM. Serial 2-fold dilutions of mAb or serum were mixed with an equal volume of virus and incubated for 2 h at 37°C. After incubation, 35 µL of the mixture, containing 100 TCID50 (50% tissue culture infectious dose) of the virus, was then added to the BHK-21 cells and allowed to adsorb for 1 h. The viral supernatant was removed and replaced with antibiotic-supplemented DMEM. The mixture was then added to cells and incubated at 37 °C. The cytopathic effect was examined for 4 days post-infection. Protection was defined as the complete absence of cytopathic effect in an individual culture well was defined as protection. The values of IC50 were calculated using Prism software (GraphPad).
Western blotting
A29L protein and its related polypeptides were separated using 12% SDS-PAGE separation and then transferred to 0.22-μm nitrocellulose membranes (GE Healthcare). The polypeptides were synthesized by GenScript Biotech Corporation and the terminal group of each peptide is linked to the primary amine on KLH by a crosslinker (crosslinker). The blotted membranes were blocked with 5% skim milk in tris-buffered saline and then incubated with the antibody at 37°C for 60 min. After washing thrice with PBST, the membranes were incubated with an HRP-conjugated goat anti-mouse antibody at 37°C for 60 min and visualized using chemiluminescent HRP substrates.
Tissue staining
Infected mice were euthanized, and their lungs were removed, inflated, and fixed in 10% neutral buffered formalin (VWR). Tissue sections (4–7 μm) from formalin-fixed paraffin-embedded lungs were stained with H&E for histopathological evaluation. Endogenous peroxidase activity was quenched by incubation for 10 min in a 3% hydrogen peroxide in PBS, followed by blocking for 10 min with 10% normal rabbit serum. Sections were counterstained with modified Harris hematoxylin, dehydrated, and mounted using neutral balsam. The stained sections were analyzed using a Nikon 80i microscope with 20× and 200× objectives and reviewed by a pathologist.
Molecular docking
Five tertiary 3D structures of A29L protein were predicted by AlphaFold2 with standard parameters and the model with the highest pLDDT score (77.1) was chosen for subsequent docking [Citation23]. Structures of the Fv domains on 9F8, 2D1 and 3A1 were generated by ABodyBuilder2 from the SAbPred antibody prediction toolbox [Citation24]. The protein–protein docking of A29L protein with Fv domains of 9F8, 2D1 and 3A1 was carried out by online tools ZDOCK and ClusPro 2.0 [Citation25–29]. The top ten predicted complexes by ZDOCK and the top ten predicted complexes by ClusPro 2.0 in balanced, electrostatic-favored, Hydrophobic-favored or VdW + Elec mode were further investigated manually with epitope mapping information. The models that match best with epitope mapping information of 9F8, 2D1 or 3A1 were chosen for presentation. Figures were prepared in PyMOL [Citation30].
Prophylactic and therapeutic efficacy studies in mice
In a prophylactic setting, groups of six female BALB/c mice aged 6–8 weeks were injected intraperitoneally (i.p.) with 200 μL of vehicle control or a dose of 10 mg/kg of the indicated antibody. One day later, the mice were deeply anesthetized with isoflurane and oxygen and challenged intranasally (i.n.) with 5*106 TCID50 of either, VACV the Tian Tan or WR strains which were grown in BHK-21 cells using standard viral culturing techniques. In the therapeutic setting, mice received the antibody at the indicated doses one day after infection. The lungs of the mice were collected for virus titration at four days after infection. Tissue samples were collected for histopathological evaluation four days after infection. The animals were observed daily for mortality and morbidity, and their body weights were measured for up to 21 days after infection. Animals that lost more than 25% of their initial body weight were euthanized according to animal ethics protocols.
Results
To generate neutralizing mAbs against MPXV directly, the mice were immunized subcutaneously with MPXV A29L protein or A35R protein, the sera were collected and evaluated, and mAbs were screened (A). To evaluate the response profile of antibodies induced via immunization with MPXV antigens, several antigen proteins from various orthopoxvirus strains, such as the A27L of Camelpox and IMV protein of Talerapox, were expressed and purified, and the amino acid homology of the corresponding proteins from different viruses, A29L protein and A35R, were compared. The homologies between the pairwise pairs of MPXV A29L, VACV Tian Tan A27L, VACV WR A27L, Camelpox A27L, and Talerapox IMV proteins were all above 93% (B and Figure S1A and S1A). The MPXV A35R protein, VACV Tian Tan A33 protein, and VACV WR A33 proteins also showed high amino acid homology between pairwise pairs, all of which were the pair homologies are above 93% (Figure S1B and 1D). Sera from mice immunized with MPXV A29L protein revealed good ELISA binding reactivity to MPXV A29L protein, Camelpox A27L protein, and Taterapox IMV protein (C), immunization with MPXV A35R protein also induces high titres of MPXV-specific antibodies in mice (D). MPXV A29L protein immune sera showed good neutralizing activity against the IMV form of the VACV Tiantan and WR strains but did not effectively neutralize the EEV form of the VACV (E-H). In the contrast, the, MPXV A35R immune serum showed high neutralizing activity against the EEV form of both VACV strains, but no neutralizing activity against EEV viruses (E-H). When the mice immunized with MPXV A29L and A35R were challenged with VACV Tian tan, their body weights recovered more rapidly, indicating that immunization with MPXV A29L and A35R could induce protective immunity in vivo (A).
Figure 2. In vivo protection of mice immunized with monkeypox-specific A29L protein or A35R protein and number of mAbs generation from immunized mice. (A), Changes in body weight of mice immunized with monkeypox virus-specific A29L protein or A35R protein after being challenged with vaccinia virus Tiantan strain. (B), Number of monkeypox-specific monoclonal antibodies screened from A35R protein and A29L protein immunized mice. (C), CDR sequences of six A29L protein -specific monoclonal antibodies
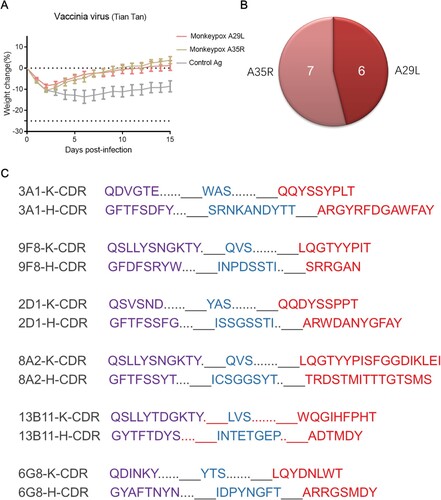
ELISA screening for MPXV A29L and A35R identified 7 MPXV A35 specific mAbs and 6 MPXV A29L specific mAbs (B). The antibodies were purified from mouse ascitic fluid (Figure S2), the DNA sequences of the VH (variable region of immunoglobulin heavy chain) and VL (variable region of immunoglobulin light chain) regions of the antibodies were obtained and compared with the closest germline sequences using the VBASE2 database (www.vbase2.org/), and complementarity determining region (CDR) sequences of six A29L antigen-specific monoclonal antibodies were observed (C). To determine the activity of MPXV-specific mAbs against orthopoxviruses, we tested the activity of purified mAbs against antigens of distinct orthopoxvirus strains. In the primary binding test, MPXV-specific mAbs 3A1, 9F8, 2D1, 13B11, 8A2, and 12D7 reacted strongly with MPXV A29L protein, with half-maximal effective concentration (EC50) values of 109, 111, 55, 337, 72, and 859 ng/mL, respectively (A). Compounds 3A1, 9F8, 13B11, and 8A2 showed strong binding activity to camelpox A27L protein, Taterapox A27L protein, and IMV particles of the VACV Tian Tan strain, showing a broad binding activity, while 2D1 and 12D7 showed only weak binding to these three antigens (B-D). For the A35R specific mAbs, 6G8, 9C6, 13F9, 2G5, 8G10, 14B3 and 16C10 reacted strongly with MPXV A35R and EEV particles of the VACV Tian Tan strain, with EC50 values ranging from 45 to 1332 ng/mL (E and F).
Figure 3. Analysis of ELISA and microneutralization activities of monkeypox A29L protein and A35R protein specific monoclonal antibodies. (A-D), ELISA activity of monkeypox A29L protein specific monoclonal antibodies against monkeypox A29L protein (A), Camelpox A27L protein (B), Taterapox A27L protein (C) and IMV form protein of VACV Tian tan strain (D). (E-F), ELISA activity of monkeypox A35R specific monoclonal antibodies against monkeypox A35R protein (E) and EEV form of VACV Tian tan strain (F). (G-H), microneutralization activity of monkeypox A29L protein specific monoclonal antibodies against IMV form of VACV Tian tan strain (G) and IMV form of VACA WR strain (H). (I), microneutralization activity of monkeypox A35R specific monoclonal antibodies against EEV form of VACV Tian tan strain.
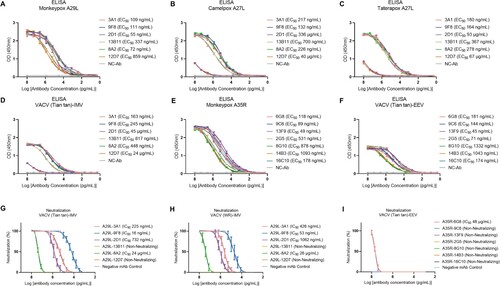
Next, we identified the in vitro neutralization activity of MPXV-specific mAbs against the two VACV strains. A29L-13B11 and A29L-12D7 are non-neutralizing antibodies against both the VACV strains, A29L-3A1, and the A29L-8A2 neutralized both VACV strains with weak activity. In contrast, A29L-9F8 and A29L-2D1 showed strong neutralization activities against the VACV Tian tan and WR strains, with IC50 values of 225, 16, and 732 ng/mL against the IMV form of VACV Tian tan strain, respectively, and 426, 53, and 1062 ng/mL against the IMV form of VACV WR strain, respectively (G and H). Most A35R specific monoclonal antibodies were non-neutralizing antibodies, and only A35R-6G8 showed weak neutralizing activity against the VACV Tian tan strain and VACV WR strains (I and Figure S3). Since the three A29L specific mAbs, 9F8, 3A1 and 2D1, exhibited strong neutralization activities against orthopoxviruses, these three mAbs were selected for further characterized further.
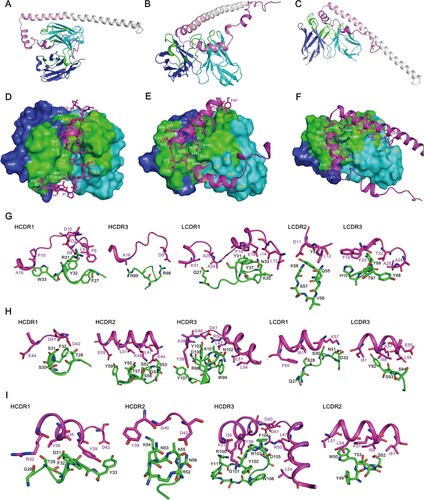
We first identified whether antibodies 3A1,9F8 and 2D1 recognized different epitopes on A29L using competitive ELISA. The competitive rate of ELISA binding between the three pairings was all below 50%, indicating that 3A1,9F8 and 2D1 recognized different epitopes on A29L. The ELISA competition rates of 3A1 to 3A1, 9F8, 2D1, and control IgG were 91%, 38%, 18%, and 14%, respectively (A), the ELISA competition rates of 9F8 to 9F8,3A1, 2D1 and Control IgG were 88%, 42%, 18%, and 13%, respectively (B), the ELISA competition rates of 2D1 to 2D1,3A1, 9F8, and Control IgG were 90%, 20%, 22%, and 14%, respectively (C) in contrast, Control IgG showed no competitive activity against all antibodies (D). To further confirm the epitopes recognized by 9F8, 2D1, and 3A1, the MPXV A29L was divided into six polypeptides, P-1-P-6, which were synthesized and purified (Figure S4A), western blotting assay was used to identify the reactivity of the three antibodies to the six polypeptides. 9F8 showed positive reactivity with the P-1 polypeptide and did not react with other peptides, the sequence of P-1 polypeptide was MDGTLFPGDDDLAIPATEFF (Figure S4B). 2D1 showed positive reactivity with P-2 and P-3 polypeptides and did not react to other peptides, the sequence of P-2 polypeptide was TEFFSTKAAKNPETKREAIVKAYGDDNEETLKQ, and P3 polypeptide was TLKQRLTNLEKKITNITTKF (Figure S4C). Conpound 3A1 showed positive reactivity with the P-2 polypeptide,but and did not react with the other peptides (Figure S4D). Western blotting confirmed that the epitopes recognized by 9F8, 3A1, and 2D1 were different. To further identify the interactions between A29L and these antibodies, the Molecular docking assay was further conducted. The structure of A29L was predicted by AlphaFold2. The N-terminal of A29L seems to be flexible while the C-terminal of A29L folds into a long alpha helix, which is consistent with the crystal structure of the Vaccinia virus A27 (PDB 3VOP), a protein showed homology to A29L [Citation18]. Molecular docking analysis showed that the CDR1 and CDR3 on the heavy chain of 9F8 and three CDRs on the light chain form a groove, which perfectly accommodates the P1 section of A29L. Three pairs of electrostatic interactions, Asp2-Arg31, Asp9-Arg98, and Asp11-Lys58, are essential to binding A29L to 9F8. Hydrophobic interaction between Leu5, Phe6 and CDR1 on a heavy chain further stabilizes the complex (A, D and G). All the CDRs of 2D1 except CDR2 on the light chain are involved in the binding to A29L. Both P2 and P3 sections contributed to the binding. The hydrophobic interactions between Tyr39, Leu47, Leu51, Leu54, and CDR3 on heavy chain were crucial for A29L binding to 2D1. Electrostatic interaction between Lys57 and Asp32 on CDR1 of the light chain also plays an important role (B, E and H). The antibody 3A1 binds to A29L mainly through the P2 section of A29L. The P2 region folds into a short helix flanked by two loops. The buried area between A29L and 3A1 seems less than that between A29L antigen-2D1 and A29L antigen-9F8. Two negatively charged residues Asp40 and Asp41 form electrostatic interactions with Arg103 on the CDR3. Residues Ile35, Val36, and Tyr39 form hydrophobic interactions with aromatic residues Phe32 and Tyr33 on CDR1 of the heavy chain (C, F and I). This result showed that antibodies 3A1, 9F8, and 2D1 recognized different epitopes on A29L, but there was a few amino acids overlaps among the epitopes between 3A1 and 9F8, 3A1 and 2D1, which to a certain extent explained the reason why the three antibodies showed a certain degree of competitive activity in competitive ELISA experiments.
Figure 4. Epitope competition analysis and synergetic antiviral effects of monkeypox A29L protein specific antibodies 3A1,9F8 and 2D1. (A), 3A1 epitope comparison with 9F8 and 2D1 using competition ELISA,with 3A1 as competitor. (B), 9F8 epitope comparison with 3A1 and 2D1 using competition ELISA,with 2D1 as competitor. (C), 2D1 epitope comparison with 3A1 and 9F8 using competition ELISA, with 2D1 as competitor. (D), Negative antibody epitope comparison with 3A1, 9F8, and 2D1 using competition ELISA. IgG was used as the competitor and as a non-neutralizing monoclonal antibody specific for A35R generated in our laboratory. Statistical analysis was performed using the t-test. *p < 0.05, **p < 0.05, ***p < 0.001 versus the negative control group. (E-F), Synergetic microneutralization ability of monkeypox A29L protein specific antibodies 3A1,9F8 and 2D1 against IMV form of VACV Tian tan strain (E) and IMV form of VACA WR strain (F).
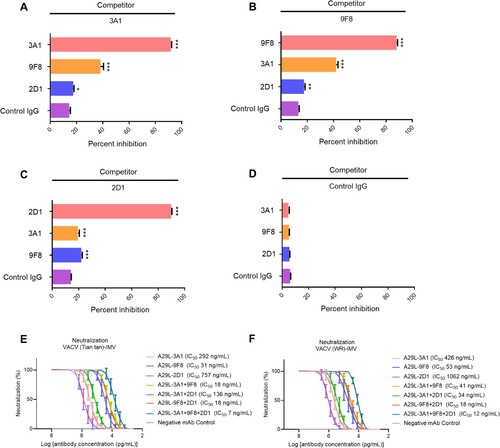
Figure 5. Model of A29L protein binding by 9F8, 2D1 and 3A1. The structures of A29L protein and the Fv domains of 9F8, 2D1 and 3A1 were predicted by AlphaFold2 and SabPred, respectively. The docked complexes were created by ZDOCK and ClusPro and were further investigated manually with epitope mapping information. The result that matches best with epitope mapping information was presented. (A-C), Overall architecture of A29L protein-9F8 (A), A29L protein-2D1 (B) and A29L protein-3A1 (C). The P1, P2 and P3 sections of A29L protein are coloured in magenta, violet and light pink, respectively. The 2D1 heavy chain, light chain and P4-P6 sections of A29L protein are coloured in blue, cyan and gray, respectively. The CDRs on both heavy chain and light chain are coloured in green. (D-F), The interfaces of A29L protein-9F8 (D), A29L protein-2D1 (E) and A29L protein-3A1 (F). The Fv domains of the antibodies are shown in surface representation with the heavy chain, light chain and CDRs coloured in blue, cyan and green, respectively. The residues on the heavy chain and light chain that are involved in A29L protein binding are labelled in white and orange. A29L protein is shown in cartoon and coloured in magenta. The residues involved in 9F8, 2D1 or 3A1 binding are shown in sticks. (G-I), The detailed interactions between A29L protein and CDRs on 9F8, 2D1 and 3A1. Residues on A29L protein are shown in stick representation and coloured in magenta. Residues on 9F8, 2D1 or 3A1 are shown in stick representation and coloured in green. The CDR domains which are unlikely to interact with A29L protein are not shown. Each residue is labelled by its one-letter code.
Antibodies that recognize different antigenic epitopes can exert synergistic antiviral effects [Citation31], the synergistic neutralization effects of these three antibodies agaisnt VACV have been identified in vitro. The neutralization IC50 values of 3A1, 9F8, 2D1, 3A1 + 9F8, 3A1 + 2D1, 9F8 + 2D1, 3A1 + 9F8 + 2D1 against VACV Tian tan strain were 292, 31, 757, 18, 136, 18, and 7 ng/mL(E). The neutralization IC50 values of 3A1, 9F8, 2D1, 3A1 + 9F8, 3A1 + 2D1, 9F8 + 2D1, 3A1 + 9F8 + 2D1 against the VACV WR strain were 426, 53, 1062, 41, 34, 18, and 12 ng/mL, respectively (E).
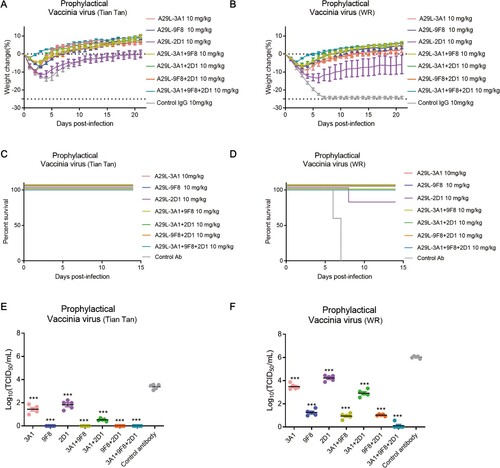
To further explore the protective effects of 3A1, 9F8, 2D1, and the antibody cocktails against orthopoxvirus in vivo, we tested the protective effects of each antibody in a mouse model. Two VACV strains, Tian Tan and WR, were intranasally administered to evaluate the prophylactic and therapeutic effects of different antibodies against viral infections. For the prophylactic experiments, single antibodies and antibody combinations (10 mg/kg) injected 24 h before viral infection protected the mice against the infections by both viruses (). The mean body weight was higher or only slightly lower in mice in all antibody-treated groups compared to the original mean body weight (A and B). Besease of the weak virulence of the VACV Tian tan strain, all mice infected with this strain survived, in contrast, when challenged with VACV WR, all mice in the control antibody-treated group died within 7 days, whereas only one mouse in the 2D1 antibody-treated group died on day 8 after the infection, and all the other A29L antibody-treated mice survived (C and D). Prophylactic treatment with any of the antibodies also reduced lung viral titres compared to control animals, with 3A1 + 9F8 + 2D1-treated mice displaying the lowest lung viral titres (E and F). In the antiviral neutralization assay, A35R specific antibody 6G8 also showed weak antiviral neutralizing activity. The prophylactic effect of 6G8 against VACV strains was investigated, showing that 6G8 administration and not effectively protect against the challenges of both VACV strains in mice (Figure S5).
Figure 6. Prophylactic effects of monkeypox A29L protein specific antibodies 3A1,9F8, 2D1, 3A1 + 9F8, 3A1 + 2D1, 9F8 + 2D1, 3A1 + 9F8 + 2D1 in mice. A-B, Body weight changes of BALB/C mice (n = 6 per group) infected with 5*106 TCID50 doses of the VACV Tian tan strain (A) or VACA WR strain (B) 24 h before intraperitoneal administration with the indicated antibodies (10 mg/kg). Weight curves represent the mean ± 95% confidence interval. c-d, Survival curves of BALB/C mice (n = 6 per group) infected with 5*106 TCID50 doses of the VACV Tian tan strain (C) or VACA WR strain (D) 24 h before intraperitoneal administration with the indicated antibodies (10 mg/kg). E-F, Pulmonary virus titres of BALB/C mice (n = 5 per group) infected with 5*106 TCID50 doses of the VACV Tian tan strain (E) or VACA WR strain (F) 24 h before intraperitoneal administration with the indicated antibodies (10 mg/kg). Black bars indicate mean values. For (E) and (F), the virus titres in the lungs of the mice treated with each antibody prophylactically were determined on four days after infection, the t-test was used for comparisons between groups. ***p < 0.001 versus the negative control group.
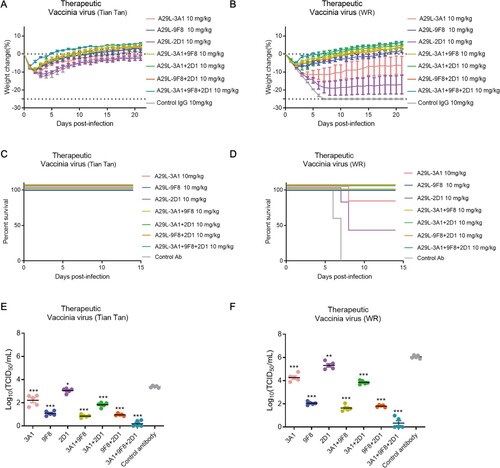
In the therapeutic assays, the antibodies were injected 24 h after infection with each virus, and the antibody cocktails showed better antiviral efficacy (). In terms of weight recovery, 2D1 and 3A1 protected mice from infection with VACV, Tian tan, and WR viruses, resulting in moderate weight loss, while all the other anti-A29L antibodies protected the mice from infection and resulted in increased weight or slight body weight loss at the end of the study, mice treated with the combination of the three antibodies recovered the fastest and gained the most weight (A and B). The 2D1 and 3A1 antibodies partially protected mice from the challenges with the VACV strains, whereas all other antibodies were able to completely protected mice infected with both VACV strains (C and D). Moreover, we found that mice treated therapeutically with a single 10 mg/kg dose of any of the antibodies one day after infection had lower lung viral titres than control animals, with the three antibody cocktails reducing lung viral titres more than the three single antibodies (E and F). Consistent with the lung viral titre data, hematoxylin, and eosin staining results indicated that therapeutic treatment with 3A1 + 9F8 + 2D1, 3A1 + 9F8, and 9F8 + 2D1 significantly decreased the lung damage caused by VACV infection, compared to the control IgG–treated group (Figure S6).
Figure 7. Therapeutic effects of monkeypox A29L protein specific antibodies 3A1,9F8, 2D1, 3A1 + 9F8, 3A1 + 2D1, 9F8 + 2D1, 3A1 + 9F8 + 2D1 in mice. A-B, Body weight changes of BALB/C mice (n = 6 per group) infected with 5*106 TCID50 doses of the VACV Tian tan strain (A) or VACA WR strain (B) 24 h after intraperitoneal administration with antibodies (10 mg/kg). Weight curves represent mean ± 95% confidence interval. C-D, Survival curves of BALB/C mice (n = 6 per group) infected with 5*106 TCID50 doses of the VACV Tian tan strain (C) or VACA WR strain (D) 24 h after intraperitoneal administration with antibodies (10 mg/kg). E-F, Pulmonary virus titres of BALB/C mice (n = 5 per group) infected with 5*106 TCID50 doses of the VACV Tian tan strain (E) or VACA WR strain (F) 24 h after intraperitoneal administration with antibodies (10 mg/kg). Black bars indicate mean values. For (E) and (F), virus titres in the lungs of mice treated with each antibody therapeutically were determined four days after infection; t-test was used for comparisons between groups. *p < 0.05, **p < 0.05, ***p < 0.001 versus the negative control group.
To further evaluate the potential clinical use of 9F8, 3A1, and 2D1, chimeric versions of these mAbs were constructed, which contained the variable region of the mouse antibody and the human IgG1 Fc region, designated C9F8, C3A1, and C2D1, respectively. The three chimeric mAbs were characterized via an in vitro antiviral neutralization experiment. C3A1, C9F8, and C2D1 showed strong neutralizing activity against the VACV Tiantan strain and WR strains, with the comparable IC50 values comparable to those of the corresponding murine antibodies (Figure S7).
Discussion
Severe cases of MPXV infection can result in death from exhaustion and fatigue [Citation3],however, apart from supportive treatment, no effective therapy has been developed to manage MPXV infection in humans. Therefore, new therapeutic drugs against MPXV must be developed, mAb are promising drugs for clinical application. Several orthopoxvirus-specific mAbs have been developed, most of which are directed against A35R-like antigens and few against A29L protein-like antigens. Most of these mAbs are induced by stimulation with VACV-related antigens, and mAbs induced by direct exposure to antigens from MPXV have not been reported till date. In this study, the three anti-MPXV mAbs 9F8, 3A1, and 2D1 were directly induced by the MPXV antigen and showed strong synergistic antiviral activities in vivo and in vitro. We demonstrated that antibody cocktails consisting of antibodies that recognize different antigenic epitopes on the A29L of MPXV could be effective prophylactic and therapeutic agents. Previously, antibody cocktails against different epitopes have been developed into antiviral drugs against important human infectious diseases [Citation32, Citation33]. The mAbs developed in this study could potentially be used to treat of orthopoxyviral infections, including MPXV infections.
The A33 protein is an important functional protein in the life cycle of orthopoxviruses and has been implicated in mediating cell-to-cell viral spread in an antibody-resistant manner [Citation2, Citation34]. Immunization with A33 can induce neutralizing antibodies against orthopoxvirus, and A33-specific neutralizing mAbs against orthopoxvirus have been developed previously [Citation14, Citation35]. In this study, the results also confirmed that sera from the mice immunized with the MPXV A35R protein could effectively neutralize the EEV form of VACV. However, we failed to generate A35R-specific mAbs with strong antiviral neutralization activity. The possible reason is that compared with the previous published neutralizing mAbs against A33 of orthopoxvirus, the non-neutralizing anti-A35R mAbs cloned in this study should recognize distinct epitopes, unfortunately, we have not been able to obtain the published mAbs to conduct direct comparative experiments between them. Our team is currently developing more A35R mAbs, to generate several A35R-specific mAbs with strong anti-orthopoxvirus neutralizing activity.
MPXV A29L specific antibodies 9F8 and 3A1 are broadly neutralizing antibodies against orthopoxvirus, and show strong reactivity to the antigens from MPXV, VACV, camelpox virus, and taterapox. In contrast, 2D1 showed a decent binding activity against the antigen of MPXV antigen, but had a weak response to VACV and other orthopoxviruses. As our team did not obtain the authentic MPXV, a neutralization experiment against the authentic MPXV was not conducted in this study, which is also a limitation of this study. In further studies, we will attempt to contact our partners to carry out in vitro neutralization experiments and in vivo antiviral protection experiments using these antibodies against the authentic MPXV, and a high neutralizing activity of 2D1 against authentic MPXV is expected. Other antigenic proteins of MPXV, such as B6R, M1R, E8L, and H3L, may also induce antiviral-neutralizing antibodies in vivo [Citation36–38]. In subsequent studies, we will immunize mice with these proteins, identify the neutralization activity of mouse immune sera, and screen various types of mAbs against orthopoxvirus. The mAbs cocktails recognize that both the EEV and IMV forms of MPXV may have better antiviral effects.
MPXV-specific antibodies may neutralize the virus through certain mechanisms since multiple pathways can be utilized to inhibit orthopoxvirus. The mechanism underlying viral neutralization by the 9F8, 3A1, and 2D1 alone individually and synergistically and the avoidance of viral escape of these mAbs should be investigated further. Antigen–antibody complex crystallization and cryo-electron microscopy technology will be used to further identify the epitopes recognized by the antibodies 9F8, 3A1, and 2D1. In follow-up experiments, chimeric antibody forms of 9F8, 3A1, and 2D1 will be further humanized and characterized to make them more promising as novel drugs against orthopoxvirus. Due to the synergistic antiviral activity possessed by 9F8, 3A1, and 2D1, these antibodies will be developed into various bispecific and trispecific antibodies, the multi-specific form of these antibodies may exert better antiviral effects.
Author contributions
C.S., W.Z., and J.P. contributed to the experimental design. C.S., W.Z., M.L., M.Y., and J.P. contributed to manuscript preparation. M.L., Y.W., Z.R., and Y.J., contributed to the virus isolation and titration. C.S., M.L., Z.R., Y.J., J.C., L.Z., Y.L., and Y.W. contributed toward the generation and production of antibodies. Z.Z., R.X., and B.Z. contributed to in vitro antibody characterization. D.L. performed the molecular docking assay. W.X., Q.W., Y.Y., and M.L. performed animal experiments. B.W., and C.W. performed statistical analyses.
Supplemental Material
Download MS Word (3.4 MB)Data availability statement
The datasets generated and/or analyzed in the current study are available from the corresponding author upon reasonable request. The source data are provided in this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Petersen E, Kantele A, Koopmans M, et al. Human monkeypox: epidemiologic and Clinical characteristics, diagnosis, and prevention. Infect Dis Clin North Am. 2019;33(4):1027–1043.
- Krupovic M, Cvirkaite-Krupovic V, Bamford DH. Protein A33 responsible for antibody-resistant spread of vaccinia virus is homologous to C-type lectin-like proteins. Virus Res 2010;151(1):97–101.
- Reynolds MG, McCollum AM, Nguete B, et al. Improving the care and treatment of monkeypox patients in low-resource settings: applying evidence from contemporary biomedical and smallpox biodefense research. Viruses. 2017;9(12).
- Lum F-M, Torres-Ruesta A, Tay MZ, et al. Monkeypox: disease epidemiology, host immunity and clinical interventions. Nat Rev Immunol. 2022;22(10):597–613.
- Bunge EM, Hoet B, Chen L, et al. The changing epidemiology of human monkeypox-A potential threat? a systematic review. PLoS Negl Trop Dis. 2022;16(2):e0010141.
- Kirtane AR, Verma M, Karandikar P, et al. Nanotechnology approaches for global infectious diseases. Nat Nanotechnol. 2021;16(4):369–384.
- Zhai L, Zhang L, Jiang Y, et al. Broadly neutralizing antibodies recognizing different antigenic epitopes act synergistically against the influenza B virus. J Med Virol 2023;95(1):e28106.
- Wu Y, Wang F, Shen C, et al. A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science. 2020;368(6496):1274–1278.
- Shen C, Chen J, Li R, et al. A multimechanistic antibody targeting the receptor binding site potently cross-protects against influenza B viruses. Sci Transl Med. 2017;9(412):eaam5752.
- Berhanu A, Wilson Rebecca L, Kirkwood-Watts Dana L, et al. Vaccination of BALB/c mice with escherichia coli-expressed vaccinia virus proteins A27L, B5R, and D8L protects mice from lethal vaccinia virus challenge. J Virol. 2008;82(7):3517–3529.
- Gilchuk I, Gilchuk P, Sapparapu G, et al. Cross-neutralizing and protective human antibody specificities to poxvirus infections. Cell. 2016;167(3):684–94.e9.
- Benhnia Mohammed R-E-I, McCausland Megan M, Moyron J, et al. Vaccinia virus extracellular enveloped virion neutralization In vitro and protection In vivo depend on complement. J Virol 2009;83(3):1201–1215.
- Chen Z, Earl P, Americo J, et al. Chimpanzee/human mAbs to vaccinia virus B5 protein neutralize vaccinia and smallpox viruses and protect mice against vaccinia virus. Proc Natl Acad Sci USA. 2006;103(6):1882–1887.
- Matho MH, Schlossman A, Meng X, et al. Structural and functional characterization of anti-A33 antibodies reveal a potent cross-species orthopoxviruses neutralizer. PLoS Pathog 2015;11(9):e1005148.
- Chen Z, Earl P, Americo J, et al. Characterization of chimpanzee/human monoclonal antibodies to vaccinia virus A33 glycoprotein and Its variola virus homolog In vitro and in a vaccinia virus mouse protection model. J Virol 2007;81(17):8989–8995.
- Gu X, Zhang Y, Jiang W, et al. Protective human anti-poxvirus monoclonal antibodies are generated from rare memory B cells isolated by multicolor antigen tetramers. Vaccines (Basel). 2022;10(7).
- Gong SC, Lai CF, Esteban M. Vaccinia virus induces cell fusion at acid pH and this activity is mediated by the N-terminus of the 14-kDa virus envelope protein. Virology. 1990;178(1):81–91.
- Chang TH, Chang SJ, Hsieh FL, et al. Crystal structure of vaccinia viral A27 protein reveals a novel structure critical for its function and complex formation with A26 protein. PLoS Pathog 2013;9(8):e1003563.
- Ward BM, Weisberg AS, Moss B. Mapping and functional analysis of interaction sites within the cytoplasmic domains of the vaccinia virus A33R and A36R envelope proteins. J Virol 2003;77(7):4113–4126.
- Law M, Hollinshead R, Smith GL. Antibody-sensitive and antibody-resistant cell-to-cell spread by vaccinia virus: role of the A33R protein in antibody-resistant spread. J Gen Virol 2002;83(Pt 1):209–222.
- Li M, Wang Y, Li C, et al. Development of monoclonal antibody-based antigens detection assays for orthopoxvirus and monkeypox virus. J Infect. 2022;85(6):702–769.
- Li GM, Chiu C, Wrammert J, et al. Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proc Natl Acad Sci USA 2012;109(23):9047–9052.
- Mirdita M, Schütze K, Moriwaki Y, et al. ColabFold: making protein folding accessible to all. Nat Methods. 2022;19(6):679–682.
- Brennan A, Wing Ki W, Fergus B, et al. Immunebuilder: deep-learning models for predicting the structures of immune proteins. bioRxiv. 2022:2022.11.04.514231.
- Pierce BG, Wiehe K, Hwang H, et al. ZDOCK server: interactive docking prediction of protein-protein complexes and symmetric multimers. Bioinformatics. 2014;30(12):1771–1773.
- Desta IT, Porter KA, Xia B, et al. Performance and its limits in rigid body protein-protein docking. Structure. 2020;28(9):1071–81.e3.
- Vajda S, Yueh C, Beglov D, et al. New additions to the ClusPro server motivated by CAPRI. Proteins. 2017;85(3):435–444.
- Kozakov D, Hall DR, Xia B, et al. The ClusPro web server for protein–protein docking. Nat Protoc. 2017;12(2):255–278.
- Kozakov D, Beglov D, Bohnuud T, et al. How good is automated protein docking? Proteins. 2013;81(12):2159–2166.
- DeLano WL. The PyMOL molecular graphic system (Delano Scientific, SanCarlos, CA). 1.7.0.1. 2002.
- Ku Z, Xie X, Davidson E, et al. Molecular determinants and mechanism for antibody cocktail preventing SARS-CoV-2 escape. Nat Commun. 2021;12(1):469.
- Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. N Engl J Med 2021;384(3):238–251.
- Gilchuk P, Murin CD, Milligan JC, et al. Analysis of a therapeutic antibody cocktail reveals determinants for cooperative and broad ebolavirus neutralization. Immunity. 2020;52(2):388–403.e12.
- Monticelli SR, Earley AK, Stone R, et al. Vaccinia virus glycoproteins A33, A34, and B5 form a complex for efficient endoplasmic reticulum to trans-Golgi network transport. J Virol 2020;94(7).
- Chen Z, Earl P, Americo J, et al. Characterization of chimpanzee/human monoclonal antibodies to vaccinia virus A33 glycoprotein and its variola virus homolog in vitro and in a vaccinia virus mouse protection model. J Virol 2007;81(17):8989–8995.
- Paran N, Lustig S. Complement-bound human antibodies to vaccinia virus B5 antigen protect mice from virus challenge. Expert Rev Vaccines. 2010;9(3):255–259.
- Bisht H, Weisberg AS, Moss B. Vaccinia virus l1 protein is required for cell entry and membrane fusion. J Virol 2008;82(17):8687–8694.
- Alkhalil A, Strand S, Mucker E, et al. Inhibition of monkeypox virus replication by RNA interference. Virol J. 2009;6:188.
