Abstract
Sorghum is an important staple food crop in dry areas of Zimbabwe but yields are reduced due to inefficient weed management strategies that largely rely on hand weeding. A study to establish weeds associated with sorghum and their management was conducted in two sorghum-growing districts, Insiza and Chipinge, during the 2016/17 cropping season. Physical weed sampling in farmers’ fields was done to identify weeds infesting sorghum. A questionnaire was used to collect survey data from 80 respondents who were randomly selected. Physical weed sampling established that the dominant weeds that infested sorghum in the two districts were Amaranthus hybridus, Richardia scabra, Tagetes minuta, Striga asiatica, Commelina benghalensis, Eleusine indica, Datura stramonium and Panicum spp. Many of the weeds are broadleaf species, offering opportunity to harness sorghum allelopathy for weed control. Allelopathic compounds exuded by sorghum, such as sorgoleone, have been proven to largely suppress broadleaf weeds. There has been little change in the weed spectrum in sorghum when results of the current study are compared to benchmark national weed survey of arable lands of the smallholder farms of Zimbabwe conducted in 1988. This indicates possibility of high persistence of the soil seedbank. The majority of farmers in the two districts (85%) controlled weeds by hand weeding, indicating a very limited shift towards adopting alternative weed management technologies since 1990 when an inventory of weed science technological needs for communal farmers was compiled. Smallholder sorghum growers may need to broaden their weed management strategies in order to overcome some of the challenges that are associated with hand weeding.
PUBLIC INTEREST STATEMENT
Sorghum is an important food crop in the semi-arid tropics. The crop competes poorly with weeds during early seedling growth. Identifying weeds associated with the crop can enhance our understanding of how those weeds respond to different weed management methods, including use of sorghum allelopathy. This study investigated weeds that infest sorghum in two of Zimbabwe’s major sorghum-growing districts; Insiza and Chipinge, through a questionnaire survey and through physical weed sampling in farmers’ fields. The study reports the major weed management methods that are used in the two districts. Weeds that were recorded in the study are listed. A comparison of the weed spectrum reported in the 1988 national weed survey and those that were identified during the 2016/17 cropping season is made. The study identified that only a few new weeds have invaded the two districts.
1. Introduction
Sorghum [Sorghum bicolor (L.) Moench] is mainly an African cereal staple crop (Orr et al., Citation2016; Winchell et al., Citation2018) for millions of people in the semi-arid tropics of Africa and Asia (Organisation for Economic Cooperation and Development [OECD], Citation2017; Griebel et al., Citation2019). It ranks fifth in cereal production after maize, rice, wheat and barley (Boyles et al., Citation2018; FAOSTAT, Citation2017; Mundia et al., Citation2019). It can be cultivated as a fuel crop (Bergtold et al., Citation2017; OECD, Citation2010; United Nations Conference on Trade and Development [UNCTAD], Citation2016). The crop is normally cultivated under dryland conditions in marginal areas with high temperatures and low rainfall (Mabhaudhi et al., Citation2019; Mundia et al., Citation2019) because it can withstand wilting compared to maize (Food and Agriculture Organisation of the United Nations [FAO], International Crops Research Institute for the Semi-Arid Tropics [ICRISAT], Citation1996). It is mostly cultivated for household food security (Phiri et al., Citation2019). In eastern and southern Africa, cereal production is dominated by maize and sorghum comes second (Orr et al., Citation2016). The crop is mostly cultivated by smallholder farmers (Wortmann et al., Citation2009), sometimes on degraded soils (Smale et al., Citation2018) using low inputs (Haji & Tegegne, Citation2018; Mrema et al., Citation2016). The crop can thrive under low fertility environments (Kante et al., Citation2019). In Zimbabwe, sorghum is primarily cultivated in drought-prone areas in the agroecological Regions IV and V, which are classified as unsuitable for intensive cropping. Agroecological regions are land classes based on agricultural potential with respect to rainfall regime, soil quality and vegetation. These regions cover the Northern, North Eastern, Western and Southern marginal belts of Zimbabwe. Chipinge and Insiza are some of the sorghum growing districts of Zimbabwe that fall within Regions IV and V, and sorghum is a key food security crop in the two districts.
Weeds are a problem in crop production (Gage & Schwartz-Lazaro, Citation2019; Nwosisi et al., Citation2019; Westwood et al., Citation2018). They can reduce crop yields (Ball et al., Citation2019). They compete with crops for resources that include moisture, nutrients, space and light, and they can also harbor pests and diseases that infest crops (Brooke & McMaster, Citation2019). In a study testing the competitive effects of weed and crop density on weed biomass and crop yield in wheat, Wilson et al. (Citation1995) established that increasing weed density where crop populations were low resulted in high crop yield losses. Sorghum grows slowly during the first few weeks after emergence (Ferrell et al., Citation2018). To prevent yield losses, weeds have to be controlled at critical periods during the crop growth cycle (Knezevic et al., Citation2002). It has been established through research that both light and heavy weed infestations during early growth can reduce grain sorghum yields, with high infestations causing yield losses of up to 20% (Barber et al., Citation2015).
Often, weeds are associated with particular crops. The reason for this is that each crop and associated management practices provide more or less specific conditions that act as filters (Belyea & Lancaster, Citation1999) offering different ecological niches for weeds (Meiss et al., Citation2010). Proper identification and management of weeds is critical for profitable crop production. Some of the weeds that have been identified in sorghum in studies that were conducted elsewhere are listed in Table .
Table 1. Weeds reported to be associated with sorghum
The list of weeds that infest sorghum (Table ), which is not exhaustive, comprises sedges, grasses and broadleaf weeds. A number of these weeds have developed resistance to herbicides targeting different sites of action (Table ). This suggests that unless if they combine herbicides with other weed control tactics, smallholder farmers who use herbicides for weed control will have to contend with the challenge of herbicide resistance.
Table 2. Reported herbicide resistance in weeds associated with sorghum
Weed communities of arable lands may evolve with time. Such changes may be caused by changes in crops being cultivated (Armengot et al., Citation2016) and changes in crop production practices (Hyvönen & Salonen, Citation2002), including tillage (Nichols et al., Citation2015). Changes in crop and fertilization (Kakabouki et al., Citation2015) may also cause differences in weed community composition that have management implications (Benaragama et al., Citation2019). Additionally, climate change can induce changes in the weed flora of arable ecosystems (Heeb et al., Citation2019; Peters et al., Citation2014; Scott et al., Citation2014), including alterations in weed biology (Ziska & Dukes, Citation2011). Resident species may also persist (Buhler et al., Citation1997; Mohammed & Denboba, Citation2020; Nikolić et al., Citation2020; Schwartz-Lazaro & Copes, Citation2019) due to intense selection pressure (Metcalfe et al., Citation2019). This makes it necessary for weed surveys to be regularly conducted so that any new weeds can be quickly identified so as to advise farmers on appropriate control measures against them.
Weed management is important for agricultural production (Westwood et al., Citation2018). The study by Chivinge (Citation1990) on weed science technological needs for communal farmers of Zimbabwe, which was preceded by the first-ever national survey of weeds of arable lands in smallholder farms (Chivinge, Citation1988) might have been motivated by the realization that weed infestations and weed management strategies that are available and applicable to the smallholder farmers of Zimbabwe are unique. Typically, communal farming areas are characterised by poor rural indigenous households who practice subsistence agriculture, with surplus being sold at the markets (Proctor et al., Citation2000). While weed management in the large scale and commercial farming areas has been made comparatively easy by improved availability and affordability of resources, access to weed research information, access to extension services, as well as quicker adoption of modern weed management technologies, it has not been so in many communal farms of Zimbabwe, where, consequently, weeds continue to reduce crop productivity.
Weed management in most smallholder farms in developing countries is dominated by hand weeding (Chivinge, Citation1990; Gianessi, Citation2013; Sims et al., Citation2018), which is slow and cumbersome (Kumar et al., Citation2017; N’cho et al., Citation2019). In contrast, improvements in and adoption of improved weed management technologies have been made in other countries. Such technologies include precision weed management (Partel et al., Citation2019; Westwood et al., Citation2018), use of herbicides (Hale et al., Citation2019; Harker & O’Donovan, Citation2013), biotechnological approaches (Beckie et al., Citation2019; Duke, Citation2003) robotic weeders (Fennimore & Cutulle, Citation2019; Igawa et al., Citation2009; Lowenberg-DeBoer et al., Citation2019; Reiser et al., Citation2019; Sabanci & Aydin, Citation2017; Siemens, Citation2014; Slaughter et al., Citation2008), automated systems with sensor and computer technologies (Young et al., Citation2014), crop allelopathy (Alsaadawi et al., Citation2015; Macías et al., Citation2019; Trezzi et al., Citation2016; Uddin et al., Citation2014), flaming (Stepanovic et al., Citation2016) and other technologies providing site-specific weed control (Coleman et al., Citation2019). It is feared that in emerging economies and rural areas, weak technological infrastructure, high costs of technology, low levels of e-literacy and digital skills, weak regulatory framework and limited access to services mean these areas risk being left behind in the digitalization process (Trendov et al., Citation2019). Solutions for adopting new technologies for modern weed management technologies will, as argued by Korres et al. (Citation2019), require a new weed management paradigm in modern agriculture, and this should be based on ecological principles and nonconventional weed management approaches. In relation to the particular conditions of Zimbabwe, however, not all of the modern weed management technologies listed above may be of significance. For example, precision agriculture is best applicable only for huge areas of fields covering dozens of hectares. In Zimbabwe, the average area of a sorghum field is only 0.4–2.0 ha. As for the use of herbicides, although they are still being promoted in some countries (Gianessi, Citation2013), the world trend is to reduce their application, and to apply them only if weeds exceed the economic threshold. Therefore in some cases, both hand weeding and mechanical weeding can be fully justified. Problem weeds that are currently infesting sorghum in Zimbabwe’s smallholder farms are not known. Little is also known about how farmers are managing these weeds. The objectives of the study were:
To identify major weeds associated with sorghum in the sorghum-growing districts of Insiza and Chipinge;
To identify the weed management methods used by sorghum growers in Insiza and Chipinge districts of Zimbabwe;
To identify and suggest best solutions for adopting modern weeding technologies.
2. Materials and methods
2.1. Study sites
The studies were conducted in Insiza (Latitude −19°46′59.99″S; Longitude 29° 11′ 60.00″) and Chipinge (Latitude −20° 11′ 17.99″ S; Longitude 32° 37′ 25.14″ E) districts.
Insiza district is in Matabeleland South Province of Zimbabwe. It covers 7 566 km2 of land. The district falls under Natural Regions IV and V, and is therefore generally regarded as not suitable for crop production due to frequent drought spells and erratic rains. The average annual rainfall is 500 mm. The area is marginal for rainfed maize production (Bird et al., Citation2002), and drought-tolerant crops such as sorghum and millets are more suitable for the district. Cattle ranching and extensive farming are the recommended agricultural activities. Soils range from sandy loam to red clay. The average area grown to sorghum per household is 0.4 ha. The average yield per household is 0.5 tonnes ha−1 and the district average annual yield is 1 415 tonnes. Sorghum is used for making thick porridge (locally known as isitshwala), making thin porridge, beer brewing, making non-alcoholic drink (mageu) and stock-feed.
Chipinge lies in southeastern Zimbabwe in Manicaland province. It is about 1134 m above sea level. The district experiences two types of climates; the highveld, which receives an average annual rainfall of 800–1 200 mm, and the lowveld, receiving an average of 450–650 mm. The highveld has predominantly sandy loam soils, while vertisols dominate the lowveld. The average land area dedicated to sorghum is between 1 and 2 ha per household. The farmers are mainly communal smallholder farmers who practise low-input sorghum production. They do not apply any form of fertiliser on the crop, except under-sponsored projects such as the FAO’s Conservation Agriculture. Sorghum is grown with little knowledge of the nutrient and pH status of the soil, and not surprisingly, the average sorghum yield in the district can be as low as 0.4 tonnes ha−1. Other crops grown in the district are maize and cotton (Baudron et al., Citation2019).
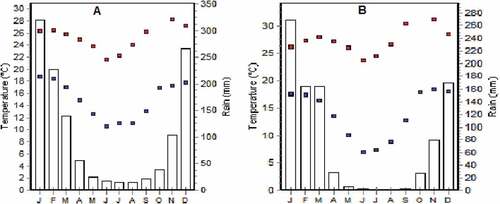
The annual precipitation and temperature for the two districts for the 2016/17 season are shown in Figure .
2.2. Questionnaire survey sample selection and size
The sampling strategy that was used for the study is presented in Table .
Table 3. Summary of sampling strategy used
A sample size of 90 for the study was determined following the procedure by Smith (Citation2013). The sampling frames used for farmer selection were lists of sorghum producers obtained from the Department of Agriculture, Technical Services and Extension. In each district, 45 farmers were randomly selected with the guidance of district agricultural extension officers. However, due to the non-availability of some of the initially selected respondents, 40 farmers were interviewed per district, resulting in an effective total sample of 80 sorghum farmers. Prior to conducting the main survey, a pretest study was conducted in December 2016. This was done to improve validity in data collection and interpretation of findings (Goerman et al., Citation2019), and to detect errors and remove ambiguity of questions (Hilton, Citation2017; Hurst et al., Citation2015; Ikart, Citation2019). After pre-testing, the questionnaire was revised before conducting the main survey. Two enumerators were trained and they administered the 80 questionnaires under supervision. Prior to the surveys, each farmer completed a consent form, confirming that s/he was voluntarily participating in the survey. Farmers were promised confidentiality and that information obtained from them was going to be used for academic purposes only. To ensure quality data during fieldwork, all questionnaires were checked daily for inconsistencies which were rectified prior to departing the district.
2.3. Physical weed sampling in sorghum fields
Field surveys were conducted during the December 2016 to May 2017 sorghum cropping season in the two districts. Thirty farms in each of the two districts were randomly selected to examine weed density in sorghum. One sorghum field was sampled per farm. Where a farmer had more than one sorghum field, one field was randomly selected. Systematic sampling, as outlined in the procedure by Chivinge (Citation1988), was done, with a few adjustments. Each field was divided diagonally and weeds were sampled after every 15 m following the diagonal lines. The starting point was 2 m from the edge of the field (Tibugari et al. unpublished data). In each field, four 40 cm × 40 cm steel quadrats were thrown along the diagonal lines and weeds in each quadrat were counted by species. The weed surveys were conducted three times; a week, 3 weeks, and 5 weeks after sorghum emergence. Sampling was conducted before farmers conducted weeding operations.
2.4. Data analysis
Data on major weeds found in sorghum fields and methods used to manage them were analysed using the Statistical Package for Social Sciences (IBM SPSS Statistics), Version 22. Results were presented in the form of simple descriptive statistics using tables. Field data on weed sampling were presented in the form of frequency tables. A comparison of the weed species that were recorded in the two districts was done in a tabular form. Field data for Insiza district were also compared with weeds that were found by Chivinge in 1988 in Matabeleland North and South Provinces, while field data for Chipinge district were compared with weeds that were found in Manicaland Province. This was done particularly to check if there had been new weeds in the smallholder farms since the 1988 weed survey.
3. Results and discussion
3.1. Questionnaire survey results
Farmers in both Insiza and Chipinge practiced hand hoeing, mechanical weeding and use of herbicides to control weeds (Table ).
Table 4. Weed management methods used in Insiza and Chipinge districts (N = 80)
Results show that hand weeding is the major weed control method practiced in the two districts. Thirteen percent (13%) of the respondents reported that they mechanically controlled weeds in the sorghum crop and only 2% said they used herbicides. The result that hand weeding dominated in the study areas was not surprising. Hand weeding is considered less costly than the use of herbicides. There is also a public view that plant protection products are unhealthy and cause negative impacts on biodiversity and environment (European Parliamentary Research Service [EPRS], Citation2019). Hand weeding, which is slow and inefficient (Gianessi, Citation2013) consumes 75% of the farmer’s time during the weeding period (Chivinge, Citation1990). Farmers in the two districts also controlled weeds mechanically. Mechanical weed control can be used together with hand weeding, thus reducing the labour required for hand weeding (Bàrberi, Citation2003). The result that a few farmers used mechanical weed control suggests that there needs to be changes in cultural practices from broadcasting to row planting to provide opportunity for use of mechanical cultivation given that the farmers have the needed implements and draught animal power. Innovative extension approaches that allow farmers opportunity to compare technologies could also improve weed management by the smallholder farmers.
Farmers who controlled weeds by hand hoeing were asked how frequently they weeded in a season. The results are shown in Table .
Table 5. Hand hoeing frequency
The result that a large percentage of farmers weeded sorghum twice and thrice suggests that sorghum is an important food crop in the two districts and therefore farmers try to protect it from weeds so as to realize maximum yields. The result that a small percentage of farmers weeded only once suggests that some farmers tend to neglect sorghum by giving weeding priorities to other crops. Weeding once could also mean that there is less weed pressure under sorghum in these areas.
The few farmers who reported that they used herbicides had labour shortages for manual weeding and they applied the herbicide glyphosate (Roundup) once in a season. The use of the herbicide by communal farmers was not surprising. Globally, glyphosate has dominated the herbicide market (Dayan, Citation2019) and has therefore been the most widely used herbicide (Barker & Dayan, Citation2019; Davoren & Schiestl, Citation2018; Duke et al., Citation2018; Heap & Duke, Citation2017), partly because it is the only herbicide that targets 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) (Duke & Powles, Citation2008), has broad-spectrum herbicidal activity (International Agency for Research on Cancer [IARC], Citation2015); Vazquez-Garcia et al., Citation2020) and also partly because of its high biodegradation (Duke & Powles, Citation2008) and increase in the use of glyphosate-tolerant crops (Duke et al., Citation2018). The herbicide, which is non-selective (Beckie et al., Citation2020; Duke et al., Citation2018), is phloem mobile (Duke, Citation2018) and has been shown to be a genotoxic carcinogen (IARC, Citation2015; Center for Food Safety, Citation2015; Myers et al., Citation2016; Van Bruggen et al., Citation2018; Davoren & Schiestl, Citation2018; Agostini et al., Citation2019). It can be assumed that, due to its non-selective properties, in Insiza and Chipinge, smallholder farmers who use glyphosate either apply it as a pre-emergence application, or apply it selectively during crop growth by using shields which prevent the spray from getting in contact with the crop, as suggested by Ampong-Nyarko and De Datta (Citation1991), Matthews (Citation2000), and Lebot (Citation2020). It is assumed that the smallholder farmers are aware of the potential health risks associated with the herbicide, that warrant its limited usage and use of appropriate protective clothing when conducting the mixing and spraying operations. It is also assumed that the smallholder farmers in Insiza and Chipinge districts are aware that weeds such as Amaranthus spp., Bidens pilosa and Eleusine indica have developed resistance to this herbicides, as research has already confirmed (Heap, Citation2020; Moss et al., Citation2019), and therefore reliance on glyphosate alone for their control may not be effective.
During focus group discussions it was revealed that farmers avoided using herbicides because calibration of herbicide application equipment required some degree of skill and literacy. Youths who could do the calibration were moving away from the family farms to urban areas in search for employment opportunities. It was also revealed that farmers had the perception that applying herbicides could damage the soil and contaminate water bodies. Some farmers were willing to use herbicides, but only if the chemicals were provided for free by government and development aid agencies. The result that low levels of literacy could affect herbicide usage suggests that smallholder farmers who want to use herbicides may require simplified training in herbicide application and safety. Poor access to herbicides by smallholder farmers can be a hindrance to their use. However, as argued by Lee and Thierfielder (Citation2017) increased access has to be accompanied by responsible use.
3.2. Physical weed sampling in sorghum fields
Weed density measurements that were conducted in farmer fields established that a number of weeds infested sorghum (Table ).
Table 6. Major weeds recorded (per square metre) in sorghum fields in Chipinge and Insiza districts
Table shows that more weeds were recorded in Chipinge compared to Insiza. Chipinge district receives more rains and is generally wetter than Insiza, possibly allowing a wider range of weeds to flourish compared to Insiza, which is generally dry. Some weed species such as Tagetes minuta, Commelina benghalensis, Amaranthus hybridus, Cleome monophylla, Richardia scabra, Cyperus spp., Cochorus spp., Datura stramonium, Setaria verticillata and Tribulus terrestris were not recorded in farmer fields in Insiza. The result that more weeds were recorded in Chipinge than Insiza suggests that other factors such as rainfall, and not necessarily the type of crop grown, may influence the weed species that dominates a particular ecological niche.
Table shows that the weed spectrum that was recorded by Chivinge in 1988 in both Insiza and Chipinge has not changed much. Tellier (Citation2018) observes that one crucial role played by seed banks is to ensure that there is a decrease in population extinction rate. The result that there has been little change in the weed spectrum for the past 30 years suggests that the store of seeds of the weed species in the soil seedbank has persisted, rather than depleting. A likely cause of soil seedbank persistence could be linked to the use of the same weed management method; hand weeding, year after year to control weeds by the smallholder farmers. It is also likely that farmers in the two districts possibly do not control weeds that infest the sorghum crop towards crop maturity, leaving weeds to flower and set seeds, consequently allowing inputs into the soil seedbank through seed rain. Chivinge (Citation1988) also made the observation that most small-scale non-commercial farmers abandon weeding once their crops have passed the flowering stage.
Table 7. Comparison of weeds reported in current survey and those reported in 1988
Of serious concern were two new problem weed species, namely C. dactylon and Panicum spp., that were recorded in Insiza, and four new weeds Cochorus spp., Datura stramonium, Setaria verticillata and Tribulus terrestris that were recorded in Chipinge. In the first national weed survey that was conducted by Chivinge in 1988, T. terrestris was recorded in Masvingo. Results reported here suggest that the weed has also spread to Manicaland Province.
4. Conclusions and recommendations
A diverse range of weeds that include Amaranthus hybridus, Richardia scabra, Tagetes minuta, Striga asiatica, Commelina benghalensis, Eleusine indica, Datura stramonium and Panicum spp. infested sorghum. Weed management in the smallholder sorghum growing districts is dominated by hand weeding. Gradual integration of existing weed management methods with modern weeding technologies will improve weed management in the smallholder farms.
Acknowledgements
The authors would like to appreciate the assistance of Mrs Evelyn Musora in conducting part of the interviews with farmers.
Additional information
Notes on contributors

Handsen Tibugari
Handsen Tibugari is a PhD student studying sorghum allelopathy at the University of Fort Hare in Alice, South Africa.
Cornelius Chiduza
Professor Cornelius Chiduza is an Agronomist at the University of Fort Hare.

Arnold Bray Mashingaidze
Professor Arnold Bray Mashingaidze is a Weed Scientist at Chinhoyi University of Technology in Zimbabwe. Work reported here is part of a series of studies on sorghum and its potential utilization for weed suppression through allelopathy.
References
- Agostini, L. P., Dettogni, R. S., Dos Reis, R. S., Stur, E., Dos Santos, E. V. W., Ventorim, D. P., Garcia, F. M., Cardoso, R. C., Graceli, J. B., & Louro, I. D. (2019). Effects of glyphosate exposure on human health: Insights from epidemiological and in vitro studies. Science of the Total Environment, 705, 135808. https://doi.org/10.1016/j.scitotenv.2019.135808
- Alsaadawi, I. S., Al-Khateeb, T. A., Hadwan, H. A., & Lahmood, N. R. (2015). A chemical basis for differential allelopathic potential of root exudates of sorghum bicolor (L.) Moench cultivars on companion weeds. Journal of Allelochemical Interactions, 1(1), 49–16.
- Ampong-Nyarko, K., & De Datta, S. K. (1991). A handbook for weed control in rice. International Rice Research Institute.
- Armengot, L., Blanco-Moreno, J. M., Bàrberi, P., Bocci, G., Carlesi, S., Aendekerk, R., Berner, A., Celette, F., Grosse, M., Huiting, H., Krantzler, A., Luik, A., Mäder, P., Peigné, J., Stoll, E., Delfosse, P., Sukkel, W., Surböck, A., Westaway, S., & Sans, F. X. (2016). Tillage as a driver of change in weed communities: A functional perspective. Agriculture, Ecosystems and Environment, 222, 276–285. https://doi.org/10.1016/j.agee.2016.02.021
- Ball, M. G., Caldwell, B. A., DiTommaso, A., Drinkwater, L. E., Mohler, C. L., Smith, R. G., & Ryan, M. R. (2019). Weed community structure and soybean yields in a long-term organic cropping systems experiment. Weed Science, 67(6), 673–681. https://doi.org/10.1017/wsc.2019.44
- Barber, T., Scott, B., & Norsworthy, J. (2015). Weed control in grain sorghum. In Arkansas grain sorghum production handbook (pp. 1–14). Division of Agriculture, Research and Extension, University of Arkansas.
- Bàrberi, P. (2003). Preventive and cultural methods for weed management. In R. Labrada (Ed.), Weed management for developing countries. Rome, Italy: Food and Agriculture Organisation of the United Nations.
- Barker, A. L., & Dayan, F. E. (2019). Fate of glyphosate during production and processing of glyphosate-resistant sugar beet (Beta vulgaris). Journal of Agricultural and Food Chemistry, 67(7), 2061–2065. https://doi.org/10.1021/acs.jafc.8b05672
- Baudron, F., Zaman-Allah, M. A., Chaipa, I., Chari, N., & Chinwada, P. (2019). Understanding the factors influencing fall armyworm (Spodoptera frugiperda J.E. Smith) damage in African smallholder maize fields and quantifying its impact on yield. A case study in Eastern Zimbabwe. Crop Protection, 120, 141–150. https://doi.org/10.1016/j.cropro.2019.01.028
- Beckie, H. J., Ashworth, M. B., & Flower, K. C. (2019). Herbicide resistance management: Recent developments and trends. Plants, 8(6), 161. https://doi.org/10.3390/plants8060161
- Beckie, H. J., Flower, K. C., & Ashworth, M. B. (2020). Farming without Glyphosate? Plants, 9(1), 96. https://doi.org/10.3390/plants9010096
- Belyea, L. R., & Lancaster, J. (1999). Assembly rules within a contingent ecology. Oikos, 86(3), 402–416. https://doi.org/10.2307/3546646
- Benaragama, D., Leeson, J. L., & Shirtliffe, S. I. (2019). Understanding the long-term weed community dynamics in organic and conventional crop rotations using the principal response curve method. Weed Science, 67(2), 195–204. https://doi.org/10.1017/wsc.2018.64
- Bergtold, J. S., Sant’Anna, A. C., Miller, N., Ramsey, S., & Fewell, J. E. (2017). Water scarcity and conservation along the biofuel supply chain in the United States: From farm to refinery. In J. R. Ziolkowska & J. M. Peterson (Eds.), Competition for water resources: Experiences and management approaches in the US and Europe (pp. 124–143). Elsevier.
- Bird, K., Sheperd, A., Scott, A., & Butaumocho, B. (2002, March). Coping strategies of poor households in semi-arid Zimbabwe. Volume 2. Full scientific report (Project Number: R7545). Natural Resources Systems Programme (NRSP).
- Boyles, R. E., Brenton, Z. W., & Kresovich, S. (2018). Genetic and genomic resources of sorghum to connect genotype with phenotype in contrasting environments. The Plant Journal, 97(1), 19–39. https://doi.org/10.1111/tpj.14113
- Brooke, G., & McMaster, C. (2019). Weed control in winter crops 2019. New South Wales Government, Department of Primary Industries.
- Buhler, D. D., Hartzler, R. G., & Forcella, F. (1997). Implications of weed seedbank dynamics to weed management. Weed Science, 45(3), 329–336. https://doi.org/10.1017/S0043174500092948
- CABI (Centre for Agriculture and Bioscience International). (2019). Online. https://www.cabi.org/isc/datasheet/47782
- Center for Food Safety. (2015, May). Glyphosate and cancer risk: Frequently asked questions. Factsheet.
- Chivinge, O. A. (1988). A weed survey of arable lands of the small-scale farming sector of Zimbabwe. Zambezia, 15(2), 167–179. https://digital.lib.msu.edu/projects/africanjournals/html/itemdetail.cfm?recordID=1363
- Chivinge, O. A. (1990). Weed science technological needs for the communal areas of Zimbabwe. Zambezia, 17(2), 133–143. http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.608.3097&rep=rep1&type=pdf
- Coleman, G. R. Y., Stead, A., Rigter, M. P., Xu, Z., Johnson, D., Brooker, G. M., Sukkarieh, S., & Walsh, M. J. (2019). Using energy requirements to compare the suitability of alternative methods for broadcast and site-specific weed control. Weed Technology, 33(4), 633–650. https://doi.org/10.1017/wet.2019.32
- Davoren, M. J., & Schiestl, R. H. (2018). Glyphosate-based herbicides and cancer risk: A post-IARC decision review of potential mechanisms, policy and avenues of research. Carcinogenesis, 39(10), 1207–1215. https://doi.org/10.1093/carcin/bgy105
- Dayan, F. E. (2019). Current status and future prospects in herbicide discovery. Plants, 8(9), 341. https://doi.org/10.3390/plants8090341
- Duke, S. O. (2003). Weeding with transgenes. Trends in Biotechnology, 21(5), 192–195. https://doi.org/10.1016/S0167-7799(03)00056-8
- Duke, S. O. (2018). The history and current status of glyphosate. Pest Management Science, 74(5), 1027–1034. https://doi.org/10.1002/ps.2018.74.issue-5
- Duke, S. O., & Powles, S. B. (2008). Glyphosate: A once-in-a-century herbicide. Pest ManagementScience, 64(4), 319–325. https://doi.org/10.1002/ps.1518
- Duke, S. O., Powles, S. B., & Sammons, R. D. (2018). Glyphosate – How it became a once in a hundred year herbicide and its future. Outlooks on Pest Management, 29(6), 1–5. https://doi.org/10.1564/v29_dec_00
- EPRS (European Parliamentary Research Service). (2019, March). Farming without plant protection products. Can we grow without using herbicides, fungicides and insecticides? In-depth analysis. European parliamentary research service, scientific foresight unit (PE 634.416). European Union.
- FAO (Food and Agriculture Organisation of the United Nations), ICRISAT (International Crops Research Institute for the Semi-Arid Tropics). (1996). The world sorghum and millet economies: Facts, trends and outlook.
- FAOSTAT. (2017). Production – Crops –Area harvested/yield/production quantity – Sorghum – 2014, FAO Statistics (online database). Food and Agriculture Organization. Retrieved October 14, 2019, from www.fao.org/faostat/en
- Fennimore, S., & Cutulle, M. (2019). Robotic weeders can improve weed control options for specialty crops. Pest Management Science, 75(7), 1767–1774. https://doi.org/10.1002/ps.5337
- Ferrell, J. A., MacDonald, G. E., & Brecke, B. J. (2018). Weed management in sorghum (SS-AGR-06). Institute of Food and Agricultural Sciences, University of Florida.
- Filho, J. S., Marchão, R. L., De Carvalho, A. M., & Ricardo Carmona, R. (2014). Weed dynamics in grain sorghum-grass intercropped systems. Revista Ciência Agronômica, 45(5), 1032–1039. https://doi.org/10.1590/S1806-66902014000500019
- Gage, K. L., & Schwartz-Lazaro, L. M. (2019). Shifting the paradigm: An ecological systems approach to weed management. Agriculture, 9(8), 179. https://doi.org/10.3390/agriculture9080179
- Gianessi, L. P. (2013). The increasing importance of herbicides in worldwide crop production. Pest Management Science, 69(10), 1099–1105. https://doi.org/10.1002/ps.2013.69.issue-10
- Goerman, P., Meyers, M., & Trejo, Y. G. (2019). The place of expert review in translation and questionnaire evaluation for hard-to-count populations in national surveys. Research report series (Survey Methodology #2019-02). Center for Behavioral Science Methods Research and Methodology Directorate U.S. Census Bureau Washington, D. C. 202033.
- Griebel, S., Webb, M. M., Campanella, O. H., Craig, B. A., Weil, C. F., & Tuinstra, M. R. (2019). The alkali spreading phenotype in Sorghum bicolor and its relationship to starch gelatinization. Journal of Cereal Science, 86(2019), 41–47. https://doi.org/10.1016/j.jcs.2019.01.002
- Haji, J., & Tegegne, B. (2018). Technical efficiency of sorghum production: The case of smallholder farmers in Konso District, Southern Ethiopia. Journal of Agricultural Economics, Extension and Rural Development, 6(7), 772–793. https://doi.org/10.20448/journal.523.2018.31.1.15
- Hale, R. R., Bararpour, T., Kaur, G., Seale, J. W., Singh, B., & Wilkerson, T. (2019). Sensitivity and recovery of grain sorghum to simulated drift rates of glyphosate, glufosinate, and paraquat. Agriculture, 9(4), 70. https://doi.org/10.3390/agriculture9040070
- Harker, K. N., & O’Donovan, J. T. (2013). Recent weed control, weed management, and integrated weed management. Weed Technology, 27(1), 1–11. https://doi.org/10.1614/WT-D-12-00109.1
- Heap, I. (2019, October 16). The international survey of herbicide resistant weeds. Corvallis, OR: International Survey of Herbicide-Resistant Weeds. Online. www.weedscience.org
- Heap, I. (2020, March 2). The international herbicide-resistant weed database. Corvallis, OR: International Survey of Herbicide-Resistant Weeds. Online. www.weedscience.org
- Heap, I., & Duke, S. O. (2017). Overview of glyphosate-resistant weeds worldwide. Pest Management Science, 74(5), 1040–1049. https://doi.org/10.1002/ps.4760
- Heeb, L., Jenner, E., & Cock, M. J. W. (2019). Climate-smart pest management: Building resilience of farms and landscapes to changing pest threats. Journal of Pest Science, 92(3), 951–969. https://doi.org/10.1007/s10340-019-01083-y
- Hilton, C. E. (2017). The importance of pretesting questionnaires: A field research example of cognitive pretesting the Exercise referral Quality of Life Scale (ER-QLS). International Journal of Social Research Methodology, 20(1), 21–34. https://doi.org/10.1080/13645579.2015.1091640
- Hurst, S., Arulogun, O. S., Owolabi, M. O., Akinyemi, R., Uvere, E., Warth, S., & Ovbiagele, B. (2015). Pretesting qualitative data collection procedures to facilitate methodological adherence and team building in Nigeria. International Journal of Qualitative Methods, 14(1), 53–64. https://doi.org/10.1177/160940691501400106
- Hyvönen, T., & Salonen, J. (2002). Weed species diversity and community composition in cropping practices at two density levels – A six-year experiment. Plant Ecology, 159(1), 73–81. https://doi.org/10.1023/A:1015580722191
- IARC (International Agency for Research on Cancer). (2015). Iarc monographs (Vol. 112). World Health Organisation.
- Igawa, H., Tanaka, T., Kaneko, S., Tada, T., & Suzuki, S. (2009, November 3–5). Visual and tactual recognition of trunk of grape for weeding robot in vineyards. Proceedings of the 35th Annual Conference of the Industrial Electronics Society (IECON 2009) (pp 4274–4279).
- Ikart, E. M. (2019). Survey questionnaire survey pretesting method: An evaluation of survey questionnaire via expert reviews technique. Asian Journal of Social Science Studies, 4(2), 1–17. https://doi.org/10.20849/ajsss.v4i2.565
- Kakabouki, I., Karkanis, A., Travlos, I. S., Hela, D., Papastylianou, P., Wu, H., Chachalis, D., Sestras, R., & Bilalis, D. (2015). Weed flora and seed yield in quinoa crop (Chenopodium quinoa Willd.) as affected by tillage systems and fertilization practices. International Journal of Pest Management, 61(3), 228–234. https://doi.org/10.1080/09670874.2015.1042413
- Kante, M., Rattunde, F., Nébié, B., Sissoko, I., Diallo, B., Diallo, A., Touré, A., Weltzien, E., Haussman, B. I. G., & Leiser, W. L. (2019). Sorghum hybrids for low-input farming systems in West Africa: Quantitative genetic parameters to guide hybrid breeding. Crop Science, 59(6), 1–19. https://doi.org/10.2135/cropsci2019.03.0172
- Knezevic, S. Z., Evans, S. P., Blankenship, E. E., Van Acker, R. C., & Lindquist, J. L. (2002). Critical period for weed control: The concept and data analysis. Weed Science, 50(6), 773–786. https://doi.org/10.1614/0043-1745(2002)050[0773:CPFWCT]2.0.CO;2
- Korres, N. E., Burgos, N. R., Travlos, I., Vurro, M., Gitsopoulos, T. K., Varanasi, V. K., Duke, S. O., Kudsk, P., Brabham, C., Rouse, C. E., & Salas-Perez, R. (2019). New directions for integrated weed management: Modern technologies, tools and knowledge discovery. Advances in Agronomy, 155, 243–319. https://doi.org/10.1016/bs.agron.2019.01.006
- Kumar, A., Naresh, V. R. K., Ghasal, P. C., Kumar, R., Singh, V., Kumar, S., & Chaudhary, K. (2017). Weed management effects on weed control efficiency, yield and economics of transplanted rice in typic ustochrept soil of Uttar Pradesh. International Journal of Chemical Studies, 5(4), 1346–1351. http://www.chemijournal.com/archives/?year=2017&vol=5&issue=4&ArticleId=816
- Lebot, V. (2020). Tropical root and tuber crops: Cassava, sweet potato, yams and aroids (2nd ed.). Crop Production Science in Horticulture, CABI.
- Lee, N., & Thierfelder, C. (2017). Weed control under conservation agriculture in dryland smallholder farming systems of southern Africa. a review. Agronomy for Sustainable Development, 37(48),1–25.
- Lowenberg-DeBoer, J., Huang, I. Y., Grigoriadis, V., & Blackmore, S. (2019). Economics of robots and automation in field crop production. Precision Agriculture, 1–22. https://doi.org/10.1007/s11119-019-09667-5
- Mabhaudhi, T., Chimonyo, V. G. P., Hlahla, S., Massawe, F., Mayes, S., Nhamo, L., & Modi, A. T. (2019). Prospects of orphan crops in climate change. Planta, 250, 695–708. https://doi.org/10.1007/s00425-019-03129-y
- Macías, F. A., Mejías, F. J. R., & Molinillo, J. M. G. (2019). Recent advances in allelopathy for weed control: From knowledge to applications. Pest Management Science, 75(9), 2413–2436. https://doi.org/10.1002/ps.5355
- Matthews, G. A. (2000). Pesticide application methods (3rd ed.). Blackwell Science Limited.
- Meiss, H., Mediene, S., Waldhardt, R., Caneill, J., & Munier-Jolain, N. (2010). Contrasting weed species composition in perennial alfalfas and six annual crops: Implications for integrated weed management. Agronomy for Sustainable Development, Springer Verlag/EDP Sciences/INRA, 30(3), 657–666. https://doi.org/10.1051/agro/2009043
- Metcalfe, H., Hassall, K. L., Boinot, S., & Storkey, J. (2019). The contribution of spatial mass effects to plant diversity in arable fields. Journal of Applied Ecology, 56(7), 1560–1574. https://doi.org/10.1111/1365-2664.13414
- Mohammed, S. A., & Denboba, M. A. (2020). Study of soil seed banks in ex-closures for restoration of degraded lands in the central Rift Valley of Ethiopia. Scientific Reports, 10(1), 956. https://doi.org/10.1038/s41598-020-57651-1
- Moss, S., Ulber, L., & Den Hoed, I. (2019). A herbicide resistance risk matrix. Crop Protection, 115, 13–19. https://doi.org/10.1016/j.cropro.2018.09.005
- Mrema, E., Shimelis, H., Laing, M., & Bucheyeki, T. (2016). Farmers’ perceptions of sorghum production constraints and Striga control practices in semi-arid areas of Tanzania. International Journal of Pest Management, 63(2), 146–156. https://doi.org/10.1080/09670874.2016.1238115
- Mundia, C. W., Secchi, S., Akamani, K., & Wang, G. (2019). A regional comparison of factors affecting global sorghum production: The case of North America, Asia and Africa’s Sahel. Sustainability, 11(7), 2135. https://doi.org/10.3390/su11072135
- Myers, J. P., Antoniou, M. N., Blumberg, B., Carroll, L., Colborn, T., Everett, L. G., Hansen, M., Landrigan, P. J., Lanphear, B. P., Mesnage, R., Vandenberg, L. N., Vom Saal, F. S., Welshons, W. V., Charles, M., & Benbrook, C. M. (2016). Concerns over use of glyphosate-based herbicides and risks associated with exposures: A consensus statement. Environmental Health, 15(1), 19. https://doi.org/10.1186/s12940-016-0117-0
- N’cho, S. A., Mourits, M., Rodenburg, J., & Lansink, A. O. (2019). Inefficiency of manual weeding in rainfed rice systems affected by parasitic weeds. Agricultural Economics, 50(2), 151–163. https://doi.org/10.1111/agec.2019.50.issue-2
- Nichols, V., Verhulst, N., Cox, R., & Govaerts, B. (2015). Weed dynamics and conservation agriculture principles: A review. Field Crops Research, 183, 56–68. https://doi.org/10.1016/j.fcr.2015.07.012
- Nikolić, N., Squartini, A., Concheri, G., Stevanato, P., Zanin, G., & Masin, R. (2020). Weed seed decay in no-till field and planted riparian buffer zone. Plants, 9(3), 293. https://doi.org/10.3390/plants9030293
- Nwosisi, S., Nandwani, D., & Hui, D. (2019). Mulch treatment effect on weed biomass and yields of organic sweetpotato cultivars. Agronomy, 9(4), 190. https://doi.org/10.3390/agronomy9040190
- OECD. (2010). Consensus document on compositional considerations for new varieties of grain sorghum [Sorghum bicolor (L.) Moench]: Key food and feed nutrients and antinutrients (Series on the safety of novel foods and feeds No. 19, ENV/JM/MONO(2010)26). Environment, Health and Safety Publications.
- OECD (Organisation for Economic Cooperation and Development). (2017). Safety assessment of transgenic organisms in the environment: OECD Consensus Documents (Vol. 7).
- Orr, A., Mwema, C., Gierend, A., & Nedumaran, S. (2016). Sorghum and millets in Eastern and Southern Africa. Facts, trends and Outlook (Working Paper Series No. 62). ICRISAT Research Program, Markets, Institutions and Policies. p. 76.
- Partel, V., Kakarla, S. C., & Ampatzidis, Y. (2019). Development and evaluation of a low-cost and smart technology for precision weed management utilizing artificial intelligence. Computers and Electronics in Agriculture, 157, 339–350. https://doi.org/10.1016/j.compag.2018.12.048
- Peters, K., Breitsameter, L., & Gerowitt, B. (2014). Impact of climate change on weeds in agriculture: A review. Agronomy for Sustainable Development, 34(4), 707–721. https://doi.org/10.1007/s13593-014-0245-2
- Phiri, K., Dube, T., Moyo, P., Ncube, C., Ndlovu, S., & Buchenrieder, G. (2019). Small grains “resistance”? Making sense of Zimbabwean smallholder farmers’ cropping choices and patterns within a climate change context. Cogent Social Sciences, 5(1), 1622485. https://doi.org/10.1080/23311886.2019.1622485
- Proctor, S., Henson, S., Loader, R., Masakure, O., Brouder, A., Bhila, L., & Sigauke, N. (2000). Facilitating the effective production and marketing of processed food products by small-scale producers in Zimbabwe (Project R7485). Department for International Development, UK.
- Reiser, D., Sehsar, E.-S., Bumann, O., Morhard, J., & Griepentrog, H. W. (2019). Development of an autonomous electric robot implement for intra-row weeding in vineyards. Agriculture, 9(1), 18. https://doi.org10.3390/agriculture9010018
- Sabanci, K., & Aydin, C. (2017). Smart robotic weed control system for sugar beet. Journal of Agricultural Science and Technology, 19, 73–83. https://journals.modares.ac.ir/article-23-3117-en.html
- Schwartz-Lazaro, L. M., & Copes, J. T. (2019). A review of the soil seedbank from a weed scientist’s perspective. Agronomy, 9(7), 369. https://doi.org/10.3390/agronomy9070369
- Scott, J. K., Webber, B. L., Murphy, H., Ota, N., Kriticos, D. J., & Loechel, B. (2014). Weeds and climate change: Supporting weed management adaptation. www.AdaptNRM.org
- Siemens, M. C. A. (2014, January 22–24). Robotic weed control. Proceedings 66th Annual California Weed Science Society “Meeting the challenge for a hungry world: Weed management strategies in the coming decade” Portola Hotel & Spa, Two Portola Plaza Monterey.
- Sims, B., Corsi, S., Gbehounou, G., Kienzle, J., Taguchi, M., & Theodor Friedrich, T. (2018). Sustainable weed management for conservation agriculture: Options for smallholder farmers. Agriculture, 8(8), 118. https://doi.org/10.3390/agriculture8080118
- Slaughter, D. C., Giles, D. K., & Downey, D. (2008). Autonomous robotic weed control systems: A review. Computers and Electronics in Agriculture, 61(1), 63–78. https://doi.org/10.1016/j.compag.2007.05.008
- Smale, M., Assima, A., Kergna, A., Thériault, V., & Weltzien, E. (2018). Farm family effects of adopting improved and hybrid sorghum seed in the Sudan Savanna of West Africa. Food Policy, 74, 162–171. https://doi.org/10.1016/j.foodpol.2018.01.001
- Smith, S. M. (2013). Determining sample size. Qualtrics blog. https://www.ndsu.edu/gdc/wp-content/pdf/Determining-Sample-Size.pdf
- Stepanovic, S., Datta, A., Neilson, B., Bruening, C., Shapiro, C., Gogos, G., & Knezevic, S. Z. (2016). The effectiveness of flame weeding and cultivation on weed control, yield and yield components of organic soybean as influenced by manure application. Renewable Agriculture and Food Systems, 31(4), 288–299. https://doi.org/10.1017/S1742170515000216
- Tadesse, A. (ed..) (2008). Increasing crop production through improved plant protection – Volume I. Plant Protection Society of Ethiopia (PPSE). PPSE and EIAR. 19–22 December 2006.
- Tellier, A. (2018). Persistent seed banking as eco-evolutionary determinant of plant nucleotide diversity: Novel population genetics insights. New Phytologist, 2019(221), 725–730. https://doi.org/10.1111/nph.15424
- Trendov, N. M., Varas, S., & Zeng, M. 2019. Digital technologies in agriculture and rural areas (Briefing Paper). Food and Agriculture Organization of the United Nations.
- Trezzi, M. M., Vidal, R. A., Balbinot, A. A., Bittencourt, H. H., & Filho, A. P. S. S. (2016). Allelopathy: Driving mechanisms governing its activity in agriculture. Journal of Plant Interactions, 11(1), 53–60. https://doi.org/10.1080/17429145.2016.1159342
- Uddin, M. R., Park, S. U., Dayan, F. E., & Pyon, J. Y. (2014). Herbicidal activity of formulated sorgoleone, a natural product of sorghum root exudate. Pest Management Science, 70, 252–257.
- UNCTAD (United Nations Conference on Trade and Development). (2016). Second generation biofuel markets: State of play, trade and developing country perspectives. United Nations.
- Valverde, B. E. 2003. Progress on Rottboellia cochinchinensis management (FAO Plant Production and Protection Paper No. 120(Add.1)). p. 67–79. Rome, Italy: FAO.
- Van Bruggen, A. H. C., He, M. M., Shin, K., Mai, V., Jeong, K. C., Finckh, M. R., & Morris, J. G., Jr. (2018). Environmental and health effects of the herbicide glyphosate. Science of the Total Environment, 616–617, 255–268. https://doi.org/10.1016/j.scitotenv.2017.10.309
- Vazquez-Garcia, J. G., Palma-Bautista, C., Rojano-Delgado, A. M., De Prado, R., & Menendez, J. (2020). The first case of glyphosate resistance in Johnsongrass (Sorghum halepense (L.) Pers.) in Europe. Plants, 9(3), 313. https://doi.org/10.3390/plants9030313
- Westwood, J. H., Charudattan, R., Duke, S. O., Fennimore, S. A., Marrone, P., Slaughter, D. C., Swanton, C., & Zollinger, R. (2018). Weed management in 2050: Perspectives on the future of weedscience. Weed Science, 66(3), 275–285. https://doi.org/10.1017/wsc.2017.78
- Wilson, B. J., Wright, K. J., Brain, P., Clements, M., & Stephens, E. (1995). Predicting the competitive effects of weed and crop density on weed biomass, weed seed production and crop yield in wheat. Weed Research, 35(4), 265–278. https://doi.org/10.1111/j.1365-3180.1995.tb01789.x
- Winchell, F., Brass, M., Manzo, A., Beldados, A., Perna, V., Murphy, C., Stevens, C., & Fuller, D. Q. (2018). On the origins and dissemination of domesticated sorghum and pearl millet across Africa and into India: A view from the Butana Group of the far Eastern Sahel. AfricanArchaeological Review, 35(4), 483–505. https://doi.org/10.1007/s10437-018-9314-2
- Wortmann, C., Mamo, M., Mburu, C., Letayo, E., Abebe, G., Kayuki, K. C., Chisi, M., Mativavarira, M., Xerinda, S., & Ndacyayisenga, T. 2009. Atlas of sorghum production in Eastern and Southern Africa. University of Nebraska-Lincoln.
- Young, S. L., Meyer, G., & Woldt, W. (2014). Future directions for automated weed management in precision agriculture. West Central Research and Extension Center.
- Ziska, L., & Dukes, J. S. (2011). Weed biology and climate change. Weed Biology and Climate Change, p. 248. Ames, Lowa: Wiley-Blackwell. https://doi.org/10.1002/9780470958674
- Ziska, L. H. (2003). Evaluation of yield loss in field sorghum from a C3 and C4 weed with increasing CO2. Weed Science, 51(6), 914–918. https://doi.org/10.1614/WS-03-002R