 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
There is increasing evidence showing distinct neurocognitive underpinnings of different deficits of written language processing. This study investigated whether functional brain mechanisms related to isolated spelling problems can be distinguished from those observed for the combined profile of reading and spelling deficits (dyslexia). Two cognitive accounts explaining isolated spelling deficits were tested. Twenty-two children with typical development, 16 children with isolated spelling deficits and 20 children with dyslexia performed a reading-aloud task with words and pseudohomophones during fMRI. The whole-brain analysis for each condition revealed differential patterns of brain activity in the two deficit groups. No reliable differences between typically developing children and children with isolated spelling deficits could be observed in critical regions of the brain’s reading network. Children with dyslexia showed lower brain activity and reduced word-pseudohomophone effects in these regions. Our findings suggest that children with isolated spelling deficits can rely on (degraded) orthographic representations during reading.
Introduction
The present study investigates whether children with isolated spelling deficits and children with dyslexia display distinct patterns of alterations of functional brain activity compared to typically developing peers in brain regions related to phonological and orthographic processing. There is extensive research on neurofunctional alterations related to dyslexia, a learning disorder typically characterised by co-occurrent reading and spelling deficits. However, not all children with deficient development in written language processing have problems in reading as well as spelling. Dissociations between reading fluency and spelling deficits are actually just as prevalent as dyslexia (Landerl & Moll, Citation2010; Moll et al., Citation2014). Isolated spelling deficits (SpD) manifest with concurrent age-adequate reading skills and are associated with partly distinct cognitive profiles compared to dyslexia. A consistent finding is that the reading fluency problems experienced by children with dyslexia in transparent orthographies are related to visual-verbal processing deficits, as measured by rapid naming tasks requiring children to name aloud a series of elements as for example objects, colours, letters or digits as accurately and quickly as possible. In contrast, visual-verbal processing deficits are not usually observed in children with SpD (Bar-Kochva & Amiel, Citation2016; Gangl, Moll, Banfi, et al., Citation2018; Gangl, Moll, Jones, et al., Citation2018; Manolitsis & Georgiou, Citation2015; Moll & Landerl, Citation2009; Torppa et al., Citation2017; Wimmer & Mayringer, Citation2002). Evidence on phonological processing deficits (indicating problems in the manipulation of phonemes) is less straightforward, as some studies reported them not only for dyslexia, but also for SpD (Bar-Kochva & Amiel, Citation2016; Fayol et al., Citation2009; Torppa et al., Citation2017; Wimmer & Mayringer, Citation2002), while others found only mild or no phonological impairments (Gangl, Moll, Banfi, et al., Citation2018; Manolitsis & Georgiou, Citation2015; Moll & Landerl, Citation2009). Inconsistencies among studies are most likely due to the different age of participants, different task formats and different orthographies. It is therefore crucial to study brain function in SpD as compared to dyslexia to expand our understanding of the neural mechanisms of written language processing and to characterise these disorders not only at the behavioural and cognitive level, but also with respect to neurobiological function.
Competing cognitive hypotheses about isolated spelling deficits
According to Frith (Citation1980), children with isolated spelling deficits read by “partial cue” (partial cue hypothesis). This means that they possess underspecified orthographic representations, which suffice for reading (recognition) but not for spelling (production). This partial cue reading style is supposed to derive from sublexical decoding difficulties of phonological origin that impair the formation of accurate orthographic representations. In other words, children with isolated spelling deficits do not decode words correctly and, as a consequence, cannot build-up precise orthographic representations as would be predicted by the self-teaching hypothesis (Share, Citation1995). Briefly, the self-teaching hypothesis claims that successful decoding of an unfamiliar word provides an opportunity to acquire word-specific knowledge related to that word. Self-teaching represents a crucial mechanism by which children independently develop an orthographic lexicon essential for lexical reading strategies and spelling, with only a few exposures to the same novel word.
Research in German orthography which has phonologically transparent letter-sound correspondences did not seem to support this view as no sublexical deficits were found for children with isolated spelling deficits (Gangl, Moll, Banfi, et al., Citation2018; Moll & Landerl, Citation2009). Nevertheless, a longitudinal study revealed phonological awareness and phonological memory deficits at the beginning of 1st grade in children who later on developed SpD (Wimmer & Mayringer, Citation2002). Comparably severe phonological deficits were not observed in older German-speaking children in Grade 3 and 4, possibly because phonics teaching and extensive experience with the transparent German grapheme-phoneme system helped children to overcome early deficits (Gangl, Moll, Banfi, et al., Citation2018; Moll & Landerl, Citation2009).
Moll and Landerl (Citation2009) suggested an alternative account to explain orthographic processing deficits in SpD (overreliance hypothesis). They asked children to read aloud words and pseudohomophones (grapheme sequences whose pronunciation corresponds to existing words, e.g. rane for rain in English). While familiar words are associated with orthographic representations in long-term memory, pseudohomophones are not stored in the orthographic lexicon. They must thus be read sublexically, yielding higher reading times than their corresponding words (word-pseudohomophone effect). Moll and Landerl observed a reliable word-pseudohomophone effect in typical readers and spellers, but not in children with SpD. The fact that SpD children responded to pseudohomophones as quickly as to words was taken as an indication for over-reliance on extremely efficient sublexical procedures that allow them to compensate for deficient orthographic knowledge during word reading. According to Moll and Landerl (Citation2009), orthographic processing deficits in SpD may rather be due to malfunctioning of self-teaching mechanisms (Share, Citation1995), which would in turn prevent the build-up of a broad orthographic lexicon necessary for correct spelling and lexical reading. Having problems in forming orthographic representations, children with SpD tend to over-rely on decoding strategies as a form of compensation. This is counterproductive because it further prevents the formation of new orthographic representations.
The lack of a word-pseudohomophone effect in SpD was replicated in an event-related potentials (ERPs) study asking children to decide whether or not a letter string (either a word, a pseudohomophone or a pseudoword) sounded like a real word (phonological lexical decision; Bakos et al., Citation2018). However, evidence on this overreliance hypothesis is mixed as other studies using eye-tracking and ERPs (Gangl, Moll, Banfi, et al., Citation2018; Kemény et al., Citation2018) did not replicate the finding of absent word-pseudohomophone effects in children with isolated spelling deficits. In contrast, they did find evidence for lexico-orthographic processing during reading tasks.
To sum up, two contrasting accounts to explain isolated spelling deficits have been put forward: (1) the partial cue hypothesis (Frith, Citation1980) predicts that isolated spelling deficits derive from sublexical deficits in phonological decoding; (2) the overreliance account (Moll & Landerl, Citation2009) suggests that children with isolated spelling deficits over-rely on highly efficient decoding skills to overcome their lexical impairment. So far, evidence on both accounts is mixed. In the current study, we aim to contribute to this discussion by testing the word-pseudohomophone effect in an fMRI experiment.
Neural network for reading and spelling
The ability to process written language involves the interplay of multiple brain regions for visual, language and motor processing (Dehaene et al., Citation2015). Two main streams within the left hemisphere have been described for reading-related neural processing (Oliver et al., Citation2017; Paz-Alonso et al., Citation2018; Price, Citation2012; Pugh et al., Citation2001; Schlaggar & McCandliss, Citation2007). The sublexical dorsal stream bonds left parietal and superior temporal regions with the posterior inferior frontal gyrus (IFG) “pars opercularis” (BA 44), enabling phonological decoding procedures based on grapheme-phoneme conversion rules. The lexical ventral stream connects the left ventral occipito-temporal (vOT) cortex with the anterior IFG “pars triangularis” and “pars orbitalis” (BA 45 and BA47, respectively) and supports lexico-orthographic processing. The ventral stream includes also the so-called visual word form area (VWFA) located in the ventral occipito-temporal cortex (McCandliss et al., Citation2003). This region shows increasing specialisation for written words over the course of literacy acquisition (Dehaene et al., Citation2012) and tunes for orthographic processing via sublexical as well as lexico-orthographic mechanisms (Kronbichler et al., Citation2007; Schurz et al., Citation2010; Zhao et al., Citation2017).
Functional alterations in dyslexia and SpD
Meta-analytical evidence (Martin et al., Citation2016; Paulesu et al., Citation2014; Richlan et al., Citation2009, Citation2011) consistently reported functional alterations in dyslexia in the reading network, including temporo-parietal regions, vOT and IFG. Findings of altered brain activity in the dorsal and ventral streams are consistent with the notion that both sublexical and lexical procedures are impaired in dyslexia (Hawelka et al., Citation2010).
Literature addressing the functional correlates of isolated spelling impairment is much more limited than that on dyslexia and findings are rather heterogeneous due to variable age ranges, orthographies and functional paradigms adopted in different studies (Borowska et al., Citation2014; Gebauer et al., Citation2012; Guardia-Olmos et al., Citation2017; Richards et al., Citation2009). Of special interest is the study by Gebauer et al. (Citation2012), which addressed functional activity related to orthographic processing in German-speaking children, relying on a similar sample as in the present study. Using an orthographic lexical decision task, Gebauer et al. reported enhanced activity of right fronto-parietal areas in children with SpD compared to the typical group and children with dyslexia. Furthermore, they reported lower activity in a left ventral occipito-temporal cluster in SpD and dyslexia as compared to the typical group. Although preliminary and based on small groups of 9–11 children only, these findings represent evidence in favour of the overreliance hypothesis by Moll and Landerl (Citation2009). It will be important to consider whether the results of the present study can be reconciled with those by Gebauer et al. (Citation2012).
The present study
The current fMRI study tests two cognitive accounts of isolated spelling deficits, which make divergent predictions concerning the sublexical dorsal stream (see also ):
(H1) According to the partial cue hypothesis (Frith, Citation1980), we predicted reduced functional activity especially for pseudohomophones in SpD compared to the typical group in the sublexical dorsal stream. Pseudohomophones are expected to yield higher functional activity than words within this stream (Kronbichler et al., Citation2007; Van der Mark et al., Citation2009). A selective reduction of functional activity for pseudohomophones should thus induce a smaller difference between words and pseudohomophones in SpD as compared to the typical group.
(H2) Based on the overreliance hypothesis (Moll & Landerl, Citation2009), no reliable difference in functional activity should be observed between the two conditions in the dorsal stream, as children are expected to use the same (sublexical) strategy to read words and pseudohomophones.
Figure 1. Competing hypotheses of expected functional activity in the dorsal stream in the two deficit groups compared to typically developing children.
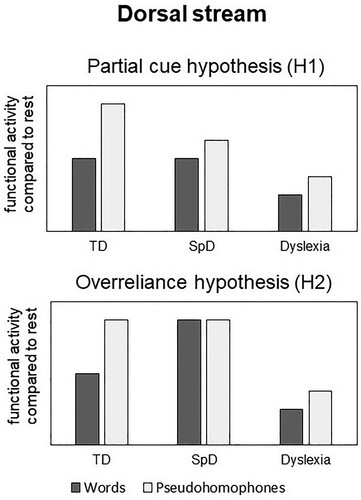
We further predicted lower activity in the ventral stream especially related to words in children with isolated spelling deficits. This would represent the neural correlate of their impaired orthographic knowledge and would be consistent with both cognitive accounts mentioned above.
To differentiate neural correlates selectively associated with spelling problems from those related to the combined profile with reading and spelling deficits, children with dyslexia were included in this study. They were expected to show reduced activity compared to typical readers and spellers in both dorsal and ventral regions of the reading network, due to their established sublexical and lexical weaknesses. Previous studies clearly showed that even children with dyslexia are able to engage in lexical reading strategies (Gangl, Moll, Banfi, et al., Citation2018; Paizi et al., Citation2013), we thus expected to observe reliable word-pseudohomophone effects, although such effects might be weaker than in good readers.
We used a reading aloud paradigm, which is more ecologically valid than lexical decision tasks for the study of written language processing, because reading-related brain activity can be accessed without any confound due to decisional load (Braun et al., Citation2019; Hawelka et al., Citation2010).
Method
Participants
The study was performed in accordance with the latest version of the Declaration of Helsinki and in compliance with national legislation. It was approved by the ethics committee of the University of Graz (Austria). Written informed consent was obtained on behalf of the children from their parents.
Participants were selected based on an extensive classroom screening with 2562 children at the end of 3rd or beginning of 4th Grade. Standardised classroom tests of sentence reading fluency (Wimmer & Mayringer, Citation2014) and spelling (Müller, Citation2004) as well as an individually administered standardised one-minute word and pseudoword reading fluency test (Moll & Landerl, Citation2010) were carried out in school. From this large sample, we selected three groups: Children with isolated spelling disorder (SpD) had spelling performance at or below percentile 20 and age-adequate reading, i.e. percentile ≥ 25 on the mean of the three reading measures. Children with dyslexia had percentile 20 or lower on two reading measures (and not higher than 43 on the third reading measure). Children with typical reading and spelling skills had percentiles between 25 and 85 on the mean of the three reading measures (sentence, word and pseudoword reading) and on spelling.
All children had German as their first language, a non-verbal IQ ≥ 85 (Weiß, Citation2006), normal or corrected-to-normal vision, no identified sensory or neurological deficits, no clinical ADHD diagnosis as well as an above-threshold score on a standardised parental questionnaire for attention deficits (Döpfner et al., Citation2008). Children were also given the Vocabulary, Digit Span and Symbol Search subtests of the Wechsler Intelligence Scale for Children (Petermann & Petermann, Citation2011). They performed a phoneme deletion task developed in our laboratory and standard paradigms of RAN-objects and RAN-digits (Denckla & Rudel, Citation1976). A full description of the literacy and cognitive measures is provided in Banfi et al. (Citation2019).
Altogether 71 children were assessed. As the Austrian school system neither undertakes nor recognises formal diagnoses of specific learning disorders, we could not rely on clinical diagnoses of a reading or spelling disorder. Note, however, that most children in the two deficit groups exhibited serious deficits. Fourteen out of the 21 children in the SpD group displayed poor spelling performance (percentile ≤ 16) and age-adequate reading (percentile ≥ 29 on the mean of the three reading measures). Among the remaining 7 children in this group, 5 children had a spelling percentile of 17 and 2 had a percentile of 20. Importantly, these children also showed a very clear discrepancy between reading and spelling, with mean reading percentile ≥ 30. Twenty out of the 23 children in the dyslexia group had serious reading problems, with percentile ≤ 16 on two reading measures (and below average performance with a percentile ≤ 34 on the third reading measure). Of the three further children in the dyslexia group, two of them had a percentile of only 11 on one reading subtest and ≤ 20 on the two others, one child had percentile 16 in one reading subtest and ≤ 18 on the two others, also indicating serious problems with reading. According to the German diagnostic guidelines (Galuschka & Schulte-Körne, Citation2016), about 70% of children in the SpD sample and 90% in the dyslexia sample fulfilled the criteria for a clinical diagnosis.
Twelve children were excluded from the analysis due to excessive movement during the fMRI session (see further sections for details). One additional participant was not considered in the analysis because inspection of the EPI image revealed that the field of view (FOV) had been wrongly defined, thus cutting a relevant part of the frontal lobe. Because of drop out during the last behavioural assessment, data on RAN were not available for two children with dyslexia. The final sample included 22 children with typical reading and spelling skills, 16 children with SpD and 20 children with dyslexia.
reports descriptive statistics for literacy and cognitive measures.
Table 1. Mean scores (M) and standard deviations (SD) for descriptive, literacy, and cognitive measures in the three groups.
The three groups did not differ with respect to gender χ2(2) = 1.11, p = .574 and handedness χ2(2) = .05, p = .977. As expected on the basis of our screening procedure, children with dyslexia showed lower performance than the typical and SpD groups in sentence, word and pseudoword reading, whereas the SpD group had age-adequate reading skills, which did not differ from the typical group. Both deficit groups showed clearly lower spelling percentiles than typical readers and spellers. Importantly, they did not differ in the severity of their spelling impairment. There were no significant group differences on nonverbal IQ and all WISC subtests (Vocabulary, Digit Span and Processing Speed). Children with dyslexia showed the typical profile of impairment in both PA and RAN (Moll & Landerl, Citation2009). The SpD group showed a trend for lower scores than the typical group on the PA task, p = .064. Although participants with ADHD-scores above a critical threshold were not admitted to the study, children in the SpD group displayed higher ADHD scores (indicating more ADHD-symptoms reported by parents) compared to typical readers and spellers, p = .010. In addition, the group with dyslexia showed a trend for a higher ADHD score that the TD group, p = .058.
Experimental stimuli
Stimuli consisted of 60 words and 60 matched pseudohomophones of three to eight letters. Pseudohomophones were built from the base word by exchanging one phonologically identical grapheme (for example the pseudohomophone “ahrm” was generated from the word “arm”, an equivalent English example would be the pseudohomophone “taksi” from the word “taxi” [Kronbichler et al., Citation2007]). Stimuli were matched on the number of letters and bigram frequency according to the childLex database (Schroeder et al., Citation2015). The list of stimuli included in the in-scanner task is provided in the appendix.
Procedure
As shown in , each stimulus was presented alone in white on a black background for 3 s in an event-related design.
Figure 2. Example of the in-scanner task. Children were instructed to read aloud German words (as for example “Familie” [family]) and pseudohomophones (as for example “Fater”, which sounds as the German word “Vater” [father]).
![Figure 2. Example of the in-scanner task. Children were instructed to read aloud German words (as for example “Familie” [family]) and pseudohomophones (as for example “Fater”, which sounds as the German word “Vater” [father]).](/cms/asset/f04756ab-a0d1-476a-8535-69e8cbe58fc6/plcp_a_1859569_f0002_ob.jpg)
After it disappeared, a fixation cross was displayed. The presentation of the fixation cross was jittered between 2000 and 6000 ms (M = 4000 ms). To prevent fatigue effects in children, stimuli were presented over three consecutive runs of 40 items each, separated by short breaks of 3–5 min. Words and pseudohomophones were shown in a pseudorandomized fashion with the restriction that no more than two items of the same condition (words or pseudohomophones) appear in immediate succession. Furthermore, each word or pseudohomophone was shown at least 30 items after its paired stimulus had been presented. Two equivalent pseudorandomized versions of the task were built and participants were randomly assigned to one of them.
Children were instructed to read aloud the stimuli appearing on the screen. In order to keep children calm and reassured, an experimenter sat in the scanner next to each child. Before the fMRI assessment began, they were familiarised with the task in a silent room, to ensure that they understood the instructions.
FMRI data acquisition
Imaging was performed on a 3.0 T Skyra scanner (Siemens Healthineers, Erlangen, Germany) using a 20-channel head coil. High-resolution 3D-T1 MPRAGE structural scans (TR = 1600 ms, TE = 1,81 ms, FOV = 224 mm, flip angle = 8 degrees, 176 slices, voxel resolution 1 × 1 × 1 mm3) and BOLD-sensitive T2*-weighted functional images were acquired using a single shot gradient-echo EPI pulse sequence (TR = 2340 ms, TE = 33 ms; FOV = 192 mm, flip angle = 90 degrees, 34 slices with 0.3 mm gap, voxel resolution 3 × 3 × 3 mm3, descending acquisition order). Head motion was restricted using firm padding that surrounded the head. Verbal responses of participants were recorded via an MR compatible microphone (FOMRI-III, OptoacousticsLtd., Moshav Mazor, Israel). Stimuli were presented using the Software Presentation (Neurobehavioral Systems, Albany, CA).
FMRI data analysis
FMRI data were preprocessed with the automated pipeline fMRIPrep 1.3.2 (Esteban et al. Citation2018, Citation2019; RRID:SCR_016216), which is based on Nipype 1.1.9 (Gorgolewski et al. (Citation2011, Citation2018); RRID:SCR_002502).
Anatomical data preprocessing
The T1-weighted (T1w) image was corrected for intensity non-uniformity (INU) with N4BiasFieldCorrection (Tustison et al. Citation2010), distributed with ANTs 2.2.0 (Avants et al. Citation2008, RRID:SCR_004757), and used as T1w-reference throughout the workflow. The T1w-reference was then skull-stripped with a Nipype implementation of the antsBrainExtraction.sh workflow (from ANTs), using OASIS30ANTs as target template. Spatial normalisation to the ICBM 152 Nonlinear Asymmetrical template version 2009c (Fonov et al. Citation2009, RRID:SCR_008796) was performed through nonlinear registration with antsRegistration (ANTs 2.2.0), using brain-extracted versions of both T1w volume and template. Brain tissue segmentation of cerebrospinal fluid (CSF), white-matter (WM) and gray-matter (GM) was performed on the brain-extracted T1w using fast (FSL 5.0.9, RRID:SCR_002823, Zhang et al., Citation2001).
Functional data preprocessing
For each of the 3 BOLD runs available per subject, the following preprocessing was performed. First, a reference volume and its skull-stripped version were generated using a custom methodology of fMRIPrep. The BOLD reference was then co-registered to the T1w reference using flirt (FSL 5.0.9, Jenkinson & Smith, Citation2001) with the boundary-based registration (Greve & Fischl, Citation2009) cost-function. Co-registration was configured with nine degrees of freedom to account for distortions remaining in the BOLD reference. Head-motion parameters with respect to the BOLD reference (transformation matrices, and six corresponding rotation and translation parameters) were estimated before any spatiotemporal filtering using mcflirt (FSL 5.0.9, Jenkinson et al. Citation2002). BOLD runs were slice-time corrected using 3dTshift from AFNI 20160207 (Cox & Hyde, Citation1997, RRID:SCR_005927). The BOLD time-series (including slice-timing correction when applied) were resampled onto their original, native space by applying a single, composite transform to correct for head-motion and susceptibility distortions. The BOLD time-series were resampled to MNI152NLin2009cAsym standard space, generating a preprocessed BOLD run in MNI152NLin2009cAsym space. Several confounding time-series were calculated based on the preprocessed BOLD: framewise displacement (FD), DVARS and three region-wise global signals. FD and DVARS were calculated for each functional run, both using their implementations in Nipype (following the definitions by Power et al. Citation2014). The three global signals were extracted within the CSF, the WM, and the whole-brain masks. Additionally, the algorithm extracted a set of physiological regressors to allow for component-based noise correction (CompCor, Behzadi et al. Citation2007). Gridded (volumetric) resamplings were performed using antsApplyTransforms (ANTs), configured with Lanczos interpolation to minimise the smoothing effects of other kernels (Lanczos, Citation1964).
Finally, functional images were smoothed with a Gaussian kernel of 8 mm using SPM12 software (v6906; Wellcome Department of Imaging Neuroscience, London, UK), which ran in a MATLAB 2016a environment (MathWorks Inc., Natick, MA).
Participants with framewise displacement (FD) > 1 mm in two out of three runs were excluded from the analysis due to excessive motion. This criterion is in line with the one adopted by (Siegel et al., Citation2014) on children’s data. Moreover, it is based on the notion that sub-millimeter movements do not systematically affect group-wise results of task fMRI studies (Greene et al., Citation2016).
First level analyses were run in SPM12 by computing linear t-contrasts between the experimental conditions “word vs. fixation” and “pseudohomophone vs. fixation” for each participant. Variance from the following parameters was regressed out for each participant: white matter signal, framewise displacement, translation and rotation values over three dimensions (x, y, z). Note that one child in the dyslexia group had log files for only two out of three runs available, due to a technical problem during data acquisition.
Second level analyses were performed with FlexFast2 (https://habs.mgh.harvard.edu/researchers/data-tools/glm-flex-fast2/). This method enables to model inter-subject variability within conditions as a random factor, thus increasing the amount of signal deriving from the two relevant experimental factors “group” and “condition”. We tested the model Group*Condition + random(SS|Condition), where group (3 levels: TD, SpD, dyslexia) and condition (2 levels: words and pseudohomophones) were entered as fixed factors and subject (SS) as random factor. As groups did not differ significantly in mean framewise displacement over the three runs, F(2, 55) = 2.39, p = .101, motion was not entered as a covariate in second level analyses.
FlexFast2 returned a T-map for each group comparison for each condition. We further produced a T-map with voxels that showed significant differences between the conditions “word” and “pseudohomophone”. This allowed us to identify regions where brain activity was modulated by the comparison of words vs. pseudohomophones in the whole sample of 58 participants. FlexFast2 results underwent family-wise error (FWE) correction (p < .05) at the voxel level.
A region-of-interest (ROI) analysis was conducted with the REX toolbox implemented in SPM12 (Duff et al., Citation2007). Based on neuroimaging literature for written language processing (Gebauer et al., Citation2012; Martin et al., Citation2015; Richlan, Citation2012; Hancock et al., Citation2017), relevant functional ROIs were selected from the T-map “words vs. pseudohomophones”. Contrast estimates were extracted for each participant, separately for each experimental condition.
The inferior frontal cluster in the T-map was very large and presented three different peaks. Therefore, based on this finding and on literature indicating differential contribution of the anterior and posterior IFG to lexico-orthographic and phonological processing, respectively (Oliver et al., Citation2017), three additional ROIs were manually delineated as spheres of 10 mm radius, centred on the coordinates of each of the three peaks.
Group comparisons of contrast estimates derived for each ROI in each experimental condition were analysed with IBM SPSS Statistics (Version 25) predictive analytics software.
Results
Reading performance
Reading accuracy during the in-scanner task was clearly at ceiling for both string categories in all three groups, as shown in .
Table 2. Accuracy in the in-scanner reading aloud task for the three groups, presented separately for each condition.
Note that the distribution of reading accuracy was negatively skewed for both words and pseudohomophones and also not normally distributed, D(58) ≥ .216, p < .001. Therefore, non-parametric tests were conducted and Bonferroni correction was applied for post-hoc tests. Pseudohomophones yielded slightly lower accuracy than words, (Mdiff = 1.10), z = 4.71, p < .001. There were significant group differences for each string type, χ2(2, N = 58) = 27.64, p < .001 for words, χ2(2, N = 58) = 22.00, p < .001 for pseudohomophones. On word stimuli, the dyslexia group scored significantly lower than the other two groups, p ≤ .001, whereas TD and SpD children did not differ from each other, p = .788. On pseudohomophones, children with dyslexia were significantly less accurate than TD children, p < .001 and showed a trend for lower accuracy compared to the SpD group, p = .078. Again, the TD and SpD groups did not differ significantly from each other, p = .097.
Whole-brain results
Conditions contrasted against fixation
Group comparisons were conducted separately for each condition (words vs. fixation and pseudohomophones vs. fixation), whole-brain results are shown in and . A consistent pattern of functional group differences can be observed for both experimental conditions, with generally more pronounced differences for pseudohomophones. In both conditions, children in the SpD group showed reduced brain activity compared to TD in medial frontal and cingulate cortices, bilateral middle, inferior temporal regions and the fusiform gyri. Regions of increased activity in the SpD group compared to the TD group were mostly located bilaterally in medial and superior temporal, frontal, pre- and postcentral clusters and the cerebellum. Children with dyslexia manifested lower activity than TD in fronto-parietal clusters around the central fissure, in inferior frontal, superior temporal regions and in the cerebellum. Brain areas of increased activity compared to the TD group were located in inferior frontal, occipito-temporal and medial temporal regions, including the hippocampus. The two deficit groups differed also among each other, with SpD children displaying higher activity than the group with dyslexia in precentral and postcentral areas, as well as in superior temporal and superior frontal regions and (only for pseudohomophones) in the inferior frontal, lingual gyri and the cerebellum. Children with dyslexia in turn manifested higher activity than the SpD group in large fronto-temporal and occipito-temporal clusters, including the inferior frontal, superior and inferior temporal, inferior occipital gyri, and the medial temporal lobe.
Table 3. Results of group comparisons for the condition “words” displayed with FWE correction at the voxel level, p < .05. Clusters ≥ 10 voxels are reported.
Table 4. Results of group comparisons for the condition “pseudohomophones” displayed with FWE correction at the voxel level, p < .05. Clusters ≥ 10 voxels are reported.
Words contrasted against pseudohomophones
This contrast yielded a broad bilateral network of brain regions including pivotal areas of the reading network, such as the inferior frontal gyrus, inferior and superior parietal lobules and the fusiform gyrus (see ). These areas showed task-positive activation (higher activity compared to baseline). We further found task-negative activation (lower activity compared to baseline) in parieto-occipital, temporal and frontal regions. Pseudohomophones yielded higher contrast estimates than words in task-positive clusters, whereas for task-negative clusters pseudohomophones were more deactivated than words, yielding more negative contrast estimates.
Table 5. Brain activity for the contrast words vs. pseudohomophones in the whole sample. Clusters are displayed with FWE correction at the voxel level, p < .05. Clusters ≥ 10 voxels are reported.
Functional ROI analysis
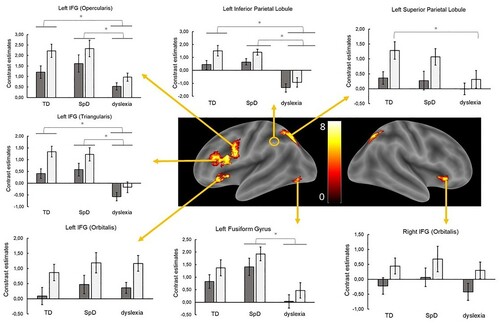
Activity for words and pseudohomophones was compared among groups in specific regions of interest, which were chosen on the basis of whole-brain results for the contrast words vs. pseudohomophones in the overall sample. The choice of ROIs focused on left hemisphere areas with task-positive activity that are particularly relevant for written language processing, as shown in the literature (Gebauer et al., Citation2012; Martin et al., Citation2015; Richlan, Citation2012). Clusters included in the ROI analysis are indicated in and provides an overview of the spatial localisation of the chosen ROIs together with main findings for condition, group effects and interactions.
Figure 3. Group-related activity for words vs. pseudohomophones in each selected ROI based on the results of the whole-brain analysis, FWE-corrected at the voxel level, p < .05. Dark-grey boxes indicate functional activity for words, light-grey boxes for pseudohomophones. Bars represent standard errors.
Contrast estimates of the ROIs were normally distributed, Ds(58) ≤ .104, ps ≥ .191. We conducted a 2 × 3 ANOVA in each selected ROI, with condition as within-subject factor (word and pseudohomophone) and group as between-subject factor (TD, SpD and dyslexia). Post-hoc comparisons are reported with Bonferroni correction.
Left inferior frontal gyrus, opercular part
Results revealed a significant main effect of condition, F(1,55) = 73.48, MSE = 14.82, p < .001, = .57, with pseudohomophones yielding higher estimates than words (Mdiff = .72). Groups differed significantly, F(2,55) = 4.80, MSE = 15.70, p = .012,
= .15 and pairwise comparisons revealed significantly lower activity in dyslexia compared to SpD, p = .018 and (though as a trend) to TD, p = .055. The interaction condition × group was also significant, F(2,55) = 4.21, MSE = .85, p = .020,
= .13. The difference between words and pseudohomophones was significantly smaller in dyslexia as compared to TD (Mdiff = .57), p = .016. Children with dyslexia displayed significantly lower estimates than the TD group for pseudohomophones (p = .011). The same trend was evident for words, but was not significant (p = .296). Children with dyslexia had significantly lower estimates in both conditions compared to SpD, ps ≤ .048.
Left inferior frontal gyrus, orbital part
In this region, only condition showed a significant main effect, F(1,55) = 54.35, MSE = 16.83, p < .001, = .50. Again, pseudohomophones yielded higher estimates than words (Mdiff = .77). Neither the main effect of group, nor its interaction with condition were significant, Fs ≤ .54, MSEs ≤ .1.40, ps ≥ .585.
Left inferior frontal gyrus, triangular part
The main effect of condition was again significant, F(1,55) = 54.29, MSE = 12.44, p < .001, = .50, revealing higher estimates for pseudohomophones than words (Mdiff = .66). Also groups differed significantly, F(2,55) = 11.36, MSE = 20.80, p < .001,
= .29: Children with Dyslexia had lower estimates compared to the other two groups, ps ≤ .001. The interaction was also significant, F(2,55) = 3.27, MSE = .75, p = .046,
= .11. The word-pseudohomophone difference was significantly smaller in dyslexia compared to the TD group (Mdiff = .53), p = .041.
Left superior parietal lobule
As in the other regions, contrast estimates for words were significantly lower than contrast estimates for pseudohomophones (Mdiff = .68), F(1,55) = 60.31, MSE = 13.08, p < .001, = .52. The group main effect was not significant, F(2,55) = 1.98, MSE = 4.97, p = .147. The condition × group interaction was significant, F(2,55) = 4.72, MSE = 1.02, p = .013,
= .15. The word-pseudohomophone difference was smaller in dyslexia compared to the TD group (Mdiff = .60), p = .014. Children with dyslexia displayed a trend for lower estimates for pseudohomophones (p = .055), but not words (p = .765), compared to the TD group. Differences between the dyslexia and SpD groups were not significant, p ≥ .261.
Right inferior frontal gyrus
Results in this region revealed the significant main effect of condition, (pseudohomophones > words; Mdiff = .67), F(1,55) = 42.31, MSE = 12.63, p < .001, = .44. The main effect of group and the interaction were not significant, Fs < .52, MSEs < 1.70, ps ≥ .600.
Left fusiform gyrus
As in the other ROIs, pseudohomophones had significantly higher estimates than words (Mdiff = .50), F(1,55) = 36.68, MSE = 7.05, p < .001, = .40. The main effect of group was significant, F(2,55) = 5.59, MSE = 18.44, p = .006,
= .17. Pairwise comparisons showed significantly lower activity in dyslexia compared to SpD (Mdiff = 1.42), p = .005. The group with dyslexia showed also lower estimates than typically developing children (Mdiff = .85), this difference, however, was not significant, p = .111. The interaction was not significant, F(2,55) = .17, MSE = .03, p = .845.
Left inferior parietal lobule
Also in this cluster, pseudohomophones were associated with more positive estimates than words, (Mdiff = .74), F(1,55) = 35.19, MSE = 15.47, p < .001, = .39. Also the main effect of group was significant, F(2,55) = 16.10, MSE = 59.15, p < .001,
= .37: While the group with dyslexia yielded negative mean contrast estimates in this region (Mdyslexia = −1.12), activity in the other two groups was positive on average (MSpD = 1.02, MTD = .99) and differed significantly from the dyslexia group, ps < .001. There was a marginally significant interaction, F(2,55) = 2.63, MSE = 1.16, p = .081,
= .09. Post-hoc comparisons showed that the difference of estimates between words and pseudohomophones was only marginally significant in the dyslexia group, p = .066, and tended to be smaller in dyslexia compared to the TD group (Mdiff = .66), p = .08. The comparison of dyslexia and SpD groups yielded non-significant differences, p = .742.
Discussion
This fMRI study tested whether different patterns of functional brain mechanisms could be observed in children with distinct written language deficits compared to a group of typically developing children. Our reading aloud task with words and pseudohomophones allowed a more naturalistic assessment of lexical and sublexical reading strategies than lexical decision tasks (Braun et al., Citation2019; Hawelka et al., Citation2010).
Whole-brain results
Whole-brain findings revealed a divergent pattern of functional alteration in each deficit group compared to typically developing children. We found reduced functional activity in medial frontal and cingulate cortices, bilateral middle and inferior temporal regions in SpD for both conditions. Interestingly, SpD children displayed lower activity than the TD group in the bilateral fusiform gyri for the condition “words”, with a larger cluster of reduced activity in the left hemisphere. This finding is in line with previous evidence (Gebauer et al., Citation2012) and supports the hypothesis of impaired functional activity in the ventral stream for lexico-orthographic processing in children with isolated spelling deficits. Note, however, that the cluster in the left fusiform gyrus (MNI coordinates: −27, −48, −9) was located more anteriorly and medially than the “classic” location of the VWFA (MNI coordinates: −45, −57,−12; [Vogel et al., Citation2012]). We also observed (small) clusters in left superior and middle temporal regions, where children in the SpD group had lower activity than the TD group. We might speculate that reduced functional activity in temporal regions represents the neural substrate for (mild) phonological processing difficulties in this group, as assumed by the partial cue hypothesis. These mild phonological problems, however, are not clearly evident at the behavioural level, as decoding performance was in the typical range in the SpD group. Note, however, that the phonological awareness score was lower in the SpD as compared to the TD group, although this difference was not statistically significant. The largest clusters of higher activity in the SpD vs. TD group were located in the right temporal cortex for both words and pseudohomophones. Unlike Gebauer et al. (Citation2012), however, whole-brain differences between the SpD and TD groups in the two conditions were not mainly driven by overactivity of children with SpD in regions of the right hemisphere. The SpD group showed several clusters with higher activity than the typical group in both hemispheres. Our findings are thus only partly consistent with the study by Gebauer et al. (Citation2012). Differences between our results and those of Gebauer et al. may be due to the different paradigms used. Orthographic lexical judgement tasks are indeed confounded with decisional processes associated with prefrontal and cingulate activity (Braun et al., Citation2019; Rudorf & Hare, Citation2014), which are not required for reading aloud. Furthermore, different analysis pipelines were adopted in our study compared to Gebauer et al., which increase the amount of variability in functional results.
Children with dyslexia displayed lower activity than TD in fronto-parietal clusters around the central fissure, in superior temporal regions, cerebellum and, specifically for pseudohomophones, in the left inferior frontal gyrus. They thus showed predominant functional alterations in regions of the sublexical dorsal stream.
The two deficit groups differed also in clusters with higher activity compared to TD. While children in the SpD group showed increased activity in fronto-temporal regions and the cerebellum, children with dyslexia activated inferior frontal, occipito-temporal and medial temporal regions more than the TD group. These results suggest differential compensatory strategies for the two deficit groups.
The interpretation of these group differences at the whole-brain level, however, should be treated with caution as brain activity in each experimental condition was not compared to a control condition, it was instead contrasted with rest (children looked at the fixation cross). Reading aloud involves overt articulation, which is related to increased activity in pre- and postcentral sensory-motor regions as well as in cortical and subcortical areas responsible for motor execution such as the cingulate cortex, cerebellum and putamen. Furthermore, auditory-motor feedback takes place during overt speech production and this increases activity in core language areas such as the superior temporal regions (Price, Citation2012). The anterior temporal cortex is involved in syntactic analysis, while lexical-semantic processing mostly (though not only) takes place at the level of the middle temporal gyrus (Friederici, Citation2011, Citation2012). These regions are present also as results of our group comparisons and it is therefore difficult to distinguish between clusters modulated by motor and perceptive, semantic, phonological and orthographic processes.
Functional ROIs
To rule out confounding factors deriving from the comparison of the experimental conditions with rest, we focused our analysis on specific brain regions defined functionally on the basis of the contrast words vs. pseudohomophones within the neural network of written language processing. Based on relevant literature (Gebauer et al., Citation2012; Hancock et al., Citation2017; Martin et al., Citation2015; Richlan, Citation2012), we selected three clusters in the left inferior frontal gyrus (corresponding to the opercular, orbital and triangular parts), and one cluster in each of the following: left superior parietal lobule, right inferior frontal gyrus, left fusiform gyrus and left inferior parietal lobule. Note that the location of the left fusiform cluster in this ROI analysis (MNI coordinates: −42, −72, −9) does not match the region found in the whole-brain analysis, but instead resembles the location of the VWFA.
SpD group
Our main goal was to test two cognitive accounts of isolated spelling deficits that make opposite predictions concerning functional activity in the sublexical dorsal network. Our findings in the ROIs, however, did not reveal any clear functional difference between the SpD and TD groups. The lack of evidence for lower or higher dorsal activity in decoding-related regions is not in line with both cognitive hypotheses. The partial cue hypothesis (Frith, Citation1980) claims that children with isolated spelling deficits do not possess well-specified orthographic representations due to decoding difficulties. As an indication of deficient sublexical routines, we expected reduced functional activity especially for pseudohomophones in the left sublexical dorsal stream, which was not found. One possible explanation for the lack of dorsal underactivity in the SpD group could be related to age and thus schooling level. As already argued in previous studies (Moll & Landerl, Citation2009; Wimmer & Mayringer, Citation2002), it is possible that initial phonological deficits are quickly overcome due to very consistent grapheme-phoneme mappings in German. A recent eye-tracking study conducted on a partly overlapping sample of 3rd and 4th graders as in the present study (Gangl, Moll, Banfi et al., Citation2018) showed no main differences in eye-movement patterns and psycholinguistic effects in children with SpD compared to TD. This evidence is consistent with the results of our ROIs analysis and (indirectly) supports the partial cue hypothesis that SpD children have low-quality orthographic representations that suffice for reading but not for spelling.
In the functional ROIs, the SpD group consistently showed a reliable difference between the two experimental conditions, with lower activity for words compared to pseudohomophones. This finding is not in line with the overreliance hypothesis by Moll and Landerl (Citation2009) that predicted no reliable difference in functional activity between the two conditions in the SpD group as a sign of compensation via sublexical mechanisms.
Taken together, our results are thus more consistent with the partial cue than the over-reliance hypothesis, although the lack of a clear decoding deficit in SpD is not completely in line with the former either. Recent evidence from an orthographic learning study (Mehlhase et al., Citation2019) suggests that SpD children have problems in developing and storing stable orthographic representations in long-term memory. This finding represents a likely alternative explanation for orthographic weaknesses observed in SpD, but needs further replication. Future fMRI studies should provide a more direct test of the partial cue vs. overreliance hypotheses by investigating the development of functional activity related to phonological and orthographic processing starting from the first years of school.
In the left fusiform ROI, part of the lexical ventral stream, functional activity in the SpD did not differ from the TD group. One possible explanation for this finding is that performance in our reading aloud paradigm was mainly based on word recognition. If the main difficulty of SpD children is in actively retrieving well specified orthographic representations (as it is the case in spelling tasks), reading tasks may not tap onto the actual deficit of this group. This could be the reason why, in line with previous eye-tracking evidence (Gangl, Moll, Banfi, et al., Citation2018), we could not observe a clear functional deficit in the ROIs in this group.
Dyslexia group
Children with dyslexia showed a more widespread alteration of brain activity compared to the other two groups in our ROIs. A group main effect, indicating reduced brain activity, was found in the opercular and triangular parts of the left IFG, in the left fusiform gyrus and in the left inferior parietal lobule. These regions refer to both dorsal and ventral components of the reading network and have already been reported as main sites for dyslexia-related functional differences in metanalyses (Richlan et al., Citation2009, Citation2011). Our findings thus confirm and extend evidence on location-specific functional alterations in dyslexia and strengthen the notion that reading deficits are characterised by visual-verbal processing problems that impact on sublexical and lexical routines (Gangl, Moll, Jones, et al., Citation2018).
Quite unexpectedly, brain activity for the dyslexia group resulted in negative contrast estimates for the triangular portion of the inferior frontal gyrus and the inferior parietal lobule, whereas the TD and SpD groups displayed positive contrast estimates for both conditions in these regions. The fact that the pattern of activity in dyslexia in these regions was in the opposite direction compared to the other groups is particularly challenging to explain. Note, however, that lower activity compared to baseline (= task-negative activation) in the left inferior parietal lobule was already reported in dyslexia in two previous metanalyses (Martin et al., Citation2016; Richlan et al., Citation2011). This finding could indicate increased cognitive effort in dyslexia during the reading aloud task, which is particularly difficult for reading-impaired children (Richlan et al., Citation2011). The reason why brain activity has a different sign only in the dyslexia group, however, remains unclear and needs further experimental investigation.
We also reported significant group × condition interactions, which were observed in the opercular and triangular part of the inferior frontal gyrus, superior and inferior parietal lobules. In these regions, the difference in contrast estimates between words and pseudohomophones was less pronounced in the dyslexia group, whereas it was comparable in the TD and SpD groups. Differences between words and pseudohomophones are attributable to orthographic mechanisms, because the two types of stimuli share semantic and phonological but not orthographic representations. The reduced (yet reliable) gap we observed between the two conditions in the group with dyslexia could thus indicate that (1) children with dyslexia have less orthographic representations at their disposal compared to typical readers; (2) they encounter problems in processing these representations.
Interactions revealed also condition-specific group differences. For example, in the left superior parietal lobule children with dyslexia showed lower activity than TD for pseudohomophones, but not for words. This finding suggests a differential impact of subtle graphotactic regularities in dyslexia compared to the other two groups with age-adequate reading performance. In the light of behavioural evidence showing a relationship between reading, dyslexia and statistical learning skills (Arciuli & Simpson, Citation2012; Conrad et al., Citation2013; Schmalz et al., Citation2017), future studies should consider the impact of sublexical regularities on reading-related functional activity in typical readers and dyslexia more in detail.
Practical implications
Our findings showing differences between children with SpD and children with dyslexia on the neurofunctional level highlight that spelling should be more seriously taken into account as a distinct skill domain as compared to reading. Although reading and spelling are moderately to strongly correlated (Debska et al., Citation2019; Ehri, Citation1997; Moll & Landerl, Citation2009), they also rely on partly distinct cognitive processes (Debska et al., Citation2019; Purcell et al., Citation2011). Importantly, spelling deficits can emerge in the context of age-adequate reading performance. Children with SpD should therefore not be overlooked, as they warrant special attention in the educational practice and should also receive adequate, evidence-based interventions aimed to resolve their specific impairment (Galuschka et al., Citation2020).
Conclusion
Our ROIs findings regarding children with isolated spelling deficits revealed no clear alteration compared to the typical group in relevant dorsal and ventral regions for written language processing. The fact that the SpD group showed a reliable difference between the two experimental conditions especially in regions of the dorsal stream is not in line with the overreliance hypothesis (Moll & Landerl, Citation2009) that predicted no reliable difference in functional activity between words and pseudohomophones in the SpD group as a sign of compensation via sublexical mechanisms. Our results are thus more consistent with the partial cue than the over-reliance hypothesis. The whole-brain analysis further showed clusters of reduced activity in SpD in the anterior left fusiform gyrus and in left temporal areas. These represent likely neural correlates of impaired orthographic processing and mild phonological deficits, respectively. This is again evidence that supports the partial cue hypothesis. Note, however, that due to lack of proper control conditions, our whole-brain analysis may be confounded by articulatory, perceptive and semantic effects and should be interpreted carefully.
Children with dyslexia displayed a different pattern of functional alteration compared to the SpD group at the whole-brain level as well as in the ROI analysis. Our findings in this group are consistent with the notion that dyslexia is related to both sublexical and lexical difficulties, indicated by functional differences in the dorsal and ventral streams for written language processing.
Supplemental Material
Download PDF (238.8 KB)Acknowledgements
This research was funded by the Austrian Science Fund (FWF) (Grant Number: I 1658-G22) and the German Research Foundation (DFG) (Grant Number: MO 2569/2-1). The data of the present study are available in the OpenNeuro repository under the project name “MRI Lab Graz: Reading-related functional activity in children with isolated spelling deficits and dyslexia”, doi:10.18112/openneuro.ds003126.v1.0.0.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Arciuli, J., & Simpson, I. C. (2012). Statistical learning is related to reading ability in children and adults. Cognitive Science, 36(2), 286–304. https://doi.org/https://doi.org/10.1111/j.1551-6709.2011.01200.x
- Avants, B. B., Epstein, C. L., Grossman, M., & Gee, J. C. (2008). Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Medical Image Analysis, 12(1), 26–41. https://doi.org/https://doi.org/10.1016/j.media.2007.06.004.
- Bakos, S., Landerl, K., Bartling, J., Schulte-Körne, G., & Moll, K. (2018). Neurophysiological correlates of word processing deficits in isolated reading and isolated spelling disorders. Clinical Neurophysiology, 129(3), 526–540. https://doi.org/https://doi.org/10.1016/j.clinph.2017.12.010
- Banfi, C., Koschutnig, K., Moll, K., Schulte-Körne, G., Fink, A., & Landerl, K. (2019). White matter alterations and tract lateralization in children with dyslexia and isolated spelling deficits. Human Brain Mapping, 40(3), 765–776. https://doi.org/https://doi.org/10.1002/hbm.24410
- Bar-Kochva, I., & Amiel, M. (2016). The relations between reading and spelling: An examination of subtypes of reading disability. Annals of Dyslexia, 66(2), 219–234. https://doi.org/https://doi.org/10.1007/s11881-015-0117-8
- Behzadi, Y., Restom, K., Liau, J., & Liu, T. (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage, 37(1), 90–101. https://doi.org/https://doi.org/10.1016/j.neuroimage.2007.04.042.
- Borowska, R. A., Francuz, P., Soluch, P., & Wolak, T. (2014). Brain activation in teenagers with isolated spelling disorder during tasks involving spelling assessment and comparison of pseudowords. fMRI study. Brain and Development, 36(9), 786–793. https://doi.org/https://doi.org/10.1016/j.braindev.2013.10.010
- Braun, M., Kronbichler, M., Richlan, F., Hawelka, S., Hutzler, F., & Jacobs, A. M. (2019). A model-guided dissociation between subcortical and cortical contributions to word recognition. Scientific Reports, 9(1), 1–12. https://doi.org/https://doi.org/10.1038/s41598-018-37186-2
- Conrad, N. J., Harris, N., & Williams, J. (2013). Individual differences in children’s literacy development: The contribution of orthographic knowledge. Reading and Writing, 26(8), 1223–1239. https://doi.org/https://doi.org/10.1007/s11145-012-9415-2
- Cox, R. W., & Hyde, J. S. (1997). Software tools for analysis and visualization of fMRI data. NMR in Biomedicine, 10(4-5), 171–78.
- Debska, A., Chyl, K., Dziegel, G., Kacprzak, A., Luniewska, M., Plewko, J., Marchewka, A., Grabowska, A., & Jednorog, K. (2019). Reading and spelling skills are differentially related to phonological processing: Behavioral and fMRI study. Developmental Cognitive Neuroscience, 39, 100683. https://doi.org/https://doi.org/10.1016/j.dcn.2019.100683
- Dehaene, S., Cohen, L., Morais, J., & Kolinsky, R. (2015). Illiterate to literate: Behavioural and cerebral changes induced by reading acquisition. Nature Reviews Neuroscience, 16(4), 234–244. https://doi.org/https://doi.org/10.1038/nrn3924
- Dehaene, S., Dehaene, S., Pegado, F., Braga, L. W., Ventura, P., Filho, G. N., Jobert, A., & Dehaene-Lambertz, G. (2012). How learning to read changes the cortical networks for vision and language. Science, 330(6009), 1359–1364. https://doi.org/https://doi.org/10.1126/science.1194140
- Denckla, M. B., & Rudel, R. G. (1976). Rapid ‘automatized’ naming (RAN): Dyslexia differentiated from other learning disabilities. Neuropsychologia, 14(4), 471–479. https://doi.org/https://doi.org/10.1016/0028-3932(76)90075-0
- Döpfner, M., Görtz-Dorten, A., Lehmkuhl, G., Breuer, D., & Goletz, H. (2008). DISYPS-II. Diagnostik-System für psychische Störungen nach ICD-10 und DSM-IV für Kinder und Jugendliche-II [DISYPS-II. Diagnostic assessment system for mental disorders in children and adolescents according to ICD-10 and DSM-IV, 2nd ed.]. Hogrefe.
- Duff, E. P., Cunnington, R., & Egan, G. F. (2007). REX: Response exploration for neuroimaging datasets. Neuroinformatics, 5(4), 223–234. https://doi.org/https://doi.org/10.1007/s12021-007-9001-y
- Ehri, L. C. (1997). Learning to read and learning to spell are one and the same, almost. In C. A. Perfetti, L. Rieben, & M. Fayol (Eds.), Learning to spell (pp. 237–269). Erlbaum.
- Esteban, O., Ross B., Christopher J. M., Shoshana L. B., Craig M., Feilong M., et al. (2018). FMRIPrep [Computer software]. Retrieved from https://doi.org/https://doi.org/10.5281/zenodo.852659.
- Esteban, O., Ross B., Christopher J. M., Shoshana L. B., Craig M., Feilong M., et al. (2019). fMRIPrep: A robust preprocessing pipeline for functional MRI. Nature Methods, 16, 111–116. https://doi.org/https://doi.org/10.1038/s41592-018-0235-4.
- Fayol, M., Zorman, M., & Lété, B. (2009). Associations and dissociations in reading and spelling French: Unexpectedly poor and good spellers. British Journal of Educational Psychology, 2(6), 63–75. https://doi.org/https://doi.org/10.1348/000709909X421973
- Friederici, A. D. (2011). The brain basis of language processing: From structure to function. Physiological Reviews, 91(4), 1357–1392. https://doi.org/https://doi.org/10.1152/physrev.00006.2011
- Friederici, A. D. (2012). The cortical language circuit: From auditory perception to sentence comprehension. Trends in Cognitive Sciences, 16(5), 262–268. https://doi.org/https://doi.org/10.1016/j.tics.2012.04.001
- Frith, U. (1980). Unexpected spelling problems. In U. Frith (Ed.), Cognitive processes in spelling (pp. 495–515). Academic Press.
- Fonov, V. S., Evans, A. C., McKinstry, R.C., Almli, C. R., & Collins, D. L. (2009). Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. NeuroImage, 47(1), S102. https://doi.org/https://doi.org/10.1016/S1053-8119(09)70884-5.
- Galuschka, K., Görgen, R., Kalmar, J., Haberstroh, S., Schmalz, X., & Schulte-Körne, G. (2020). Effectiveness of spelling interventions for learners with dyslexia: A meta-analysis and systematic review. Educational Psychologist, 55(1), 1–20. https://doi.org/https://doi.org/10.1080/00461520.2019.1659794
- Galuschka, K., & Schulte-Körne, G. (2016). The diagnosis and treatment of reading and / or spelling disorders in children and adolescents. Deutsches Arzteblatt international, 113, 279–286. https://doi.org/https://doi.org/10.3238/arztebl.2016.0279
- Gangl, M., Moll, K., Banfi, C., Huber, S., Schulte-Körne, G., & Landerl, K. (2018). Reading strategies of good and poor readers of German with different spelling abilities. Journal of Experimental Child Psychology, 174, 150–169. https://doi.org/https://doi.org/10.1016/j.jecp.2018.05.012
- Gangl, M., Moll, K., Jones, M. W., Banfi, C., Schulte-Körne, G., & Landerl, K. (2018). Lexical reading in dysfluent readers of German. Scientific Studies of Reading, 22(1), 24–40. https://doi.org/https://doi.org/10.1080/10888438.2017.1339709
- Gebauer, D., Enzinger, C., Kronbichler, M., Schurz, M., Reishofer, G., Koschutnig, K., Kargl, R., Purgstaller, C., Fazekas, F., & Fink, A. (2012). Distinct patterns of brain function in children with isolated spelling impairment: New insights. Neuropsychologia, 50(7), 1353–1361. https://doi.org/https://doi.org/10.1016/j.neuropsychologia.2012.02.020
- Greene, D. J., Black, K. J., & Schlaggar, B. L. (2016). Considerations for MRI study design and implementation in pediatric and clinical populations. Developmental Cognitive Neuroscience, 18, 101–112. https://doi.org/https://doi.org/10.1016/j.dcn.2015.12.005
- Greve, D. N., & Fischl, B. (2009). Accurate and robust brain image alignment using boundary-based registration. NeuroImage, 48(1), 63–72. https://doi.org/https://doi.org/10.1016/j.neuroimage.2009.06.060.
- Gorgolewski, K., Burns, C. D., Madison, C., Clark, D., Y. O. Halchenko, Waskom, M. L., & Ghosh S. (2011). Nipype: A flexible, lightweight and extensible neuroimaging data processing framework in Python. Frontiers in Neuroinformatics, 5(13). https://doi.org/https://doi.org/10.3389/fninf.2011.00013.
- Gorgolewski, K., Esteban, O., Christopher J. M., Ziegler, E., Ellis, D. G., Notter, M. P., Jarecka, D., et al. (2018). Nipype [Computer software]. Retrieved from https://doi.org/https://doi.org/10.5281/zenodo.596855.
- Guardia-Olmos, J., Zarabozo-Hurtado, D., Peró-Cebollero, M., Gudayol-Farré, E., Gómez-Velázquez, F. R., & González-Garrido, A. (2017). Analysis of pseudohomophone orthographic errors through functional magnetic resonance imaging (fMRI). The Spanish Journal of Psychology, 20, Article e74. https://doi.org/https://doi.org/10.1017/sjp.2017.72
- Hancock, R., Richlan, F., & Hoeft, F. (2017). Possible roles for fronto-striatal circuits in reading disorder. Neuroscience and Biobehavioral Reviews, 72, 243–260. https://doi.org/https://doi.org/10.1016/j.neubiorev.2016.10.025
- Hawelka, S., Gagl, B., & Wimmer, H. (2010). A dual-route perspective on eye movements of dyslexic readers. Cognition, 115(3), 367–379. https://doi.org/https://doi.org/10.1016/j.cognition.2009.11.004
- Jenkinson, M., & Smith, S. (2001). A global optimisation method for robust affine registration of brain images. Medical Image Analysis, 5(2), 143–56. https://doi.org/https://doi.org/10.1016/S1361-8415(01)00036-6.
- Jenkinson, M., Bannister, P., Brady M., & Smith S. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage, 17(2), 825–41. https://doi.org/https://doi.org/10.1006/nimg.2002.1132.
- Kemény, F., Banfi, C., Gangl, M., Perchtold, C. M., Papousek, I., Moll, K., & Landerl, K. (2018). Print-, sublexical and lexical processing in children with reading and/or spelling deficits: An ERP study. International Journal of Psychophysiology, 130, 53–62. https://doi.org/https://doi.org/10.1016/j.ijpsycho.2018.05.009
- Kronbichler, M., Bergmann, J., Hutzler, F., Staffen, W., Mair, A., Ladurner, G., & Wimmer, H. (2007). Taxi vs. Taksi: On orthographic word recognition in the left ventral occipitotemporal cortex. Journal of Cognitive Neuroscience, 19(10), 1584–1594. https://doi.org/https://doi.org/10.1162/jocn.2007.19.10.1584
- Lanczos, C. (1964). Evaluation of noisy data. Journal of the Society for Industrial and Applied Mathematics Series B Numerical Analysis 1(1): 76–85. https://doi.org/https://doi.org/10.1137/0701007.
- Landerl, K., & Moll, K. (2010). Comorbidity of learning disorders: Prevalence and familial transmission. Journal of Child Psychology and Psychiatry, 51(3), 287–294. https://doi.org/https://doi.org/10.1111/j.1469-7610.2009.02164.x
- Manolitsis, G., & Georgiou, G. K. (2015). The cognitive profiles of poor readers/good spellers and good readers/poor spellers in a consistent orthography: A retrospective analysis. Preschool and Primary Education, 3(2), 103–116. https://doi.org/https://doi.org/10.12681/ppej.178
- Martin, A., Kronbichler, M., & Richlan, F. (2016). Dyslexic brain activation abnormalities in deep and shallow orthographies: A meta-analysis of 28 functional neuroimaging studies. Human Brain Mapping, 37(7), 2676–2699. https://doi.org/https://doi.org/10.1002/hbm.23202
- Martin, A., Schurz, M., Kronbichler, M., & Richlan, F. (2015). Reading in the brain of children and adults: A meta-analysis of 40 functional magnetic resonance imaging studies. Human Brain Mapping, 36(5), 1963–1981. https://doi.org/https://doi.org/10.1002/hbm.22749
- McCandliss, B. D., Cohen, L., & Dehaene, S. (2003). The visual word form area: Expertise for reading in the fusiform gyrus. Trends in Cognitive Sciences, 7(7), 293–299. https://doi.org/https://doi.org/10.1016/S1364-6613(03)00134-7
- Mehlhase, H., Bakos, S., Landerl, K., Schulte-Körne, G., & Moll, K. (2019). Orthographic learning in children with isolated and combined reading and spelling deficits. Child Neuropsychology, 25(3), 370–393. https://doi.org/https://doi.org/10.1080/09297049.2018.1470611
- Moll, K., Kunze, S., Neuhoff, N., Bruder, J., & Schulte-Körne, G. (2014). Specific learning disorder: Prevalence and gender differences. PLoS ONE, 9(7), Article e103537. https://doi.org/https://doi.org/10.1371/journal.pone.0103537
- Moll, K., & Landerl, K. (2009). Double dissociation between reading and spelling deficits. Scientific Studies of Reading, 13(5), 359–382. https://doi.org/https://doi.org/10.1080/10888430903162878
- Moll, K., & Landerl, K. (2010). SLRT-II: Lese-und Rechtschreibtest [Reading and spelling test]. Hans Huber.
- Müller, R. (2004). DRT 3: Diagnostischer Rechtschreibtest für 3. Klassen: In neuer Rechtschreibung [Diagnostic spelling test for the 3rd grade]. Beltz.
- Oliver, M., Carreiras, M., & Paz-Alonso, P. M. (2017). Functional dynamics of dorsal and ventral reading networks in bilinguals. Cerebral Cortex, 27(12), 5431–5443. https://doi.org/https://doi.org/10.1093/cercor/bhw310
- Paizi, D., De Luca, M., Zoccolotti, P., & Burani, C. (2013). A comprehensive evaluation of lexical reading in Italian developmental dyslexics. Journal of Research in Reading, 36(3), 303–329. https://doi.org/https://doi.org/10.1111/j.1467-9817.2011.01504.x
- Paulesu, E., Danelli, L., & Berlingeri, M. (2014). Reading the dyslexic brain: Multiple dysfunctional routes revealed by a new meta-analysis of PET and fMRI activation studies. Frontiers in Human Neuroscience, 8, https://doi.org/https://doi.org/10.3389/fnhum.2014.00830
- Paz-Alonso, P. M., Oliver, M., Lerma-Usabiaga, G., Caballero-Gaudes, C., Quiñones, I., Suárez-Coalla, P., Duñabeitia, J. A., Cuetos, F., & Carreiras, M. (2018). Neural correlates of phonological, orthographic and semantic reading processing in dyslexia. NeuroImage: Clinical, 20, 433–447. https://doi.org/https://doi.org/10.1016/j.nicl.2018.08.018
- Petermann, F., & Petermann, U. (2011). WISC-IV. Wechsler intelligence scale for children–fourth edition. Pearson.
- Price, C. J. (2012). A review and synthesis of the first 20years of PET and fMRI studies of heard speech, spoken language and reading. NeuroImage, 62(2), 816–847. https://doi.org/https://doi.org/10.1016/j.neuroimage.2012.04.062
- Power, J. D., Mitra, A., Laumann, T. O., Snyder, A. Z., Schlaggar, B. L., & Petersen, S. E. (2014). Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage, 84, 320–41. https://doi.org/https://doi.org/10.1016/j.neuroimage.2013.08.048.
- Pugh, K. R., Mencl, W. E., Jenner, A. R., Katz, L., Frost, S. J., Lee, J. R., Shaywitz, S. E., & Shaywitz, B. A. (2001). Neurobiological studies of reading and reading disability. Journal of Communication Disorders, 34(6), 479–492. https://doi.org/https://doi.org/10.1016/S0021-9924(01)00060-0
- Purcell, J. J., Turkeltaub, P. E., Eden, G. F., & Rapp, B. (2011). Examining the central and peripheral processes of written word production through meta-analysis. Frontiers in Psychology, 2, Article e239. https://doi.org/https://doi.org/10.3389/fpsyg.2011.00239
- Richards, T. L., Berninger, V. W., & Fayol, M. (2009). fMRI activation differences between 11-year-old good and poor spellers’ access in working memory to temporary and long-term orthographic representations. Journal of Neurolinguistics, 22(4), 327–353. https://doi.org/https://doi.org/10.1016/j.jneuroling.2008.11.002
- Richlan, F. (2012). Developmental dyslexia: Dysfunction of a left hemisphere reading network. Frontiers in Human Neuroscience, 6, Article 120. https://doi.org/https://doi.org/10.3389/fnhum.2012.00120
- Richlan, F., Kronbichler, M., & Wimmer, H. (2009). Functional abnormalities in the dyslexic brain: A quantitative meta-analysis of neuroimaging studies. Human Brain Mapping, 30(10), 3299–3308. https://doi.org/https://doi.org/10.1002/hbm.20752
- Richlan, F., Kronbichler, M., & Wimmer, H. (2011). Meta-analyzing brain dysfunctions in dyslexic children and adults. NeuroImage, 56(3), 1735–1742. https://doi.org/https://doi.org/10.1016/j.neuroimage.2011.02.040
- Rudorf, S., & Hare, T. A. (2014). Interactions between dorsolateral and ventromedial prefrontal cortex underlie context-dependent stimulus valuation in goal-directed choice. Journal of Neuroscience, 34(38), 15988–15996. https://doi.org/https://doi.org/10.1523/JNEUROSCI.3192-14.2014
- Schlaggar, B. L., & McCandliss, B. D. (2007). Development of neural systems for reading. Annual Review of Neuroscience, 30(1), 475–503. https://doi.org/https://doi.org/10.1146/annurev.neuro.28.061604.135645
- Schmalz, X., Altoé, G., & Mulatti, C. (2017). Statistical learning and dyslexia: A systematic review. Annals of Dyslexia, 67(2), 147–162. https://doi.org/https://doi.org/10.1007/s11881-016-0136-0
- Schroeder, S., Würzner, K.-M., Heister, J., Geyken, A., & Kliegl, R. (2015). Childlex: A lexical database of German read by children. Behavior Research Methods, 47(4), 1085–1094. https://doi.org/https://doi.org/10.3758/s13428-014-0528-1
- Schurz, M., Sturm, D., Richlan, F., & Kronbichler, M. (2010). A dual-route perspective on brain activation in response to visual words: Evidence for a length by lexicality interaction in the visual word form area (VWFA). NeuroImage, 49(3), 2649–2661. https://doi.org/https://doi.org/10.1016/j.neuroimage.2009.10.082
- Share, D. L. (1995). Phonological recoding and self-teaching: Sine qua non of reading acquisition. Cognition, 55(2), 151–218. https://doi.org/https://doi.org/10.1016/0010-0277(94)00645-2
- Siegel, J. S., Power, J. D., Dubis, J. W., Vogel, A. C., Church, J. A., Schlaggar, B. L., & Petersen, S. E. (2014). Statistical improvements in functional magnetic resonance imaging analyses produced by censoring high-motion data points. Human Brain Mapping, 35(5), 1981–1996. https://doi.org/https://doi.org/10.1002/hbm.22307
- Torppa, M., Georgiou, G. K., Niemi, P., Lerkkanen, M. K., & Poikkeus, A. M. (2017). The precursors of double dissociation between reading and spelling in a transparent orthography. Annals of Dyslexia, 67(1), 42–62. https://doi.org/https://doi.org/10.1007/s11881-016-0131-5
- Tustison, N. J., Avants, B. B., Cook, P. A., Zheng, Y., Egan, A., Yushkevich, P. A., & Gee, J. (2010). N4ITK: improved N3 bias correction. IEEE Transactions on Medical Imaging, 29(6), 1310–1320. https://doi.org/https://doi.org/10.1109/TMI.2010.2046908
- Van der Mark, S., Bucher, K., Maurer, U., Schulz, E., Brem, S., Buckelmüller, J., Kronbichler, M., Loenneker, T., Klaver, P., Martin, E., & Brandeis, D. (2009). Children with dyslexia lack multiple specializations along the visual word-form (VWF) system. NeuroImage, 47(4), 1940–1949. https://doi.org/https://doi.org/10.1016/j.neuroimage.2009.05.021
- Vogel, A. C., Petersen, S. E., & Schlaggar, B. L. (2012). The left occipitotemporal cortex does not show preferential activity for words. Cerebral Cortex, 22(12), 2715–2732. https://doi.org/https://doi.org/10.1093/cercor/bhr295
- Weiß, R. (2006). CFT 20-R: Grundintelligenztest Skala 2-Revision [Culture fair intelligence test-scale 2]. Hogrefe.
- Wimmer, H., & Mayringer, H. (2002). Dysfluent reading in the absence of spelling difficulties: A specific disability in regular orthographies. Journal of Educational Psychology, 94(2), 272–277. https://doi.org/https://doi.org/10.1037/0022-0663.94.2.272
- Wimmer, H., & Mayringer, H. (2014). Salzburger Lese-screening für die Schulstufen 2-9: SLS 2-9 [Salzburg reading screening]. Hogrefe.
- Zhang, Y., Brady, M., & Smith, S. (2001). Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Transactions on Medical Imaging, 20(1), 45–57. https://doi.org/https://doi.org/10.1109/42.906424.
- Zhao, L., Chen, C., Shao, L., Wang, Y., Xiao, X., Chen, C., Yang, J., Zevin, J., & Xue, G. (2017). Orthographic and phonological representations in the fusiform cortex. Cerebral Cortex, 27(11), 5197–5210. https://doi.org/https://doi.org/10.1093/cercor/bhw300.
