ABSTRACT
Mercury chloride is a common heavy metal found in the environment, and it endangers both the environment and living organisms. The study aimed to show whether Flavonoids Fractions of Adansonia digitata (FAD) could protect rats from HgCl2-induced hepatorena toxicity. Thirty (30) rats were randomly assigned to one of six groups. The first group was given no HgCl2 as a control, while the second group was given a single daily dose of HgCl2 (0.5 mg/kg). The treatment groups (III, IV, and V) received a single daily dose of HgCl2 (0.5 mg/kg) along with 25 mg/kg, 50 mg/kg, and 75 mg/kg of FAD, respectively. HgCl2 (0.5 mg/kg) was given to Group VI, along with Ascorbic Acid (200 mg/kg) as a standard control. After the administration, the blood serum of the experimental rats was used for biochemical analysis. The liver and kidney organs were obtained for histological examination. AST, ALP, ALT, urea, creatinine, and MDA levels all increased in rats given HgCl2 (group II), with decreased SOD, CAT, and GSH levels (p < 0.001), whereas FAD was able to prevent the upsurge of ALT, AST, ALP, creatinine, Urea, and MDA. It also increased SOD, CAT, and GSH levels in the body. FAD protected the glomerulus from degeneration and prevented histological liver steatosis.
Introduction
Heavy metals pollute the environment and are toxic to living organisms, and in their various forms, they exhibit distinct biological behavior, pharmacokinetics, and clinical manifestations [Citation1–3]. It is commonly found in the environment and is linked to severe health issues in mammals, and exposure to mercury chloride (HgCl2) is via products like batteries, pesticides, and paints [Citation1,Citation4,Citation5]. Mercury poisoning affects the nervous system, liver, kidney, and digestive system [Citation6]. It is primarily metabolized in the liver before accumulating in the kidneys. As a result, the liver and kidneys are the organs most affected [Citation7]. Mercury chloride poisoning has previously been shown to occur via several routes, including inhalation, ingestion, and skin absorption [Citation8]. Mercury chloride also degrades antioxidants and reduces free radical scavenging systems like superoxide dismutase (SOD), Catalase (CAT) and reduced glutathione (GSH) [Citation9–11]. The occurrence of lipid peroxidation is among the critical pathological factors in the sequence of events that leads to the onset of degenerative diseases due to disruptions in redox and calcium homeostasis [Citation12,Citation13].
Damage occurs in lipids, proteins, and DNA, which are the macromolecules that make up the cell due to oxidative stress induced by mercury chloride; thus, cell integrity is compromised [Citation14]. Previous experiment has shown that many plants are high in antioxidants and can help prevent or treat diseases caused by an oxidative-antioxidative system imbalance [Citation15]. Given that oxidative stress caused by environmental pollutants such as mercury can lead to liver and kidney poisoning, researchers believe that antioxidant compounds can protect against these pathological conditions [Citation16].
For many years, plants with medicinal properties have been used to treat a variety of acute and chronic ailments. However, the basis for such usage is based on the potential use of bioactive compounds to manage various chronic diseases such as inflammatory diseases, cancer, and cardiovascular abnormalities [Citation17]. Adansonia digitata has a variety of functions, one of which is the antioxidant activity by mitigating oxidative stress rise [Citation18]. Adansonia digitata is high in vitamin C, dietary fiber, calcium, potassium, procyanidins, kaempferol isomers, terpenoids, flavonoids, and thiamine [Citation19]. Adansonia digitata leaves and fruit are high in flavonoids, linked to cardioprotective, antioxidant, and hepatoprotective properties [Citation20,Citation21].
Because of their pharmacological properties, medicinal plants containing various substances such as alkaloids, glycosides, flavonoids, phenolic compounds, and cannabinoids have recently piqued the interest of researchers [Citation22]. Flavonoids are phenolic metabolites that occur naturally in all plant materials and are secondary plant metabolites [Citation23]. Flavonoids are famous for their anti-inflammatory, antiallergic, antiviral, antibacterial, antitumor, and antioxidant properties [Citation22,Citation24]. Flavonoids have various biological activities, including cell proliferation inhibition, enzyme inhibition, and antioxidant effects [Citation17]. According to previous studies, flavonoids have anti-atherosclerotic, anti-inflammatory, and antioxidant properties [Citation25]. This study set out to investigate the protective effects of flavonoid fractionate from Adansonia digitata fruit against hepatorenal damage caused by mercury chloride in rats.
Methods
Materials
Mercury chloride (BDH Chemicals Ltd, England) was used as a hepatorenal toxicity. Vitamin C (Ascorbic Acid; 70 mg/tablet) produced by Micro Labs Limited with NAFDAC number A4-6634 was obtained from a reputable pharmaceutical store (M.U.B Pharmaceutical Enterprises Ltd.) in Sabon Gari, Zaria, Kaduna state, and was used as a standard drug for Antioxidant. Phosphate Buffer Saline (PBS), 70% Ethanol (Sigma-Aldrich Co. LLC St. Louis, USA). The anaesthetic agent used in the study was ketamine hydrochloride (Sigma-Aldrich Co. LLC St. Louis, USA). Colourimetric diagnostic kits (Randox kits) for ALT, ALP, AST, urea, and creatinine were also used. Using laboratory diagnostic kits (Biodiagnostic Co., Cairo, Egypt), antioxidant enzyme activities (SOD, CAT, and GSH) and MDA content in blood serum were determined.
Plants collection and authentication
The fruit of Adansonia digitata L. was obtained from the Sabo market of Kaduna State’s Sabo Gari local government. The fruit was identified and authenticated using standard botanical monographs. The Botany Department, Faculty of Life Sciences, Ahmadu Bello University Zaria, Nigeria, confirmed them further. The plant’s specimen voucher number (2512).
Extraction
The dried fruit pulps were ground into a coarse powder, and the powdered sample was kept in an airtight container until needed. The fruit pulp of Adansonia Digitata L. was extracted with water in a soxhlet apparatus for 10 hours according to the Association of Official Analytical Chemists [Citation26] procedure. The methods of Won et al. [Citation27], was used whereby Adansonia digitata’s crude extract was dissolved in n-hexane, the insoluble residue was then suspended in distilled water, and diethyl ether was added to it. After that, n-butanol was used to partition the distilled water fraction. After that, the water portion was discarded. The first n-butanol fraction was obtained by treating the n-butanol fraction with 1% KOH and then separating it (saponins). To obtain the second n-butanol fraction, conc. HCl was added to the remaining 1% KOH portion, which was then partitioned with n-butanol until exhaustion (flavonoids). The final product used in this study was the crude Flavonoids fraction. A total of 6 kg of Adansonia digitata fruit were used. The flavonoids yielded a total of 1,020 mg.
Experimental animals
Thirty (30) Wistar rats of both sexes with weights ranging from 130 g to 220 g were purchased from the Human Anatomy Animal House at Ahmadu Bello University’s Faculty of Basic Medical Sciences in Zaria, Kaduna State, Nigeria. The animals were housed in the Department of Human Anatomy’s animal house and kept at room temperature in standard laboratory conditions. The animals were fed a standard feed grower mash diet and were given free access to water. The animals were given two weeks to acclimate before the administration began. The study was approved by the Ahmadu Bello University Zaria Animal Use and Care Committee (ABUCAUC/2018/088) and carried out in accordance with the ARRIVE guidelines.
Experimental procedure
Thirty (30) Wistar rats were divided into six groups (A-F), consisting of five rats. Group A served as control received distilled water (2 ml/kg); while Groups B, C, D, E and F were treatment groups. Group B – mercuric chloride treated rats (0.5 mg/kg BW per day in distilled water); Group C – Flavonoid fraction plus mercuric chloride (25 mg/kg BW + 0.5 mg/kg BW per day, respectively); Group D – Flavonoid fraction plus mercuric chloride (50 mg/kg BW + 0.5 mg/kg BW per day, respectively); Group E – Flavonoid fraction plus mercuric chloride (75 mg/kg bw + 0.5 mg/kg BW per day, respectively); Group F – Ascorbic Acid plus mercuric chloride (200 mg/kg BW + 0.5 mg/kg BW per day, respectively) for a period of 28 days. The mercury chloride doses were selected based on previous research [Citation28]. The route of mercury chloride administration was via intraperitoneal injection, while for the flavonoids and ascorbic acid was via oral gavage. Before and during the study, the experimental animals were weighed weekly.
Sampling procedure
Following ketamine anesthesia (75 mg/kg: intraperitoneally) at the end of the study, all rats were humanely sacrificed. Blood samples were taken from each rat in a plain tube, and the blood samples were spun at 3000 rpm for 5 minutes in a centrifuge machine to obtain the serum for the biochemical studies.
Biochemical studies
The activities of alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), urea, and creatinine in the serum were determined using commercially available kits and following the manufacturer’s instructions. Randox Laboratories Limited (United Kingdom) produced the ALT, AST, ALP, urea and creatinine test kits. The method described by Akanji et al. [Citation29] was used to measure lipid peroxidation. The method described by Aebi [Citation30] was used to determine CAT activity, and Fridovich’s [Citation31] method was used to determine SOD activity, while GSH concentration was determined according to the method described by Rajagopalan et al. [Citation32].
Histological studies
The rats were dissected after being euthanized; the liver and kidneys were removed and fixed in neutral buffered formalin before being dehydrated in graded alcohol, embedded in paraffin wax, cleared in xylene, sectioned at 5 m, and stained with hematoxylin-eosin (H & E) for general histology.
Statistical analysis
The data from these studies were analyzed using the Statistical Package for Social Science (SPSS) software, version 20 (IBM, USA), with one-way ANOVA and Turkey’s multiple comparison post hoc test, which compared the significance level between the control and test groups. All data were presented as mean SEM, with p < 0.05 considered significant.
Results
Liver function parameters
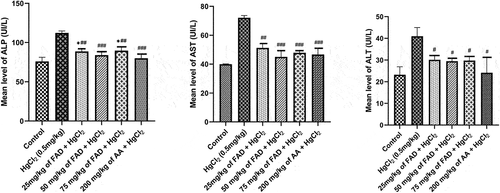
When HgCl2-treated rats were compared to control rats and FAD-treated rats a significant rise in ALT, ALP and AST was observed in the HgCl2-treated rats. ALT, ALP, and AST levels increased significantly (p < 0.0001). (See ). Treatment with FAD at all doses were found to be effective. ALP, ALT, and AST levels did not differ significantly (p > 0.05) between control and FAD-treated and Ascorbic Acid-treated rats ().
Figure 1. Bar charts of the liver enzymes parameters ((a) ALP, (b) AST, and (c) ALT) after 4 weeks of the experiment. Data were analyzed using one way ANOVA, followed with Tukey post hoc test. *P < 0.033; ** P < 0.002; *** P < 0.0001 indicates a significant difference when compared to normal control; #P < 0.03; ## P < 0.002; ### P < 0.0001, indicates a significant difference when compared to HgCl2 control group.
Parameters of kidney function
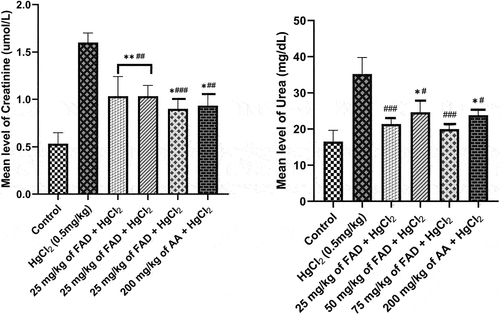
When compared to control and FAD/Ascorbic acid-treated rats, the serum levels of urea and creatinine in rats treated only with HgCl2 increased significantly (p < 0.0001) (). Between control and FAD-treated rats (all dosages) and Ascorbic Acid-treated rats (200 mg/kg), there was no significant difference in creatinine levels (p > 0.05). ().
Figure 2. Bar charts of kidney function parameters ((a) urea, and (b) creatinine) after 4 weeks of the experiment. Data were analyzed using one way ANOVA, followed with Tukey post hoc test. *P < 0.033; ** P < 0.002; *** P < 0.0001 indicates a significant difference when compared to normal control; #P < 0.03; ## P < 0.002; ### P < 0.0001, indicates a significant difference when compared to HgCl2 control group.
Oxidative stress biomarkers
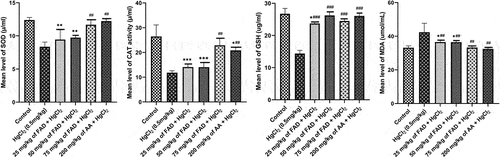
When HgCl2 control rats were compared to control and FAD-treated rats, the levels of catalase (CAT), superoxide dismutase (SOD), and reduced glutathione (GSH) in their serum significantly decreased (p < 0.0001) (). Treatment with FAD (75 mg/kg) was found to be more effective. SOD, CAT, and GSH levels did not differ significantly (p > 0.05) between control and FAD (500 mg/kg) treated rats (). In the case of MDA, rats cotreated with FAD (75 mg/kg) and ascorbic acid showed a significant decrease (p < 0.05) when compared to HgCl2 control rats.
Figure 3. Bar charts of Oxidative stress parameters ((a) SOD, (b) CAT, (c) GSH and (d) MDA) after 4 weeks of the experiment. Data were analyzed using one way ANOVA, followed with Tukey post hoc test. *P < 0.033; ** P < 0.002; *** P < 0.0001 indicates a significant difference when compared to normal control; #P < 0.03; ## P < 0.002; ### P < 0.0001, indicates a significant difference when compared to HgCl2 control group.
Histological study
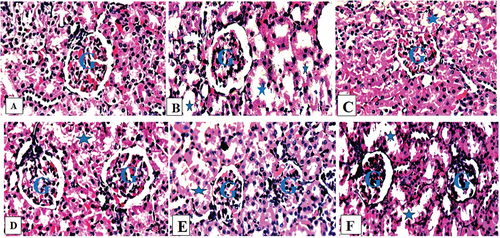
A photograph of the liver of a healthy control rat revealed normal hepatocytes, central veins, and sinusoids (). In their livers, rats exposed only to HgCl2 had degenerated hepatocytes, steatosis, and fat hepatocellular vacuoles (). The livers of rats given HgCl2 (5 mg/kg) and FAD (25, 50 and 75 mg/kg, respectively) showed mild steatosis and some microvesicular fatty droplet formation (). HgCl2 (5 mg/kg) and ascorbic acid (200 mg/kg) treatment caused mild inflamed in rats’ livers (). Normal control rats’ kidneys showed a typical histological structure, including normal renal tubules and glomeruli (). The kidneys of HgCl2-treated rats showed focal renal tubular degeneration (). The kidneys of rats given HgCl2 (5 mg/kg) + FAD at doses of 25, and 50 mg/kg, respectively, revealed a mildly obliterative form of a glomerulus (). FAD at 75 mg/kg-treated rats’ kidneys and Ascorbic acid-treated rats’ kidneys had a mildly normal glomerulus ().
Figure 4. Composite photomicrographs of liver of control (a) showing normal hepatocytes (arrow) and central vein (CV), HgCl2 treated micrograph (b) exhibited remarkable degenerating hepatocyte (green arrow), and fat hepatocellular vacuoles (Asterix). Extract 25 mg/kg + 0.5 mg/kg HgCl2 (c) showed mild inflamed hepatocyte (green arrow) and mild dilatation of the sinusoid (blue arrow), Extract 50/75 mg/kg + 0.5 mg/kg HgCl2 (d & e) showing normal hepatocyte (arrow), Ascorbic Acid treated group (f), showed mild dilatation of the sinusoid (blue arrow), and mild inflamed hepatocyte (green arrow).
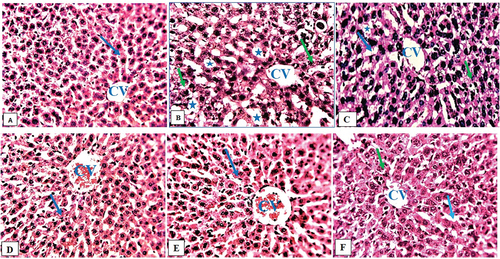
Figure 5. Composite photomicrographs of kidney of control (a) showing a normal glomerulus (G) and renal tubules, HgCl2 treated micrograph (b) exhibited degenerated renal tubules (Asterix), with a mild retracted glomerulus (G), Extract 25 mg/kg + 0.5 mg/kg HgCl2 (c) showed a mild obliterative form glomerular space (G) with mild degenerated renal tubule (Asterix), Extract 50/75 mg/kg + 0.5 mg/kg HgCl2 (d & e) showing very normal rental tubules (Asterix) and a normal glomerulus (G), Ascorbic Acid treated group (f), exhibited mild obliterative form of glomerulus (G).
Discussion
HgCl2 produces free radicals, which increases oxidative stress and causes nephrotoxicity and elevate hepatotoxicity [Citation33]. The adverse effects of HgCl2 could be prevented by flavonoids treatment, most likely due to their vigorous free radical scavenging activity, which protects cells from oxidation and necrosis [Citation34].
In this study, the kidney and liver functions of HgCl2-treated rats were negatively altered, resulting in hepatorenal degeneration as evidenced by a significant increase in ALP, ALT, and AST enzyme activities, as well as urea and creatinine levels. The upsurge of both liver and kidney function parameters by the HgCl2 explains the severity of hepatic and renal damage induced by the HgCl2 might be linked to the degree of intracellular and extracellular oxidative stress, which is caused by excessive free radical production combined with low antioxidant concentrations [Citation35]. The pathogenesis of various liver and kidney diseases has been linked to free radical-induced lipid peroxidative damage (Muhammad et al., 2016). Nabil et al. [Citation36] reported similar findings. Rats given HgCl2 and flavonoids showed significant improvements in ALP, ALT, and AST enzyme activities, as well as urea and creatinine levels, which could be attributed to the antioxidant activity of the flavonoid fractions from Adansonia digitata [Citation37].
HgCl2 significantly reduces the activities of the antioxidant enzymes SOD, CAT, and GSH in the current study, whereas the end-product of lipid peroxidation (MDA) was significantly increased in rats treated with only HgCl2. This could be due to HgCl2ʹs ability to initiate the formation of highly reactive substances such as oxidative stress, and as a result, lipid peroxidation increased while antioxidant enzyme activity decreased [Citation38]. Several experiments have produced similar results [Citation39,Citation40]. Coadministration of flavonoids fractions + HgCl2, on the other hand, revealed a significant modulation in the activities of SOD, CAT, and the levels of GSH and MDA toward normal. This could be due to Flavonoids’ ability to act as exogenous antioxidants by directly oxidizing radicals to form less reactive species through four mechanisms: inhibition of nitric-oxide synthase activity, inhibition of xanthine oxidase activity, modulation of channel pathways, and interaction with other enzyme systems [Citation34]. Furthermore, flavonoid metabolites have been linked to the induction of antioxidant defense mechanisms via antioxidant response elements (AREs), which induce the expression of antioxidant enzymes [Citation41]. Flavonoids react with the radical’s reactive compound to stabilize reactive oxygen species. Radicals are rendered inactive due to the high reactivity of the flavonoids’ hydroxyl group [Citation42].
The histopathological study revealed the architectural changes (liver and kidney) of the flavonoid’s fractions and HgCl2 administered rats (). As previously stated, HgCl2 causes oxidative stress in the liver and kidney, resulting in pathological changes [Citation43]. Mercury chloride transport in the kidney has been studied, and it has been discovered that it is taken up by proximal tubular cells [Citation44]. Also, in this study, rats exposed to only HgCl2 developed renal tubular damage, as well as vascular congestion and mild Bowman’s space enlargement. Flavonoid has been shown to preserve histological integrity in damaged renal tissue with necrosis in the renal parenchyma, and tubular dilatations [Citation45]. The PPARs appear to be promising targets for treating liver disease. Nuclear receptors known as PPARas are involved in mitigating liver inflammation by inhibiting NF-κB and reducing C-reactive protein expression [Citation46]. PPARα stimulation is expected to reduce steatosis by stimulating β-oxidation and reducing inflammation by inhibiting NF-kB [Citation47]. Flavonoids have been shown to stimulate PPAR in several studies [Citation48,Citation49]. Other studies have found that flavonoids increase the PPAR gene expression and protein expression [Citation50,Citation51]. Furthermore, Flavonoids have renoprotective effects [Citation52] by modulating the Nrf2 signaling pathway, which favors the translocation of the transcription factor Nrf2 to the nucleus, where it binds to AREs and activates the transcription of genes encoding phase II antioxidant and detoxifying enzymes [Citation53]. The vital histological findings of this study were that flavonoids treatment resulted in hepatic and renal architecture recovery.
Conclusion
In this study, flavonoids fractions of Adansonia digitata fruit reduces the toxic effect of HgCl2 on hepatorenal functions.
Limitations of the Study
The antioxidant potential of FAD fruit is thought to play a role in hepatorenal protection. However, at the conclusion of the experiments, this study did not estimate the level of gene expression disruption and the ultrastructure interference in mitochondria metabolism.
Authors’ contributions
All authors contributed to the conceptualization and design. Wusa Makena, Yomi Samson Aribiyun and Aisha Aminu provide study materials. Barka Ishaku, Wusa Makena and Yomi Samson Aribiyun collected the data. All authors contributed to the interpretation and analysis of the data. Wusa Makena, Ekwere Eke Inemesit, Ayuba Yohana and Aisha Aminu wrote the first draft of the manuscript. Wusa Makena, Ayuba Yohana, Aisha Aminu, Ekwere Eke Inemesit, and Barka Ishaku provided critical feedback on the manuscript. All authors gave their final approval to the manuscript.
Ethical considerations & guidelines
The Ahmadu Bello University, Zaria, Committee on Animal Use and Care has given its approval to all animal-related experimental protocols, with the number ABUCAUC/2018/088.
Acknowledgments
Mr Jigo Yaro, Chief Medical Laboratory Scientist, Department of Pathology, Ahmadu Bello University Teaching Hospital, and Mr Bamidele, Human Anatomy Department, deserve special thanks for taking the time to process the tissues.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Caglayan C, Kandemir FM, Darendelioğlu E, et al. Rutin ameliorates mercuric chloride-induced hepatotoxicity in rats via interfering with oxidative stress, Inflammation and apoptosis. J Trace Elem Med Biol. 2019;56:60–68.
- Kandemir FM, Yildirim S, Kucukler S, et al. Therapeutic efficacy of zingerone against vancomycin-induced oxidative stress, inflammation, apoptosis and aquaporin 1 permeability in rat kidney. Biomed Pharmacother. 2018;105:981–991. biopha.2018.06.048.
- Bernhoft RA. Mercury toxicity and treatment: a review of the literature. J Environ Pub Health. 2012. [ CrossRef] [PubMed.;2012. 1–10.
- Joshi D, Mittal DK, Shukla S, et al. N-acetyl cysteine and selenium protects mercuric chloride-induced oxidative stress and antioxidant defense system in liver and kidney of rats: a histopathological approach. J Trace Elem Med Biol. 2014;28(2):218–226.
- Venkatesan RS, Sadiq AM. Effect of morin-5′-sulfonic acid sodium salt on the expression of apoptosis related proteins caspase 3, Bax and Bcl 2 due to the mercury induced oxidative stress in albino rats. Biomed Pharmacother. 2017;85:202–208.
- Carranza-Torres IE, Viveros-Valdéz E, Guzmán-Delgado NE, et al. Protective effects of phenolic acids on mercury-induced DNA damage in precision-cut kidney slices. Ijbm. 2019;22(4):36775.
- Bridges CC, Zalups RK, Joshee L. Toxicological significance of renal Bcrp: another potential transporter in the elimination of mercuric ions from proximal tubular cells. Toxicol Appl Pharmacol. 2015;285(2):110–117.
- Goyer RA, Clarkson TW. Toxic effects of metals. In: Klaassen CD, editor. Casarett and Doull’s Toxicology: the basic science of poisons. 4th ed. New York: McGraw-Hill; 2001. p. 811–867.
- Mahboob M, Shireen KF, Atkinson A, et al. Lipid peroxidation and antioxidant enzyme activity in different organs of mice exposed to low level of mercury. J Environ Science Health B. 2001;36(5):687–689.
- Miller DM, Lund BO, Woods JS. Reactivity of Hg (II) with superoxide: evidence for the catalytic dismutation of superoxide by Hg (II). J Biochem Toxicol. 1991;6(4):293–298.
- Ibrahim ATA, Banaee M, Sureda A. Selenium protection against mercury toxicity on the male reproductive system of Clarias gariepinus. Comp Biochem Physiol Part - C: Toxicol Pharmaco. 2019;225:108583.
- Singh N, Abhishek K, Vivek KG, et al. Biochemical and molecular bases of lead-induced toxicity in mammalian systems and possible mitigations. Chem Res Toxicol. 2018;31(10):1009–1021.
- Chen F, Zhou CC, Yang Y, et al. GM1 ameliorates leadinduced cognitive deficits and brain damage through activating the SIRT1/CREB/BDNF pathway in the developing male rat Hippocampus. Biol Trace Elem Res. 2019;190(2):425–436.
- Aksu EH, Kandemir FM, Altun S, et al. Ameliorative effect of carvacrol on cisplatin-induced reproductive damage in male rats. Jbmt. 2016;30(10):513–520.
- Raeeszadeh M, Moradi M, Ayar P, et al. The antioxidant effect of Medicago sativa L. (Alfalfa) ethanolic extract against mercury chloride (HgCl2) toxicity in rat liver and kidney: an in vitro and in vivo study. Evid -Based Complementary Altern Med. 2021:10. Article ID 8388002. DOI:10.1155/2021/8388002.
- Li HD, Meng XM, Huang C, et al. Application of herbal traditional Chinese medicine in the treatment of acute kidney injury. Front Pharmacol. 2019;10:376.
- Yakubu OE, Nwodo OFC, Shaibu C, et al. In vitro determination of antioxidant activities of the fractions obtained from Adansonia Digitata L. (baobab) stem bark ethanolic extract using different parameters. Curr Trends Biomed Eng Biosci. 2019. DOI:10.19080/CTBEB.2019.17.555973.
- Makena W, Otong ES, Dibal NI, et al. Aqueous fruit pulp extract of Adansonia digitata (L) protects against lead-acetate-induced hepato-renal damage in rat model. Beni-Suef Univ J Basic Appl Sci. 2021;10(1):59.
- Braca A, Sinisgalli C, De Leo M, et al. Phytochemical profile, antioxidant and antidiabetic activities of Adansonia digitata L. (Baobab) from Mali, as a source of health-promoting compounds. Molecules. 2018;23(12):3104.
- Ghoneim MAM, Hassan AI, Mahmoud MG, et al. Protective effect of Adansonia digitata against isoproterenol-induced myocardial injury in rats. Anim Biotechnol. 2016;27(2):84–95.
- Tembo DT, Holmes MJ, Marshall LJ. Effect of thermal treatment and storage on bioactive compounds, organic acids and antioxidant activity of baobab fruit (Adansonia digitata) pulp from Malawi. J Food Compos Anal. 2017;58:40–51.
- Mohammad FE, Hasan WA, Mohamed EG. Natural antioxidant flavonoids in formalin-induced mice paw inflammation; inhibition of mitochondrial sorbitol dehydrogenase activity. J Biochem Mol. 2017;31(7):1–8.
- Kandemir FM, Caglayan C, Aksu EH, et al. Protective effect of rutin on mercury chloride-induced reproductive damage in male rats. Andrologia. 2019; 1–9. 10.1111/and.13524
- Celik H, Kucukler S, Comakli S, et al. Morin attenuates ifosfamide-induced neurotoxicity in rats via suppression of oxidative stress, neuroinflammation and neuronal apoptosis. Neurotoxicology. 2019;76:126–137.
- Cook NC, Samman S. Flavonoids-chemistry, metabolism, cardioprotective effects, and dietary resources. J Nutr Biochem. 1996;7(2):66–76.
- Association of Official Analytical Chemists (AOAC). Official methods of analysis XI Edition: Association of official analytical chemists. 11th ed. Washington D.C: The Association; 1970.
- Won WS, Shin KH, Kang SS. Chemistry and Pharmacology of Flavone –C- Glycoside from ziziphus seeds. Kor J Pharmacog. 1980;11(3–4):141–148.
- Salman MA, Kotb AM, Haridy AM, et al. Hepato- and nephroprotective effects of bradykinin potentiating factor from scorpion (Buthus occitanus) venom on mercuric chloride-treated rats. EXCLI J. 2016;15:807–816.
- Akanji MA, Adeyemi OS, Oguntoye SO, et al. Psidium guajava extract reduces trypanosomosis associated lipid peroxidation and raises glutathione concentrations in infected animals. EXCLI J. 2009;8:148–154.
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. PMID:6727660.
- Fridovich I. Superoxide dismutases; An adaptation to a paramagnetic gas. J Biol Chem. 1989;264(14):7761–7764. PMID:2542241.
- Rukkumani R, Aruna K, Varma PS, et al. Comparative effects of curcumin and an analog of curcumin on alcohol and PUFA induced oxidative stress. J Pharm Pharm. 2004;7(2):274–283. PMID:15367386.
- Deng Y, Xu Z, Liu W, et al. Effects of lycopene and proanthocyanidins on hepatotoxicity induced by mercuric chloride in rats. Biol Trace Elem Res. 2012;146(2):213–223.
- Kumar S, Pandey AK. Chemistry and biological activities of flavonoids: an overview. Sci World J.2013;2013:1–16.
- Godoy P, Hewitt NJ, Albrecht U, et al. Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Arch Toxicol. 2013;87(8):1315–1530.
- Nabil A, Elshemy MM, Asem M, et al. Protective effect of DPPD on mercury chloride-induced hepatorenal toxicity in rats. J Toxicol. 2020:7. 10.1155/2020/4127284. Article ID 4127284.
- Sokeng AJT, Sobolevb AP, Lorenzo AD, et al. Metabolite characterization of powdered fruits and leaves from Adansonia digitata L. (baobab): a multi-methodological approach. Food Chem. 2019;272:93–108.
- Ahmad S, Mahmood R. Mercury chloride toxicity in human erythrocytes: enhanced generation of ROS and RNS, hemoglobin oxidation, impaired antioxidant power, and inhibition of plasma membrane redox system. Environmental Science and Pollution Research International. 2019;26(6):5645–5657.
- Aslanturk A, Uzunhisarcikli M, Kalender S, et al. Sodium selenite and vitamin E in preventing mercuric chloride induced renal toxicity in rats. Food Chem Toxicol. 2014;70:185–190.
- Joshi D, Mittal DK, Shukla S, et al. Curcuma longa Linn. extract and curcumin protect CYP 2E1 enzymatic activity against mercuric chloride-induced hepatotoxicity and oxidative stress: a protective approach. Exp Toxicol Pathol. 2017;69(6):373–382.
- Andreucci M, Faga T, Pisani A, et al. Quercetin protects against radiocontrast medium toxicity in human renal proximal tubular cells. J Cell Physiol. 2018;233(5):4116–4125. CrossRef] [PubMed.
- Korkina LG, Afanas’ev IB. Antioxidant and chelating properties of flavonoids. Adv Pharmacol. 1997;38:151–163.
- Bridges CC, Zalups RK. Mechanisms involved in the transport of mercuric ions in target tissues. Archives of Toxicology. 2017;91(1):63–81.
- Ma Y, Shi Y, Li L, et al. Toxicological effects of mercury chloride on laying performance, egg quality, serum biochemistry, and histopathology of liver and kidney in laying hens. Biol Trace Elem Res. 2018;185(2):465–474.
- Vargas-Mendoza N, Madrigal-Santillan E, Morales-Gonzalez A, et al. Hepatoprotective effect of silymarin. World J Hepatol. 2014;6(3):144–149. CrossRef] [PubMed.
- Tailleux A, Wouters K, Staels B, et al. Roles of PPARs in NAFLD: potential therapeutic targets. Biochim Biophys Acta. 2012;1821(5):809–818.
- Van De Wier B, Koek GH, Bast A, et al. Haenen the potential of flavonoids in the treatment of non-alcoholic fatty liver disease. Crit Rev Food Sci Nutr. 2017;57(4):834–855.
- Jia Y, Kim JY, Jun H-J, et al. Cyanidin is an agonistic ligand for peroxisome proliferator-activated receptor-alpha reducing hepatic lipid. Biochim Biophys Acta. 2013;1831(4):698–708.
- Malek MA, Hoang MH, Jia Y, et al. Ombuin-3-O-beta-D-glucopyranoside from Gynostemma pentaphyllum is a dual agonistic ligand of peroxisome proliferator-activated receptors alpha and delta/beta. Biochem Biophys Res Commun. 2013;430(4):1322–1328.
- Cho KW, Kim YO, Andrade JE, et al. Dietary naringenin increases hepatic peroxisome proliferators-activated receptor alpha protein expression and decreases plasma triglyceride and adiposity in rats. Eur J Nutr. 2011;50(2):81–88.
- Goto T, Teraminami A, Lee J-Y, et al. Tiliroside, a glycosidic flavonoid, ameliorates obesity-induced metabolic disorders via activation of adiponectin signaling followed by enhancement of fatty acid oxidation in liver and skeletal muscle in obese–diabetic mice. J Nutr Biochem. 2012;23(7):768–776.
- Wang W, Ma B-L, Xu C-G, et al. Dihydroquercetin protects against renal fibrosis by activating the Nrf2 pathway. Phytomedicine. 2020;69:153185.
- Ruiz S, Pergola PE, Zager RA, et al. Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int. 2013;83(6):1029–1041. CrossRef.
