Abstract
Economically important vegetable crop lettuce (Lactuca sativa L.) of family Asteraceae was selected for the present investigation. It is being cultivated in UAE due to its commercial importance. In lettuce cultivation, the major problem is the requirement of large quantities of irrigation water. The present study was aimed to reduce the water consumption of lettuce cultivation; for that, a varied irrigation regime was used with the application of abscisic acid (ABA). The parameters studied were biochemical constituents, antioxidant potential and antioxidant enzymes’ activities in lettuce plants under drought stress and its response to ABA under stress. Drought stress caused an increase in the biochemical constituents like proline and amino acid contents when compared with control and also increased under individual ABA treatments and treatments under drought stress. The non-enzymatic antioxidant molecules like ascorbate and α-tocopherol showed significant increase under drought condition in lettuce. ABA slightly reduced these contents. The antioxidant enzymes like superoxide dismutase, catalase and peroxidase showed significant increase under drought condition and ABA caused significant enhancement in these antioxidant enzymes under drought stress and also in unstressed conditions, thereby protecting the plants from the deleterious effects of drought stress. From the results of this investigation, it can be concluded that ABA in 10 mg g−1 can be used as a potential tool to minimise the drought stress effects in lettuce cultivation.
Public Interest Statement
Lettuce (Lactuca sativa L.) is an economically important vegetable crop of the family Asteraceae. It is being cultivated in UAE due to its commercial importance. In lettuce cultivation, the major problem is the requirement of large quantities of irrigation water. The present study was aimed to reduce the water consumption of lettuce cultivation; for that, a varied irrigation regime was used with the application of abscisic acid.
Competing interests
The authors declare no competing interest.
1. Introduction
Plants experience many different kinds of abiotic stresses like higher concentration of salt, drought, temperature and heavy metals which largely influence plant development and crop productivity. A major threat to food security due to constant changes of climate and deterioration of environment has been observed for the past few years (Suzuki, Rivero, Shulaev, Blumwald, & Mittler, Citation2014). Among abiotic stresses, the drought has been the major environmental factors limiting the growth and productivity of agronomically important plants (Anjum et al., Citation2011). Plants have developed several biochemicals, physiological and metabolic strategies in order to combat such abiotic stresses (Gupta, Sengupta, Saha, & Gupta, Citation2013). The plant hormone abscisic acid (ABA) is a stress hormone that is accumulated mainly under drought stress (Zhang, Jia, Yang, & Ismail, Citation2006). Spraying ABA on leaves increases the growth of Ilex paraguariensis plants by ameliorating water stress by promoting stomatal closure that was positively correlated with an increase in the RWC in leaves (Sansberro, Mroginski, & Bottini, Citation2004). ABA synthesis triggers major reprogramming of the transcriptome, stomatal closure and restraints transpirational water loss (Raghavendra, Gonugunta, Christmann, & Grill, Citation2010). ABA increase due to drought stress can cause pod abortion (Liu, Jensen, & Andersen, Citation2004). Previously, Liu et al. (Citation2004) reported seed abortion in soybean under ABA influence. It is reported to gradually degrade upon removal of stress (Zhang et al., Citation2006). Zhang et al. (Citation2011) reported that ABA pretreatment further increased the endogenous level in maize seedling. Pre-soaking seed treatment with ABA was reported to significantly enhance the antioxidant enzymes activity in maize seedlings subjected to water stress (Bano, Ullah, & Nosheen, Citation2012). Similarly, Nishiyama et al. (Citation2011) found that relative water content of ABA-treated plants was higher under drought stress.
Lettuce (Lactuca sativa L.) is a vegetable which contains many nutrients (Dan, Qiang, Zhaonan, & Zhengquan, Citation2014). Cultivated lettuce is an important salad crop which is grown throughout the world. Lettuce is also widely grown as a vegetable in home gardens. Lettuce is extremely sensitive to drought due to its shallow root system (Kizil, Genc, Inalpulat, Şapolyo, & Mirik, Citation2012). It is especially important as a commercial crop in Asia, North and Central America and Europe. China, the USA, Spain, Italy, India and Japan are among the world’s largest producers. Nutritionally, it rates low among other vegetable crops; 95% of the crop contains water with varying amounts of phosphorus, iron, sodium and potassium, depending on the morphological type. Leaf lettuces have higher levels of ascorbic acid, vitamin A and calcium (Martínez-Sánchez, Tudela, Luna, Allende, & Gil, Citation2011). So, there is a requirement to investigate the efficacy of this ABA in the enhancement of biochemical, antioxidant potentials and antioxidant enzymes in lettuce plants to increase their yield under different irrigation intervals. Hence, this analysis aims to evaluate the capability of ABA to enhance the biochemical constituents with special emphasis on both non-enzymatic and enzymatic antioxidant constituents under varied irrigation regimes.
2. Materials and methods
A greenhouse experiment was conducted to evaluate the physiological response of lettuce under deficit irrigation with or without exogenous ABA application. The greenhouse experiment was carried out in Al-Foah Experimental Station (270° N and 220° S latitude and 510° W and 570° E longitude) of College of Food and Agriculture, UAEU in Alain city, 160 km from Eastern Abu Dhabi, the capital city of UAE. The greenhouse environment was controlled for temperature and relative humidity. Accordingly, during the experimental periods, the temperature of the greenhouse was maintained at 24 ± 2°C. The greenhouse allows the entrance of the natural light and hence artificial light was not applied. The methodologies adopted are described below.
2.1. Cultivation methods and experimental design
Lactuca sativa var. longifolia seeds were obtained from Lortolano company, Italy, kindly provided by Agricultural inputs commercial supplier “Shat Alarab”. Seed sowing was carried out manually on the 10 February 2012 in polystyrene trays filled with different media with 84 cells (peatmoss:perlite 2:1). In this experiment, the trays were kept in the greenhouse and irrigated as needed with tap water until germination. After emergence (15 days after sowing), the seedlings were thinned to retain three seedlings in each cell. Seedlings were under natural light conditions; ventilation was provided automatically when the air temperature exceeded 28°C by a cooling system.
Plastic pots of 40 cm diameter and 45 cm height size were used for the study. The pots were filled with 10 kg of soil mixture containing red soil, sand and commercial potting soil at 1:1:1 ratio. Three seedling of lettuce were transplanted into the pots. In a week period, second thinning was applied to retain the healthiest seedling for the experiment.
2.2. Drought stress induction and ABA application in pot culture
The experiment was carried out with several drought levels and ABA concentrations were applied exogenously. The drought factors were expressed as different irrigation intervals and fixed water quantity (200 ml). The irrigation intervals are 24 h, considered as a common control (well watered), 48 h, 72 h and 96 h and applied manually for each pot. Moreover, the lettuce samples were subjected to different concentrations of ABA. The ABA effect was studied at different concentrations (not sprayed (control), 5 µg l−1, 10 µg l−1 and 15 µg l−1). ABA concentrations were applied manually using a 500-ml sprayer for each plant. The experimental units are arranged in a completely randomized block design (CRBD). The plants were allowed to grow up to 15 days after the transplantation under daily irrigation, which is considered as control. Fifteen days after transplantation, the plants were treated with different ABA concentrations, different intervals of drought stress and ABA with drought combinations. On the 75th day, plants were uprooted gently, washed carefully and packed in labelled plastic bags for further analysis. From the uprooted plants, leaves were collected for experiments. All leaf samples were analysed to measure biochemical contents, enzymatic and non-enzymatic antioxidants.
2.3. Biochemical analysis
Proline content was estimated following the method of Bates, Waldren, and Teare (Citation1973). Amino acid content was estimated by following the method of Moore and Stein (Citation1948).
2.4. Antioxidants
Ascorbic acid content was estimated as described by Omaye, David Turnbull, and Sauberlich (Citation1979) and α-tocopherol content by following Baker, Frank, DeAngelis, and Feingold (Citation1980).
2.5. Antioxidant enzymes
The superoxide dismutase (SOD, EC: 1.15.1.1) extraction was done by the method of Hwang, Lin, Chern, Lo, and Li (Citation1999). The enzyme protein was determined by Bradford (Citation1976) method. Superoxide dismutase activity was assayed as described by Beauchamp and Fridovich (Citation1971). Superoxide dismutase activity was expressed in units per mg protein. One unit is defined as the amount of change in the absorbance by 0.1 per hour per milligram protein under the assay condition (Cherry, Citation1963). Catalase (CAT, EC: 1.11.1.6) activity was assayed as described by Chandlee and Scandalios (Citation1984). The enzyme activity was expressed in units 1 mM of H2O2 reduction per minute per mg protein. Peroxidase (POX, EC: 1.11.1.7) was assayed by the method of Kumar and Khan (Citation1982). The activity was expressed in unit mg−1 protein. One unit is defined as the change in the absorbance by 0.1 min−1 mg−1 protein.
3. Results and discussion
3.1. Biochemical analysis
3.1.1. Proline
In lettuce, drought stress caused increased accumulation of proline content at all stages of growth. ABA also resulted in increased proline content in unstressed and stressed lettuce. ABA in combination with drought caused, again, an enhancement in proline content when compared to stress and well-watered control plants. 10-µg ABA treatment on 72 h irrigation regime increased the proline content significantly when compared to all other treatments (Figure ).
Figure 1. Effect of varied irrigation regimes, ABA and their combination on proline contents (mg g−1 dry weight) in lettuce plants on the 75th day after planting
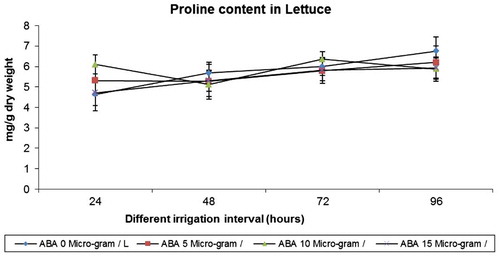
In lettuce, drought stress caused increased accumulation of proline content of the leaves at all water deficit irrigation intervals. ABA also resulted in increased proline content in lettuce in control and drought stressed plants. Water stress resulted in an increase in proline accumulation in sorghum (Yadav, Jyothi Lakshmi, Maheswari, Vanaja, & Venkateswarlu, Citation2005). Enhancement in proline content of stressed plants may be an adaptation to overcome the stress. Proline accumulated under stressed conditions supplies energy for growth and survival, and thereby helps the plant to tolerate stress (Jaleel et al., Citation2007). Proline accumulation in plants might be a scavenger and act as osmolytes. ABA increased the proline content in Phaseolus vulgaris (Mackay, Christopher Hall, Hofstra, & Fletcher, Citation1990).
3.1.2. Amino acids
Water deficit stress increased the amino acid content in lettuce on all irrigation regimes. The amino acid content increased under individual ABA treatments and treatments under drought stress. The extent of increase was more in 15 ABA on 96 h irrigation regime (Figure ).
Figure 2. Effect of varied irrigation regimes, ABA and their combination on amino acid contents (mg g−1 dry weight) in lettuce plants on the 75th day after planting
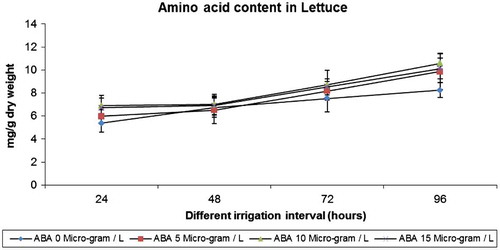
Drought stress caused increased amino acid content when compared to control in lettuce plants. The amino acid content increased under individual ABA treatments and treatments under drought stress. The amino acid content has been shown to increase under drought condition in sunflower (Manivannan et al., Citation2007). Accumulated amino acid may be occurring in response to the change in osmotic adjustment of their cellular contents (Shao et al., Citation2007). Amino acids accumulation plays a very important role in drought tolerance, probably through osmotic adjustment in different plant species, such as Radix astragali (Tan, Liang, Shao, & Du, Citation2006).
3.2. Antioxidants
3.2.1. Ascorbic acid
In lettuce, ascorbic acid content was increased with drought stress when compared to control plants. ABA decreased the ascorbic acid content when compared to well-watered and stressed plants (Figure ).
Figure 3. Effect of varied irrigation regimes, ABA and their combination on ascorbic acid contents (mg g−1 dry weight) in lettuce plants on the 75th day after planting
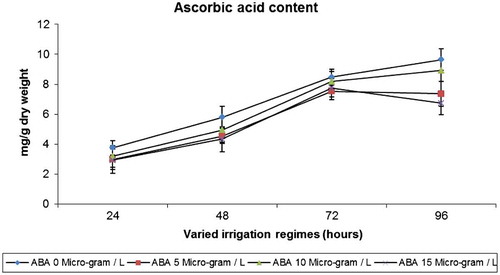
The ascorbic acid content increased with drought in lettuce plants. ABA decreased the ascorbic acid content when compared to control. Compared with controls, plants under drought stress displayed increased AA levels throughout the experimental period, whereas ABA treatment lowered AA levels. Treatment in the ABA + drought group increased AA level lower than those of drought treatment alone. These results indicate that the enhancement of AA level is correlated with both exogenous ABA and drought stress. Similar results were reported by Guo et al. (Citation2012) in pepper (Capsicum annuum) leaves subjected to chilling stress and exogenous ABA application. Ascorbate is one of the most extensively studied antioxidant and has been detected in the majority of plant cell types, organelles and apoplasts (Smirnoff, Citation2000). Water stress resulted in significant increases in antioxidant AA concentration in turf grass (Zhang & Schmidt, Citation2000). A decrease in ascorbic acid was reported in ABA treatment in plants (Zhang et al., Citation2006).
3.2.2. α-Tocopherol
α-Tocopherol of the drought stressed plant roots significantly increased when compared to control plants. ABA was an inhibitor of α-tocopherol individually and also under drought stress. ABA application resulted in a significant reduction in α-tocopherol content in lettuce when compared to control and different irrigation regimes (Figure ).
Figure 4. Effect of varied irrigation regimes, ABA and their combination on α-tocopherol contents (mg g−1 fresh weight) in lettuce plants on the 75th day after planting
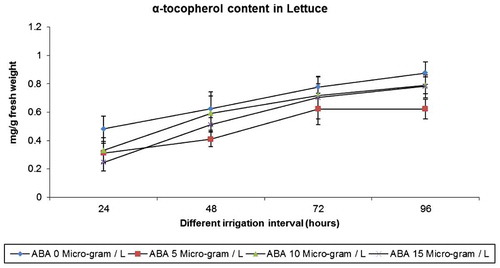
α-Tocopherol of the drought stressed plants significantly increased when compared to control plants. ABA was an inhibitor of α-tocopherol individually and also under drought stress. ABA increased the α-tocopherol content, but it was significantly less than drought stressed and control plants. The active oxygen species formed at the membrane of wheat leaves under drought stress was efficiently removed upon rehydration with increase in the α-tocopherol and β-carotene (Bartoli et al., Citation1999). A similar result was observed in pea (Simontacchi, Caro, Fraga, & Puntarulo, Citation1993) chilling stress. Previous studies have demonstrated the important roles of AA in the tolerance of plants to environmental stresses. For instance, Li, Yu, Gao, Dai, and Bai (Citation2011) showed that low temperature increases AA level in cucumber plants, through the enhanced recycling pathway, reduces the deleterious effects of environmental oxidative stress.
3.3. Antioxidant enzymes
3.3.1. Superoxide dismutase (SOD, EC: 1.15.1.1)
The activity of SOD increased in water deficit lettuce. Only 10 ABA treatment showed significant increase in SOD activity. Treatment with ABA resulted in an enhancement of SOD activity under drought stress. The maximum increase was in 10 ABA with 72 h irrigation regime. More concentrations like 15 showed decreased activity (Table ).
Table 1. Effect of varied irrigation regimes, ABA and their combination on superoxide dismutase (SOD) activity (units mg−1 protein) in lettuce plants on the 75th day after planting
The activity of SOD increased in water deficit lettuce plants. Treatment with ABA resulted in an enhancement of SOD activity under drought stress. The SOD activity increased under drought in Phaseolus acutifolius (Türkan, Bor, Özdemir, & Koca, Citation2005). An increase in SOD activity was reported in Carthamus tinctorius plants under water deficit stress (Hojati, Modarres-Sanavy, Karimi, & Ghanati, Citation2011). SOD activity increased under drought stressed higher plants (Reddy, Chaitanya, & Vivekanandan, Citation2004).
3.3.2. Catalase (CAT, EC: 1.11.1.6)
The activity of CAT increased with different irrigation regimes. Treatment with ABA increased the CAT activity in control plants. On 96 h irrigation regime, there was no significant rise in CAT activity with ABA application (Table ).
Table 2. Effect of varied irrigation regimes, ABA and their combination on catalase (CAT) activity (units mg−1 protein) in lettuce plants on the 75th day after planting
The activity of catalase increased in drought stressed plants when compared to control. ABA increased the catalase activity to a higher level than control. ABA increases the activities of antioxidant enzymes such as SOD, catalase, APX and glutathione reductase in plant tissues under drought freezing stress (Anderson, Prasad, & Stewart, Citation1995; Bellaire et al., Citation2000; Yang et al., Citation2013).
3.3.3. Peroxidase (POX, EC: 1.11.1.7)
The peroxidase activity showed an increase in lettuce under drought conditions. Treatment with ABA increased the peroxidase activity in all concentrations. ABA in combination with drought increased the peroxidase activity on all irrigation regimes (Table ). Higher concentration like 15 decreased the POX activity in control at all irrigation regimes.
Table 3. Effect of varied irrigation regimes, ABA and their combination on peroxidase (POX) activity (units mg−1 protein) in lettuce plants on the 75th day after planting
The peroxidase activity showed an increase in the drought stressed plants. Treatment with ABA increased the peroxidase activity. R. astragali plants under water deficit stress showed an enhancement in POX activity, irrespective of different genotypes (Tan et al., Citation2006). Water deficit stress increased the POX activity in soybean plants (Zhang et al., Citation2006).
4. Conclusion
The parameters studied were biochemical constituents and antioxidant enzymes’ activities in lettuce plants under drought stress and their response to ABA under stress. Drought stress caused an increase in the biochemical constituents like proline and amino acid contents when compared with control in lettuce. All these parameters also increased under individual ABA treatments and treatments under drought stress. ABA treatments to the unstressed plants caused an increase in these parameters.
The non-enzymatic antioxidant molecules like ascorbate and α-tocopherol showed significant increase under drought conditions in lettuce. ABA slightly reduced the non-enzymatic antioxidant contents. The antioxidant enzymes like superoxide dismutase, catalase and peroxidase showed significant increase under drought conditions in lettuce. ABA caused significant enhancement in these antioxidant enzymes under drought stress and also in unstressed conditions.
Additional information
Funding
Notes on contributors
Mohamed A. Al Muhairi
Mohamed A. Al Muhairi graduated MSc in Horticulture, 2015, from College of Food and Agriculture (CFA), United Arab Emirates University (UAEU), Al Ain, UAE. Presently, he is working as a farm business head for a unit in farmer’s services centre, Abu Dhabi.
Abdul J. Cheruth
Abdul J. Cheruth is an assistant professor; he holds a PhD in Botany from Annamalai University, 2009.
Shyam S. Kurup
Shyam S. Kurup is an associate professor and holds a PhD in Horticulture from University of Agricultural Sciences, Bangalore, 1988.
Gabriel A. Rabert
Gabriel A. Rabert is an assistant researcher; he holds a PhD in Plant Biology and Plant Biotechnology from Annamalai University, 2014.
Mohammad S. Al-Yafei
Mohammed S. Al-Yafei is an associate professor and holds a PhD in Agronomy in Agriculture from Mississippi State University, 2005. The team from the Department of Aridland Agriculture, CFA, UAEU is working on plant stress physiology, alkaloid, antioxidant, hormonal balance, plant growth regulators, landscape management, tissue culture and sustainable agriculture in aridland water conservation and management.
References
- Anderson, M. D. , Prasad, T. K. , & Stewart, C. R. (1995). Changes in isozyme profiles of catalase, peroxidase, and glutathione reductase during acclimation to chilling in mesocotyls of maize seedlings. Plant Physiology , 109 , 1247–1257.
- Anjum, S. A. , Xie, X. Y. , Wang, L. C. , Saleem, M. F. , Man, C. , & Lei, W. (2011). Morphological, physiological and biochemical responses of plants to drought stress. African Journal of Agricultural Research , 6 , 2026–2032.
- Baker, H. , Frank, O. , DeAngelis, B. , & Feingold, S. (1980). Plasma tocopherol in man at various times after ingesting free or acetylated tocopherol. Nutrition Reports International , 21 , 531–536.
- Bano, A. , Ullah, F. , & Nosheen, A. (2012). Role of abscisic acid and drought stress on the activities of antioxidant enzymes in wheat. Plant Soil and Environment , 58 , 181–185.
- Bartoli, C. G. , Simontacchi, M. , Tambussi, E. , Beltrano, J. , Montaldi, E. , & Puntarulo, S. (1999). Drought and watering-dependent oxidative stress: effect on antioxidant content in Triticum aestivum L. leaves. Journal of Experimental Botany , 50 , 375–383.10.1093/jxb/50.332.375
- Bates, L. S. , Waldren, R. P. , & Teare, I. D. (1973). Rapid determination of free proline for water-stress studies. Plant and Soil , 39 , 205–207.10.1007/BF00018060
- Beauchamp, C. , & Fridovich, I. (1971). Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Analytical Biochemistry , 44 , 276–287.10.1016/0003-2697(71)90370-8
- Bellaire, B. A. , Carmody, J. , Braud, J. , Gossett, D. R. , Banks, S. W. , Cranlucas, M. , & Fowler, T. E. (2000). Involvement of abscisic acid-dependent and —Independent pathways in the upregulation of antioxidant enzyme activity during NaCl stress in cotton callus tissue. Free Radical Research , 33 , 531–545.10.1080/10715760000301071
- Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Analytical Biochemistry , 72 , 248–254.10.1016/0003-2697(76)90527-3
- Chandlee, J. M. , & Scandalios, J. G. (1984). Analysis of variants affecting the catalase developmental program in maize scutellum. Theoretical and applied genetics , 69 , 71–77.
- Cherry, J. H. (1963). Nucleic acid, mitochondria, & enzyme changes in cotyledons of peanut seeds during germination. Plant Physiology , 38 , 440–446.10.1104/pp.38.4.440
- Dan, S. , Qiang, H. , Zhaonan, D. , & Zhengquan, H. (2014). Genetic transformation of lettuce (Lactuca sativa): A review. African Journal of Biotechnology , 13 , 1686–1693.
- Guo, W. L. , Chen, R. G. , Gong, Z. H. , Yin, Y. X. , Ahmedand, S. S. , & He, Y. M. (2012). Exogenous abscisic acid increases antioxidant enzymes and related gene expression in pepper (Capsicum annuum) leaves subjected to chilling stress. Genetics and Molecular Research , 11 , 4063–4080.10.4238/2012.September.10.5
- Gupta, B. , Sengupta, A. , Saha, J. , & Gupta, K. (2013). Plant abiotic stress: ‘Omics’ approach. Journal of Plant Biochemistry & Physiology , 1 , Article ID: e108.
- Hojati, M. , Modarres-Sanavy, S. A. M. , Karimi, M. , & Ghanati, F. (2011). Responses of growth and antioxidant systems in Carthamus tinctorius L. under water deficit stress. Acta Physiologiae Plantarum , 33 , 105–112.10.1007/s11738-010-0521-y
- Hwang, S. Y. , Lin, H. W. , Chern, R. H. , Lo, H. F. , & Li, L. (1999). Reduced susceptibility to waterlogging together with high-light stress is related to increases in superoxide dismutase and catalase activities in sweet potato. Plant Growth Regulation , 27 , 167–172.10.1023/A:1006100508910
- Jaleel, C. A. , Gopi, R. , Sankar, B. , Manivannan, P. , Kishorekumar, A. , Sridharan, R. , & Panneerselvam, R. (2007). Studies on germination, seedling vigour, lipid peroxidation and proline metabolism in Catharanthus roseus seedlings under salt stress. South African Journal of Botany , 73 , 190–195.10.1016/j.sajb.2006.11.001
- Kizil, Ü. , Genc, L. , Inalpulat, M. , Şapolyo, D. , & Mirik, M. (2012). Lettuce (Lactuca sativa L.) yield prediction under water stress using artificial neural network (ANN) model and vegetation indices. Zemdirbyste-Agriculture , 99 , 409–418.
- Kumar, K. B. , & Khan, P. A. (1982). Peroxidase and polyphenol oxidase in excised ragi (Eleusine coracana cv. PR 202) leaves during senescence. Indian Journal of Experimental Botany , 20 , 412–416.
- Li, Q. , Yu, B. , Gao, Y. , Dai, A. H. , Bai, J. G. (2011). Cinnamic acid pretreatment mitigates chilling stress of cucumber leaves through altering antioxidant enzyme activity. Journal of Plant Physiology , 168 , 927–934.10.1016/j.jplph.2010.11.025
- Liu, F. , Jensen, C. R. , & Andersen, M. N. (2004). Pod set related to photosynthetic rate and endogenous ABA in soybeans subjected to different water regimes and exogenous ABA and BA at early reproductive stages. Annals of Botany , 94 , 405–411.10.1093/aob/mch157
- Mackay, C. E. , Christopher Hall, J. , Hofstra, G. , & Fletcher, R. A. (1990). Uniconazole-induced changes in abscisic acid, total amino acids, and proline in Phaseolus vulgaris . Pesticide Biochemistry and Physiology , 37 , 74–82.10.1016/0048-3575(90)90110-N
- Manivannan, P. , Jaleel, C. A. , Sankar, B. , Kishorekumar, A. , Somasundaram, R. , Lakshmanan, G. A. , & Panneerselvam, R. (2007). Growth, biochemical modifications and proline metabolism in Helianthus annuus L. as induced by drought stress. Colloids and Surfaces B: Biointerfaces , 59 , 141–149.10.1016/j.colsurfb.2007.05.002
- Martínez-Sánchez, A. , Tudela, J. A. , Luna, C. , Allende, A. , & Gil, M. I. (2011). Low oxygen levels and light exposure affect quality of fresh-cut Romaine lettuce. Postharvest Biology and Technology , 59 , 34–42.10.1016/j.postharvbio.2010.07.005
- Moore, S. , & Stein, W. H. (1948). Photometric ninhydrin method for use in the chromatography of amino acids. Journal of biological chemistry , 176 , 367–388.
- Nishiyama, R. , Watanabe, Y. , Fujita, Y. , Le, D. T. , Kojima, M. , Werner, T. , … Tran, L. S. P. (2011). Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis. The Plant Cell , 23 , 2169–2183.10.1105/tpc.111.087395
- Omaye, S. T. , David Turnbull, J. , & Sauberlich, H. E. (1979). Selected methods for the determination of ascorbic acid in animal cells, tissues, and fluids. Methods in Enzymology , 62 , 3–11.10.1016/0076-6879(79)62181-X
- Raghavendra, A. S. , Gonugunta, V. K. , Christmann, A. , & Grill, E. (2010). ABA perception and signalling. Trends in Plant Science , 15 , 395–401.10.1016/j.tplants.2010.04.006
- Reddy, A. R. , Chaitanya, K. V. , & Vivekanandan, M. (2004). Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. Journal of Plant Physiology , 161 , 1189–1202.10.1016/j.jplph.2004.01.013
- Sansberro, P. A. , Mroginski, L. A. , & Bottini, R. (2004). Foliar sprays with ABA promote growth of Ilex paraguariensis by alleviating diurnal water stress. Plant Growth Regulation , 42 , 105–111.10.1023/B:GROW.0000017476.12491.02
- Shao, H. B. , Chu, L. Y. , Wu, G. , Zhang, J. H. , Lu, Z. H. , & Hu, Y. C. (2007). Changes of some anti-oxidative physiological indices under soil water deficits among 10 wheat (Triticum aestivum L.) genotypes at tillering stage. Colloids and Surfaces B: Biointerfaces , 54 , 143–149.10.1016/j.colsurfb.2006.09.004
- Simontacchi, M. , Caro, A. , Fraga, C. G. , & Puntarulo, S. (1993). Oxidative stress affects [alpha]-tocopherol content in soybean embryonic axes upon imbibition and following germination. Plant Physiology , 103 , 949–953.
- Smirnoff, N. (2000). Ascorbic acid: Metabolism and functions of a multi-facetted molecule. Current Opinion in Plant Biology , 3 , 229–235.10.1016/S1369-5266(00)00069-8
- Suzuki, N. , Rivero, R. M. , Shulaev, V. , Blumwald, E. , & Mittler, R. (2014). Abiotic and biotic stress combinations. New Phytologist , 203 , 32–43.10.1111/nph.12797
- Tan, Y. , Liang, Z. , Shao, H. , & Du, F. (2006). Effect of water deficits on the activity of anti-oxidative enzymes and osmoregulation among three different genotypes of Radix Astragali at seeding stage. Colloids and Surfaces B: Biointerfaces , 49 , 60–65.10.1016/j.colsurfb.2006.02.014
- Türkan, İ. , Bor, M. , Özdemir, F. , & Koca, H. (2005). Differential responses of lipid peroxidation and antioxidants in the leaves of drought-tolerant P. acutifolius Gray and drought-sensitive P. vulgaris L. subjected to polyethylene glycol mediated water stress. Plant Science , 168 , 223–231.10.1016/j.plantsci.2004.07.032
- Yadav, S. K. , Jyothi Lakshmi, N. , Maheswari, M. , Vanaja, M. , & Venkateswarlu, B. (2005). Influence of water defict at vegetative, anthesis and grain filling stages on water relation and grain yield in sorghum. Indian Journal of Plant Physiology , 10 , 20–24.
- Yang, H. , Li, H. , Rao, L. Q. , Long, G. Y. , Shi, G. R. , & Peng, G. P. (2013). Effects of exogenous ABA on antioxidant enzymes in detached citrus leaves treated by rapid freezing. African Journal of Biotechnology , 10 , 9779–9785.
- Zhang, A. , Zhang, J. , Zhang, J. , Ye, N. , Zhang, H. , Tan, M. , & Jiang, M. (2011). Nitric oxide mediates brassinosteroid-induced ABA biosynthesis involved in oxidative stress tolerance in maize leaves. Plant and Cell Physiology , 52 , 181–192.10.1093/pcp/pcq187
- Zhang, J. , Jia, W. , Yang, J. , & Ismail, A. M. (2006). Role of ABA in integrating plant responses to drought and salt stresses. Field Crops Research , 97 , 111–119.10.1016/j.fcr.2005.08.018
- Zhang, X. , & Schmidt, R. E. (2000). Hormone-containing products’ impact on antioxidant status of tall fescue and creeping bentgrass subjected to drought. Crop Science , 40 , 1344–1349.10.2135/cropsci2000.4051344x
