Abstract
Aqueous methanol extract of seeds and tuber of food and medicinal plant Nymphaea nouchali (Burm.f.) displayed potent pancreatic lipase and intestinal α-glucosidase inhibitory properties. These properties may help reduce development of diet induced postprandial hyperlipidaemia and postprandial hyperglycaemia respectively. In addition, seeds and tuber extracts possess potent free radicals scavenging activities and reducing power. Furthermore, extracts also displayed strong inhibitory activities against formation of various advanced glycation end-products which is an important link inducing postprandial oxidative stress due to postprandial hyperlipidaemia and postprandial hyperglycaemia. Seeds and tubers of N. nouchali are rich sources of polyphenolic and flavonoids. Results of this study show for the first time that N. nouchali seeds and tuber may become an important dietary supplement to counter development of diet induced hyperglycaemia, hyperlipidaemia and resultant oxidative stress and can be utilized as herbal therapeutics for management of type 2 diabetes mellitus and obesity.
Public Interest Statement
Diabetes and obesity are disorders of modern civilization. Highly processed, refined food and beverages devoid of micronutrients and therapeutically important phytochemicals are being held responsible for development of these disorders. Life of every creature survives on food he/she eats in his/her life time. For that reason food is also considered as supreme medicine. Identification of medicinal properties in food materials will certainly help people fight battle against these diseases. Nymphaea nouchali has been important ingredient in traditional Indian medicinal formulations. Its tuber and seed is consumed by underprivileged people. Identification of antihyperglycaemic, antihyperlipidaemic properties along with antioxidant activities in seeds and tuber of this plant will help diabetic and obese people use this for therapeutic purpose. This demand will also encourage farmers grow this plant at commercial scale and improve their economic status.
Competing Interests
The authors declare no competing interest.
1. Introduction
Increased consumption and dependence on highly processed calorie rich, energy dense diet and beverages that lack micronutrients, vitamins, minerals and essential co-factors in sufficiency (Gibbons, Citation2014) now-a-days, has resulted new form of disorders being referred to as postprandial dysmetabolism (Sottero et al., Citation2015). Postprandial dysmetabolism better cracked as postprandial hyperglycaemia (PPHG) and postprandial hyperlipidaemia (PPHL) followed by postprandial oxidative stress (PPOS) is being held responsible for evolution and progression of type 2 diabetes mellitus (T2DM) and obesity (Sottero et al., Citation2015). In fact, under normal physiological conditions, oxidative stress plays vital role in aptly governing array of biochemical and cellular processes. However, it becomes deleterious beyond physiological control of endogenous antioxidant defence (Sharma, Taneja, Khanna, & Rajput, Citation2015).
Therefore, antioxidant therapy got promotion as promising strategies (Di Pierro, Citation2015) to counter experiences of overt PPOS. Recent animal experiments (Le Gal et al., Citation2015) and clinical studies (Gontero et al., Citation2015) however, caution unsounded use of antioxidants due to several unwanted health effects. Therefore, strategies that can primarily control acute spikes in PPHG and PPHL and resultantly reduce burden of PPOS development may sound prudent. For that reason, identification of properties in dietary materials that slows down the digestion of carbohydrates and lipids respectively and also seize diet induced oxidative stress development would be of greater therapeutic significance.
Nymphaea nouchali Burm.f. (Family: Nymphaeaceae) is a perennial aquatic plant. Better known as famine food, it is consumed by underprivileged folk. Tuber is eaten boiled and cooked. Seed flour is consumed mixing with barley or wheat flour. In Ayurvedic medicine it is prescribed as mental tonic (Sharma, Citation1996).
This research identified intestinal α-glucosidase and potent pancreatic lipase inhibitory activities in aqueous methanol extract of tuber and seeds of N. nouchali that may help slow down digestion of carbohydrates and lipids respectively and thereby, mitigate diet induced acute spikes in PPHG and PPHL. Furthermore, this research also presents multifaceted free radical scavenging antioxidant activities in extracts that may help trim down burden of exacerbated PPOS.
2. Experimental
2.1. Chemicals
Reagents of high quality were obtained from Sigma-Aldrich Chemicals (St Louis, MO). Other chemicals of analytical grade were purchased from Merck Limited (Mumbai, India) and S.D. Fine Chemicals Ltd (Mumbai, India).
2.2. Plant material and extract preparation
The tubers and fruits of Nymphaea nouchali Burm.f. (Family-Nymphaeaceae) was collected from a pond situated in village Chebrollu of East Godavari district (Andhra Pradesh, India). Plant was taxonomically identified by Prof. Ajmeera Ragan (Department of Botany, Kakatiya University-Warangal, Telangana). The voucher specimen is deposited at Kakatiya University Herbarium, Warangal (KUW) with accession No. KUW1924.
Outer layer of mature tubers were peeled off and cut into small portions. Seeds from dried mature fruits were collected. The pieces of tubers and seeds were dried for 24 h in incubator (Innova 4230 refrigerated incubator shaker, New Brunswick Scientific Edison, NJ, USA) at 37°C and ground in food grade grinder to obtain powder. Five gram of each samples powder was soaked in 40 mL of HPLC grade methanol and Milli Q purified water (1:1) as described by Slanc et al. (Citation2009). Extracts were centrifuged at 7,500 rpm for 30 min at 15°C (Eppendorf Centrifuge 5430R, Eppendorf AG 22331 Hamburg, Germany) and clear solution was dried using rotary evaporator (Laborota 4000 eco Rotary Evaporator, Heidolph Instruments GmbH & Co. KG, Germany). Extracts were stored at 4°C for analysis.
2.3. Phytochemicals analysis
2.3.1. Total polyphenol content
Phenol content was measured according to previously reported method (Tiwari et al., Citation2013). Total polyphenol content was measured spectrophotometrically (BioTek synergy 4 multi-mode microplate reader, BioTek Instruments, Inc Winooski, VT, USA) at 765 nm and results were expressed in terms of μg/mL of gallic acid equivalent.
2.3.2. Total flavonoids content
Flavonoid content was determined by procedure previously described (Tiwari et al., Citation2013). Total flavonoid content was recorded spectrophotometrically at 430 nm and results were expressed as μg/mL rutin equivalent.
2.4. Rat intestinal α-glucosidase inhibition assay
Rat intestinal α-glucosidase enzyme inhibitory activity was performed as reported earlier (Shukla, Anand Kumar, Anusha, & Tiwari, Citation2016). Acarbose was taken as standard α-glucosidase inhibitor.
2.5. Assay of porcine pancreatic lipase inhibition
Pancreatic lipase inhibition was determined according to procedure described by Shukla et al. (Citation2016). Orlistat was taken as standard pancreatic lipase inhibitor.
2.6. Free radicals scavenging assays
2.6.1. ABTS radical scavenging
[2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid)] radical cation (ABTS+) free-radical scavenging activity was performed according to Tiwari et al. (Citation2013). Decolorized ABTS+ absorbance was measured spectrophotometrically at 734 nm. Different dilutions of respective samples were prepared and read to find concentration dependent scavenging. Suitable regression analysis was applied for calculation of SC50.
2.6.2. DPPH decolorization assay
2,2-diphenyl-1-picrylhydrazyl (DPPH) free-radical scavenging activity was determined according to previously reported technique (Tiwari, Manasa, Kumar, & Zehra, Citation2013). Absorbance was recorded spectrophotometrically at 517 nm. Several serial dilutions of respective extract were prepared and read. Percentage scavenging concentration 50% (SC50) was calculated accordingly.
2.6.3. Assay for NBT reducing power
Nitro blue tetrazolium (NBT) reducing assay was determined according to the method performed earlier (Tiwari et al., Citation2013). The reduction of NBT was measured spectrophotometrically at 560 nm.
2.6.4. Assay for FeCl3 reducing power
In brief, 100 μl of various dilutions of extracts were mixed with 100 μl of phosphate buffer and 100 μl of potassium ferricyanide (1%). After 20 min incubation at 50°C, 10% trichloroacetic acid (TCA) was added to terminate the reaction. The mixture was centrifuged at 3,000 rpm for 10 min. Supernatant (100 μl) was transferred into a 96-well microplate and 100 μl of distilled water and 20 μl of 0.1% Ferric Chloride (FeCl3) were added and mixed well. Absorbance was measured spectrophotometrically at 700 nm (Tiwari et al., Citation2013) and the percentage of reducing power was calculated accordingly.
2.7. AGEs formation
Advanced Glycation-End Products (AGEs) screening was performed according to Poornima et al. (Citation2016). Aminoguanidine (5 mg/mL) was used as standard. Both vesperlysine-like (ʎexc 370 nm; ʎem 440 nm) and pentosidine-like (ʎexc 335 nm; ʎem 385 nm) AGEs inhibitions were determined according to Séro et al. (Citation2013) by using BioTek synergy 4 multimode microplate reader (BioTek Instruments Inc, Winooski, VT, USA). Results were expressed as percentage inhibition of AGEs.
2.8. Statistical analysis
One way ANOVA followed by Tukey’s multiple comparison test was applied to calculate differences within the groups. To compare differences between the groups unpaired t test with Welch’s correction was applied. Criterion for statistical significance was p < 0.05. Statistical analysis was performed by using GraphPad PRISM Version 5.01 (GraphPad Software Inc. California, USA).
3. Results and discussion
3.1. Phytochemicals
3.1.1. Yield of aqueous methanol extract in tuber and seeds
50% aqueous methanol extract yield in tuber and seeds of N. nouchali was quantified to be about 10 and 7% (w/w) respectively.
3.1.2. Total polyphenol and flavonoids content
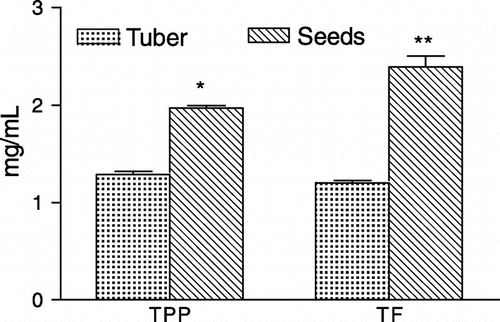
Polyphenols and flavonoids present in dietary materials have been claimed to provide protection against diet induced PPHG and PPHL (Sies, Stahl, & Sevanian, Citation2005). Total polyphenol content in seeds was significantly (p < 0.003) higher than that present in tubers (Figure ). Similarly, presence of more (p < 0.05) flavonoids was observed in seeds when compared in tubers extract (Figure ). These phytochemicals have being found valuable source of dietary supplements that positively modify biological processes important to maintain health (Morazzoni, Citation2015).
Figure 1. Total polyphenol (TPP) and flavonoids (TF) values in aqueous methanol extract of N. nouchali.
3.2. α-rat intestinal glucosidase and porcine pancreatic lipase inhibition
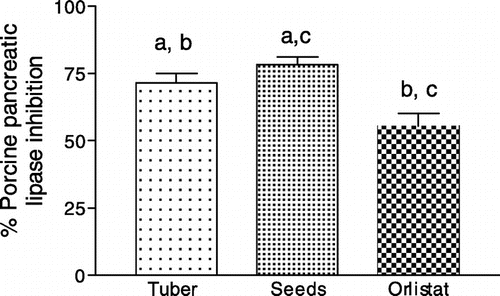
Clinically, use of intestinal α-glucosidase and pancreatic lipase inhibitors have shown promising strategies in mitigating diet induced PPHG and PPHL burden in diabetic and obese people respectively. However, several disadvantages are associated with use of these synthetic drug molecules (Tucci, Boyland, & Halford, Citation2010). Therefore, identification of dietary food materials containing inherent inhibitory activities for these enzymes may prove advantageous (Shukla et al., Citation2016). Results presented in Figure shows that seed extract displayed significantly better α-glucosidase inhibition than extract of tuber (p < 0.01). However, this activity was significantly lower (p < 0.001) in tuber and (p < 0.01) in seeds when compared with clinical drug acarbose. Conversely, pancreatic lipase inhibitory activity in tuber and seed extracts was similar but, 25% more (p < 0.05) than the standard drug molecule orlistat (Figure ). The hydro alcoholic extract of N. nouchali seeds has been reported to increase glucose consumption in 3T3-L1 adipocytes through activation of PPARγ and insulin sensitization (Parimala, Debjani, Vasanthi, & Shoba, Citation2015) and display antidiabetic activity in STZ induced diabetic rats (Parimala & Shoba, Citation2014). Taken together, the multifaceted antidiabetic activities like presence of slow carbohydrate, lipid digesting potentials, and enhancing glucose utilization by cells via insulin sensitization might have been the reason Indian Ayurvedic physicians prescribed seeds of N. nouchali as suitable dietary material for management diabetes (Achariya, Upadhyay, & Dwivedi, Citation1996). In addition, this research also discovered potent pancreatic lipase inhibitory activity in tubers of N. nouchali consumption of which might prove advantageous in mitigating development of diet induced PPHL.
Figure 2. Rat intesitnal α-glucosidase inhibition by aqueous methanol extract of seeds and tuber of N. nouchali.
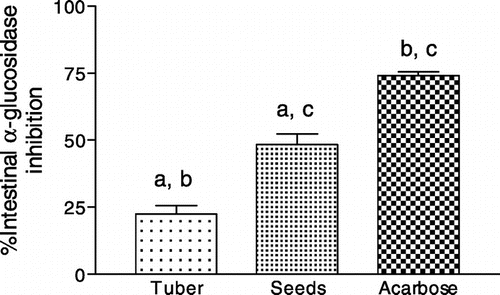
Figure 3. Porcine pancreatic lipase inhibitory activity by aqueous methanol extract of seeds and tuber of N. nouchali.
3.3. Free radicals scavenging activity and analysis of reducing power
3.3.1. ABTS+ and DPPH radicals scavenging
ABTS+ is an amphiphilic cation radical applied to indentify both the hydrophilic antioxidants as well as lipophilic antioxidants (Awika, Rooney, Wu, Prior, & Cisneros-Zevallos, Citation2003) and DPPH is organic nitrogen centered radical often applied to assess reducing power of an antioxidant (Prior, Wu, & Schaich, Citation2005). The ABTS+ cation is a planar radical hence an antioxidant even with low redox potentials may be identified using this test model, however, they may react slowly or even not when tested on DPPH·radical due to the streric hindrance of N·radical (Prior et al., Citation2005). Comparing the ABTS+ radical scavenging potentials present in tuber and seed of N. nouchali in terms of their SC50 values, they were equipotent, however the DPPH radical scavenging potential of seed extract was observed double than that present in tuber extract (Table ). The DPPH radical scavenging potential of NN seed extract was equally comparable to the ascorbic acid (Table ).
Table 1. In-vitro free radicals scavenging, antioxidant activities in aqueous methanol extract of seeds and tuber of N. nouchali
3.3.2. NBT and FeCl3 reducing power
FeCl3 reduction method detects compounds reducing power (Prior et al., Citation2005) and NBT reagent is used to identify presence of ascorbic acid like properties present over plant leaves (Concklin, Saracco, Norris, & Last, Citation2000). Comparison of NBT reducing power 50% (RP50) for seeds and tuber extracts find close similarity however, the FeCl3 reducing potential of seed was double than that present in tuber extract (Table ). When compared with ascorbic acid, the reducing potential for NBT was 50% less in both seed as well as tuber extract however, FeCl3 reducing power in seeds extract was more followed by tubers extract than that of the ascorbic acid (Table ).
3.4. AGEs formation
AGEs are identified as important link between hyperglycaemia and promotion of oxidative stress (Sottero et al., Citation2015) and increasing reactive oxygen species (ROS) levels (Barlovic, Soro-Paavonen, & Jandeleit-Dahm, Citation2011). Although AGEs are complex type of molecules, Séro et al. (Citation2013) have grouped them into two types; the vesperlysine-type and pentosidine-type based on their structural similarities. Grillo and Colombatto (Citation2008) have mentioned that pentosidine-type AGEs are primarily present in plasma and erythrocytes whereas the vesperlysine-type of AGEs is present particularly in lens of diabetic people. Inhibitory activity in tuber extract for pentosidine-type AGEs was noticed more than three times higher than that for vesperlysine-type AGEs however, inhibitory activity was recorded same in seed extract for both type of AGEs studied (Table ). Standard AGE inhibitor aminoguanidine inhibited pentosidine-type AGEs better than vesperlysine-type AGEs. Our results show that extract of seed of N. nouchali is better inhibitor for AGEs formation than aminoguanidine (Table ).
For, the seed extract of N. nouchali potently inhibited pentosidine-type AGEs and tuber extract inhibited both type of AGEs effectively, the tuber extract of N. nouchali may become valuable therapeutic in combating diabetic retinopathy. AGEs are important accelerator of ageing process and development of neurodegenerative disorders (Grillo & Colombatto, Citation2008). Hence, inclusion of N. nouchali seeds and tuber in daily diet may benefit in delaying ageing process and related neurodegenerative disorders.
4. Conclusion
Our research finds that seed and tuber of N. nouchali may turn into an important dietary material to counter modern challenges of PPHG, PPHL, and PPOS. Hence, seeds and tuber of N. nouchali can become an important natural material for development of antidiabetic and antiobesity therapeutics possessing multiple remedial properties. Further research is required to unravel detail therapeutic potentials of this aquatic plant. This is the first report of its kind finding in vitro antidiabetic and antiobesity properties in seeds and tuber of N. nouchali.
Funding
This work was financially supported from CSIR-New Delhi sponsored projects [project number SMiLE (CSC-0111)], [project number NaPAHA (CSC-0130)] is acknowledged. Ajay Anand thanks CSIR-UGC-New Delhi for JRF. The author thanks University Grants Commission for JRF.
Additional information
Notes on contributors
Ashok K. Tiwari
The first and third authors were post graduate students. The second author is research fellow working for his PhD thesis. All are very sincere, hard working students full of scientific temperament. The fourth author has vast technical experience in the subject matter of this article. The senior author is mentor, advisor and faculty in the institute. He has enormous experience in natural medicines, food and nutrition. His main research interest includes identification and development of natural therapeutics, functional food and nutraceuticals that may help common people fight against diseases of modern civilization such as hyperglycaemia, hyperlipidaemia and oxidative stress. As a creative writer he has published number of research, review and popular articles.
References
- Achariya, R. K., Upadhyay, B. N., & Dwivedi, L. D. (1996). Dietary management in prameha. Ancient Science of Life, 15, 176–189.
- Awika, J. M., Rooney, L. W., Wu, X., Prior, R. L., & Cisneros-Zevallos, L. (2003). Screening methods to measure antioxidant activity of Sorghum (Sorghum bicolor) and Sorghum products. Journal of Agricultural and Food Chemistry, 51, 6657–6662.10.1021/jf034790i
- Barlovic, D. P., Soro-Paavonen, A., & Jandeleit-Dahm, K. M. (2011). RAGE biology, atherosclerosis and diabetes. Clinical Science, 121, 43–55.10.1042/CS20100501
- Concklin, P. L., Saracco, S. A., Norris, S. R., & Last, R. L. (2000). Identification of ascorbic acid-deficient Arabidopsis thaliana mutants. Genetics, 154, 847–856.
- Di Pierro, F. (2015). Antioxidants and cancer: A debate on prevention, progression, hormesis, and cruciferous vegetables. Nutrafoods, 14, 175–179.10.1007/s13749-015-0064-3
- Gibbons, A. (2014). The evolution of deit. National Geographic Magazine, 2, 81–101.
- Gontero, P., Marra, G., Soria, F., Oderda, M., Zitella, A., Baratta, F., … Brusa, P. (2015). A randomized double-blind placebo controlled phase I-II study on clinical and molecular effects of dietary supplements in men with precancerous prostatic lesions. Chemoprevention or “chemopromotion”? The Prostate, 75, 1177–1186.10.1002/pros.v75.11
- Grillo, M. A., & Colombatto, S. (2008). Advanced glycation end products (AGEs): Involvement in aging and in neurodegenerative diseases. Amino Acids, 35, 29–36.10.1007/s00726-007-0606-0
- Le Gal, K., Ibrahim, M. X., Wiel, C., Sayin, V. I., Akula, M, K., Karlsson, C., … Bergo, M. O. (2015). Antioxidants can increase melanoma metastasis in mice. Science Translational Medicine, 7, 308re810.1126/scitranslmed.aad3740
- Morazzoni, P. (2015). Food plants: A valuable source of supplements for human health. Nutrafoods, 14, 51–52.10.1007/s13749-015-0008-y
- Parimala, M., Debjani, M., Vasanthi, H. R., & Shoba, F. G. (2015). Nymphaea nouchali Burm. F. hydroalcoholic seed extract increases glucose consumption in 3T3-l1 adipocytes through activation of peroxisome proliferator-activated receptor gamma and insulin sensitization. Journal of Advanced Pharmaceutical Technology and Research, 6, 183–189. doi:10.4103/2231-4040.165013
- Parimala, M., & Shoba, F. G. (2014). Evaluation of antidiabetic potential of Nymphaea nouchali Burm. F. seeds in STZ-induced diabetic rats. International Journal of Pharmacy and Pharmaceutical Science, 6, 536–541.
- Poornima, B., Anand Kumar, D. A., Siva, B., Venkanna, A., Vadaparthi, P. R. R., Kumar, K., … Tiwari, A. K. (2016). Advanced glycation end-products inhibitors isolated from Schisandra grandiflora. Natural Product Research, 30, 493–496.10.1080/14786419.2015.1024117
- Prior, R. I., Wu, X., & Schaich, K. (2005). Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. Journal of Agricultural and Food Chemistry, 53, 4290–4302.10.1021/jf0502698
- Séro, L., Sanguinet, L., Blanchard, P., Dang, B. T., Morel, S., Richomme, P., … Derbré, S. (2013). Tuning a 96-well microtiter plate fluorescence-based assay to identify AGE inhibitors in crude plant extracts. Molecules, 18, 14320–14339.10.3390/molecules181114320
- Sharma, P. V. (1996). Classical uses of medicinal plants (Haridas Ayurveda series 4) (pp. 105–106). Varanasi: Chaukhambha Visvabharati.
- Sharma, A. K., Taneja, G., Khanna, D., & Rajput, S. K. (2015). Reactive oxygen species: Friend or foe? RSC Advances, 5, 57267–57276.10.1039/C5RA07927F
- Shukla, S., Anand Kumar, D., Anusha, S. V., & Tiwari, A. K. (2016). Antihyperglucolipidaemic and anticarbonyl stress properties in green, yellow and red sweet bell peppers (Capsicum annuum L.). Natural Product Research, 30, 583–589.10.1080/14786419.2015.1026343
- Sies, H., Stahl, W., & Sevanian, A. (2005). Nutritional dietary and postprandial oxidative stress. Journal of Nutraceuticals, 135, 969–972.
- Slanc, P., Doljak, B., Kreft, S., Lunder, M., Janeš, D., & Štrukelj, B. (2009). Screening of selected food and medicinal plant extracts for pancreatic lipase inhibition. Phytotherapy Research, 23, 874–877.10.1002/ptr.v23:6
- Sottero, B., Gargiulo, S., Russo, I., Barale, C., Poli, G., & Cavalot, F. (2015). Postprandial dysmetabolism and oxidative stress in type 2 diabetes: Pathogenetic mechanisms and therapeutic strategies. Medicinal Research Reviews, 35, 968–1031.10.1002/med.2015.35.issue-5
- Tiwari, A. K., Jyothi, A. L., Tejeswini, V. B., & Madhusudana, K., Kumar, D. A., Zehra, A., & Agawane, S. B. (2013). Mitigation of starch and glucose-induced postprandial glycemic excursion in rats by antioxidant-rich green-leafy vegetables’ juice. Pharmacognosy Magazine, 9, S66–S73.10.4103/0973-1296.117872
- Tiwari, A. K., Manasa, K., Kumar, D. A., & Zehra, A. (2013). Raw horse gram seeds possess more in vitro antihyperglycaemic activities and antioxidant properties than their sprouts. Nutrafoods, 12, 47–54.10.1007/s13749-013-0012-z
- Tucci, S. A., Boyland, E. J., & Halford, J. C. G. (2010). The role of lipid and carbohydrate digestive enzyme inhibitors in the management of obesity: A review of current and emerging therapeutic agents. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy, 3, 125–143.10.2147/DMSO
