?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Silver cyprinid (Rastrineobola argentea) is a nutritious and low-cost source of proteins for food and feed. Processing and storage of silver cyprinid are still sub-optimal and may therefore impact on its quality and safety. In this study, we evaluated markers of microbial quality, nutrient degradation and aflatoxin content in stored dry silver cyprinid from four landing sites on Lake Victoria and five markets in Kampala district. The moisture content of fish samples from both landing sites and markets was higher than 12% which is the recommended by East African standards. The microbial quality of the silver cyprinid from landing sites met the national standards (total coliform count, 3.6 (3.0, 4.6) log cfu/g) while that of market samples (total coliform count 4.2 (3.0, 5.0) log cfu/g). Silver cyprinid from the landing sites and markets contained aflatoxins (14.5 ± 0.9 ppb; 15.5 ± 2.2 ppb, respectively) above the standard specification. Additionally, fat degradation of the landing site samples was higher than the maximum allowable limit: free fatty acid content 3.6 (3.1, 4.3) mgKOH/g. Fat degradation of the market site samples was higher than the maximum allowable limit: free fatty acid content 7.4 ± 1.4 mgKOH/g. Stored, dry silver cyprinid fish may pose a health risk, and may have slightly less healthy fats than fresh fish.
PUBLIC INTEREST STATEMENT
In this study, we evaluated markers of microbial quality, nutrient degradation and aflatoxin content in stored dry silver cyprinid from four landing sites on Lake Victoria and five markets in Kampala district. The moisture content of fish samples from both landing sites and markets was higher than the recommended. The microbial quality of the silver cyprinid from landing sites met the national standards while that of market samples was below the required. The samples contained aflatoxins above the standard specifications. Additionally, fat degradation of the landing site and market samples was higher than the maximum allowable limit. The mukene fish found in the various markets around Kampala may pose a health risk due to poor microbial quality, aflatoxin contamination and degradation of the fats in the fish. Efforts are required to improve on the processing and storage practices of silverfish in Uganda.
Competing interests
The authors declare no competing interests.
1. Introduction
Fish and other aquatic products provide substantial amounts of macro- and micronutrients to the world’s population, especially those living in developing countries (Béné et al., Citation2007). Silver cyprinid (Rastrineobola argentea), a small pelagic fish is a cheap, nutrient-dense option that can be utilized to prevent or treat malnutrition in tropical areas. Dried silver cyprinid contains substantial amounts of proteins, calcium, and iron (Kabahenda et al., Citation2011). After drying, silver cyprinid may either be packaged in heat-sealed plastic pouches, woven polypropylene bags, or stored without packaging (Kabahenda et al., Citation2009; Ssebisubi, Citation2013). Silver cyprinid storage occurs at the landing site first before it is stored again in the market. Storage duration mainly depends on market availability, available catch and seasonality.
However, silver cyprinid, being a fatty fish with high-protein content, tends to spoil easily during warm and humid storage conditions, and this spoilage is exacerbated by inadequate packaging (Ibengwe, Citation2011). Spoilage, which is due to lipid oxidation and microbial proliferation (Immaculate et al., Citation2013) leads to degradation of nutrients such as protein and lipids. Spoiled fish has a bad odour, brown colour, and bitter taste that makes it less fit for human consumption and of a low economic value (Akande & Diei-Ouadi, Citation2010). Free fatty acids, peroxides, thiobarbituric acid reactive substances, trimethylamine, microbial content, and aflatoxin content are measures of the extent of fish deterioration (Ikape & Cheikyula, Citation2017; Kubiriza et al., Citation2020).
Prior studies on silver cyprinid assess its quality after drying (Kubiriza et al., Citation2020; Masette & Kwetegyeka, Citation2013), modified processing and modified storage with limited attention paid to the quality of silver cyprinid found in stores. However, the quality of stored silver cyprinid in Uganda with respect to microbial contamination, nutrient degradation and aflatoxin contamination was hitherto unknown. Therefore, this study was carried out to evaluate the quality (microbial, nutrient and aflatoxin content) of dry silver cyprinid at landing site stores and in selected market stores in Kampala Uganda.
2. Materials and methods
2.1. Study area
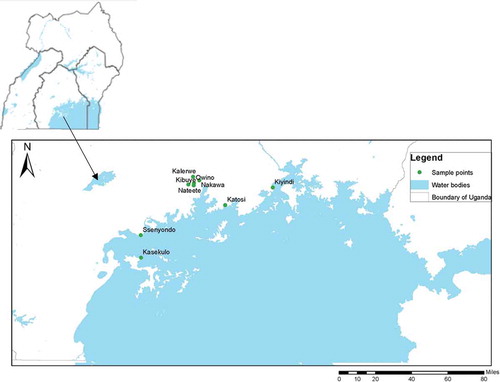
The study was conducted at four landing sites: Kiyindi (Buikwe district), Katosi (Mukono district), Kasekulo (Kalangala district) and Ssenyondo (Mpigi district) on Lake Victoria; and five markets in Kampala district, Uganda (namely Kalerwe, Kibuye, Nakawa, Owino and Nateete) (Figure ). Landing sites selected were the key silver cyprinid processing sites in Uganda as per the information obtained from the Ministry of Fisheries in Uganda. Kampala district was selected since it is one of the densely populated locations. From each of the Kampala divisions, a major market was selected to be involved in the study. The location of the sampling areas is shown in Figure .
2.2. Study design and sampling
2.2.1. Sample collection
Silver cyprinid samples were obtained from 70 traders in each of the landing sites and markets during March and April 2018. Sun-dried silver cyprinid samples (n = 40) were purchased from stores at a total of four landing sites. Sun-dried Silver cyprinid samples (n = 30) were purchased from stores from a total of five markets. Samples, weighing about 200 g, were collected in triplicate at the location, packed in plastic freezer bags and immediately placed on ice. The samples were transported to Makerere University School of Food Technology, Nutrition and Bioengineering research laboratories and kept in a deep freezer (−20°C) which was connected to a generator in case of a power failure. The maximum travel time of the samples from the landing sites and markets was 1 day.
2.3. Analyses
The analyses that were carried out in this study included assessment of environmental conditions such as temperature and relative humidity of the landing sites and markets, proximate composition, microbial quality and markers of fat and protein degradation of the fish samples.
2.3.1. Proximate composition
2.3.1.1. Moisture content
Moisture content was determined using the dry oven method by Association of Official Analytical Chemists [AOAC] (Citation2005). For each dry fish sample, was dried in a hot air oven at 100°C for about 18 hours. Dry sample was cooled in a desiccator for 30 minutes and weighed to determine the lost moisture. Thus, : where W1 is the weight of the empty dish, W2 is the weight of wet sample and dish; W3 is the weight of dry sample and dish.
2.3.1.2. Crude protein
Crude protein was determined by the Kjeldahl method as described by Kirk and Sawyer (Citation1991). Concentrated sulphuric acid was used for digestion of the fish sample in the presence of Kjeldahl catalyst at 420°C. The distillate was titrated against 0.05 M hydrochloric acid to determine the ammonia absorbed by boric acid. In order to determine crude protein content, the nitrogen value was multiplied by a conversion factor of 6.25 as shown below.
, where V2 = the volume (ml) of hydrochloric acid solution required for the sample, V1 = volume of hydrochloric acid required for the blank test, MHCL = morality of hydrochloric acid, W = weight in grams of the test sample, 6.25 = nitrogen conversion factor of protein, 14 = atomic mass of nitrogen.
2.3.1.3. Crude fat
The soxhlet extraction method was used as described by AOAC (Citation2005). Fat in the sample was extracted using petroleum ether by boiling the samples for about an hour. The solvent was distilled off and the fat extracted dried in an air oven at 100 °C for 30 minutes. The oil collected in the beakers was weighed and used to calculate fat content as follows; : where; W0 = weight of the sample (g), W2 = weight of the empty beaker (g) and W1 = weight of the beaker and fat (g)
2.3.2. Ash content
The dry fish sample was ignited in a muffle furnace at 500–600°C for 6 hours as recommended in AOAC (Citation2005). Ash remains as a residue in crucibles. Ash in the crucible was cooled for 30 minutes and weighed. Ash content was calculated as; , where; W1 is the weight of crucible, W2 is the weight of sample and crucible, W3 is the weight of ash and crucible.
2.3.2.1. Iron content
Iron content was determined according to Atomic absorption spectrophotometric manual of Perkin Elmer, 400. Samples (1 g, 0.5 g and 0.25 g) were dried in an air oven (Memmert DIN 40050-IP 20, German) at 50°C for 24 h. The dried sample was then ground using a blender (HR2058 Phillips, UK) and sieved through 0.005-micron sieve. The ground sample was digested with aquaregia solution (a mixture of nitric acid and hydrochloric acid in the ratio of 1–3), filtered and filled to 50 ml mark. The atomic absorption spectrophotometer reading, volume, dilution factor and sample weight were used to compute iron content. Iron content was calculated as:
2.3.3. Microbial quality
2.3.3.1. Total coliform content
Total coliforms were determined using the pour plate technique in ISO 4832:2006. Violet Red Bile Lactose (VRBL) agar was prepared by weighing 20.75 g of media powder into 500 ml of distilled water. The mixture was heated on a Bunsen burner flame until boiling and allowed to boil for 2 minutes then immediately cooled to 47°C using a water bath. Serial dilutions (10−1 to 10−7) of the fish samples were made. ABOUT 1 ml of inoculum from each dilution and molten agar were transferred into each of the petri dishes containing inoculum and carefully mixed by rotating the petri dishes.
The dishes were incubated at 37°C for 24 hours after which the purplish colonies, were considered to be typical coliform colonies, ranging from 30 to 300. These were counted using the colony counter and results expressed in colony-forming units (cfu)/g.
2.3.3.2. Total plate count
Total plate count was enumerated using the pour plate technique in the ISO 4833:2013 method. Plate count agar was prepared by dissolving agar powder into distilled water and sterilized by autoclaving at 121°C for 15 minutes then allowed to cool to about 47°C using a water bath. Serial dilutions (10−1 to 10−7) of the sample solutions were made.
Inoculum (1 ml) from each selected dilution and molten agar was transferred to petri dishes that were incubated at 37°C for 24 hours. Plates with colonies ranging from 30 to 300 were considered for counting using the colony counter and the results expressed as cfu/g.
2.3.3.3. Yeasts and mould content
Yeasts and moulds were enumerated using the surface spread technique in ISO 21527–2:2009. Dichloran Rose Bengal Chloramphenicol (DRBC) agar was weighed and mixed with distilled water. This mixture was sterilized by autoclaving at 121°C for 15 mins and immediately cooled to about 47 °C. Molten agar was aseptically poured into the petri dish and allowed to set.
Inoculum (0.1 ml) from the serial dilutions (10−1 to 10−7) of the fish samples was aseptically transferred onto the centre of the solidified agar and evenly spread over the agar surface using a sterile wire loop. This set-up was incubated at room temperature for 5 days. Colonies between 30 and 300 were taken and counted using the colony counter and results expressed as cfu/g.
2.3.3.4. Aflatoxin content
Aflatoxin content was determined according to the method of USDA (Citation2015). An extraction buffer bag and 30 ml distilled water were added to the groundfish sample in a Whirl-Pak bag. The mixture was homogenized by agitating manually and then left to stand. A 50 µL aliquot of this sample extract was diluted with 1000 µL dilution buffer. Then, 100 µL of diluted sample extract was then transferred to a conjugate microwell into which a test strip was inserted prior to the reading of total aflatoxins using an Agra Vision Reader.
2.3.4. Markers of fat and protein degradation
2.3.4.1. Peroxide value
A 3 g sample of dried fish powder was weighed into a 250 mL stoppered conical flask. Chloroform (Loba chemie laboratory reagents, India; 20 ml) was added to the sample and the mixture stirred to extract the oil. The mixture was then filtered and washed with 30 mL of acetic acid (Scharlau, Spain) into an Erlenmeyer flask. Freshly prepared saturated potassium iodide (Loba chemie laboratory, India; 1 mL) was added to the mixture and the mixture kept in the dark for 5 minutes. The solution was withdrawn from the dark and five drops of starch solution (5%) added. The resultant blue-black solution was titrated against sodium thiosulphate (Loba chemie laboratory, India; 0.1 M) and the titer value recorded. The peroxide value was calculated using the following equation.
where B – titration of blank, S – titration of the sample, N – normality of Na2S2O3 solution
2.3.4.2. Free fatty acid content
A mixture of diethyl ether, absolute ethanol and phenolphthalein solution was neutralized using 0.1 M sodium hydroxide. A homogenized sample was dissolved in the mixed solvent. The mixture was titrated with an aqueous 0.1 M sodium hydroxide. The acid value was calculated using the sample weight, concentration, quantity and relative formula mass of sodium hydroxide used. The acid value was calculated using the following equation:
2.3.4.3. Thiobarbituric acid reactive substances
A 0.2 g sample of dried fish was homogenized with 25 mL butan-1-ol. This was followed by pipetting 5 mL of the mixture into a dry-stoppered test tube containing 5 mL of TBA reagent (200 mg of 2-thiobarbituric acid in 100 mL 1-butanol). The stoppered mixture was mixed and placed in a water bath at 95°C for 120 min and cooled immediately. Absorbance (AB) of the blank and samples were measured with a spectrophotometer at 530 nm. Sample absorbance and mass were used in the calculation of TBARs value. TBARs value was calculated using the following formula:
, where: As = absorbance of sample; Ab = absorbance of blank; M = mass of sample
2.3.4.4. Trimethylamine (TMA) and total volatile base nitrogen (TVBN)
The fish powder was homogenized with 7.5% aqueous trichloroacetic acid solution, the homogenate centrifuged at 3000 rpm for 20 min and the supernatant liquid filtered. Each filtrate was transferred into a Kjeldahl distillation tube followed by 10% sodium hydroxide solution with 4% boric acid in the receiving flask. Timing started when the contents of the receiving flask turned green and distillation was carried out for 10 min. Each 5 mL distillate was titrated against an aqueous 0.05 M sulphuric acid solution with constant shaking until a pink colour that persisted for at least 15 seconds was obtained. The same protocol of TVBN was used for the measurement of TMA (Malle & Poumeyrol, Citation1989). The only difference was the addition of 2 mL of 35% (v/v) formaldehyde to the distillation tube. The amount of TVBN and TMA were calculated from the volume of 0.05 M sulphuric acid (n ml) used for titration and the results were expressed in mg nitrogen 100 g−1 of sample. TVBN (mgN/100 g) = n × 16.8 mg N 100 g−1 and TMA (mgN/100 g) = n × 16.8 mg N 100 g−1.
2.4. Statistical analyses
Statistical analysis of data was done with SPSS software (version 21). Data were cleaned, and the Shapiro-Wilk test was used to check for normality. Landing site data and some market site data (moisture content, trimethylamine, thiobarbituric acid reactive substances, total plate count, total coliform count, yeasts and mould content) were log transformed to perform parametric tests. The independent sample t-test was used to compare data for the measured parameters of dry silver cyprinid between markets and landing sites. One-way ANOVA with Tukey HSD test was used to compare respective data among landing sites and markets.
Data were summarized into means ± SD and significant differences were considered at p < 0.05; transformed data are presented as the median and interquartile range (Q1, Q3).
3. Results
3.1. Nutrient content, microbial quality and nutrient degradation characteristics of stored silver cyprinid from landing sites on Lake Victoria and markets in Kampala
3.1.1. Nutrient content
3.1.1.1. Moisture content
The moisture content of market samples was significantly lower than landing site samples (Table ). The moisture content of landing site samples differed significantly with Katosi landing site samples having the highest mean moisture whereas Kiyindi landing site samples had the lowest (Table ).
Table 1. Proximate composition, microbial level, aflatoxin content and nutrient degradation of stored silver cyprinid from landing sites on Lake Victoria and markets in Kampala
Table 2. Proximate composition, microbial level, aflatoxin content and nutrient degradation of stored silver cyprinid from landing sites on Lake Victoria
3.1.1.2. Fat content
The fat content of market samples was significantly lower than landing site samples (Table ). The fat content of silver cyprinid obtained from different market samples differed significantly (Table ) with Owino market having the highest fat content whereas the Nakawa market had the lowest (Table ). The fat content of landing site samples differed significantly, Ssenyondo landing site had the lowest fat content whereas Kiyindi landing site samples had the highest fat (Table ).
Table 3. Moisture content, microbial level, aflatoxin content and nutrient degradation of stored silver cyprinid from different markets
3.1.1.3. Ash content
Ash content of market samples was significantly higher than landing site samples (Table ). Ash content of silver cyprinid obtained from different market samples differed significantly (Table ). Kibuye market had the highest mean ash content whereas the Nakawa market had the lowest. Ash content of landing site samples differed significantly. Kiyindi landing site had the highest protein whereas the Katosi landing site had the lowest protein (Table ).
3.1.1.4. Protein content
The protein content of silver cyprinid obtained from different market samples differed significantly (Table ). Kalerwe market had the highest mean protein content whereas Owino market had the lowest.
3.1.1.5. Iron content
The iron content of market samples differed significantly. Owino market had the highest iron content whereas Nateete market had the lowest. The iron content of landing site samples differed significantly that is to say Katosi samples had the highest iron content, whereas Kiyindi samples had the lowest iron content (Table ).
3.1.2. Microbial quality
Microbial counts of landing site samples differed significantly. Ssenyondo landing site had the highest total coliforms, whereas Kiyindi landing site had the lowest (Table ). Total coliforms (TC) for market samples were signifcantly higher than landing sites samples (p < 0.05; Table ). The total coliform count of market samples differed significantly with Owino market having the highest value, and Kalerwe market had the lowest (Table ).
The yeast and mould content of the market samples were significantly lower than landing site samples (Table ). Yeasts and moulds among the market samples differed significantly (Table ) with Kibuye market fish having the highest value whereas Kalerwe had the lowest (Table ). Ssenyondo landing site had the highest yeast and mould count whereas Kiyindi landing site had the lowest (Table ).
Total plate count of market samples differed significantly as in Table . Kibuye market had the highest TPC whereas Nakawa market had the lowest (Table ). Kasekulo landing site had the highest TPC whereas Kiyindi landing site had the lowest (Table ).
3.1.3. Markers of fat and protein degradation
There were significant differences in FFA between samples from landing sites stores and markets. Much as the PV, pH in market samples were significantly lower than landing site samples, TBARs, TVBN and TMA from market samples were signifcantly higher than landing sites samples (Table ). Peroxide value of all market samples was below the detection point.
TVBN, FFA and TMA content varied significantly across markets (Table ). Nateete market had the highest TVBN content while Kalerwe market had the lowest. Owino market had the highest FFA, TMA and TBARs content whereas the Nakawa market had the lowest FFA content, Nateete market had the lowest TMA and TBARs content (Table ).
Acid value, TBARS, TVBN and TMA content of landing sites differed significantly. Samples from Kibuye market had significantly the highest aflatoxin. Owino market had the highest free fatty acids, trimethylamine content and TBARs. Nateete market had the highest TVBN and the lowest TMA and TBARS content (Table )
Peroxide value, acid value, pH, TBARS, TVBN, TMA and aflatoxin content of landing sites differed significantly. Samples from the Katosi landing site had significantly the highest aflatoxin and trimethylamine content but had significantly the lowest free fatty acids and PV (Table ). Ssenyondo had the highest TVBN and the lowest TBARs. Kiyindi samples had significantly the lowest total volatile basic nitrogen, TMA, highest thiobarbituric acid reactive substances (TBARs) values (Table ). Kasekulo samples had the significantly lowest aflatoxin content (Table ).
4. Discussion
4.1. Nutrient content
4.1.1. Moisture content
Landing site samples had higher moisture content than market samples which could be due to differences in the humidity: 56–89% at landing sites and 42–69% in markets. Results are in agreement with previous studies that have identified a higher moisture content in landing site dry Silver cyprinid as compared to market dry Silver cyprinid in Kenya (Ogonda et al., Citation2014; Onyuka et al., Citation2014).
The mean moisture content of dried silver cyprinid from markets and landing sites was higher than the standard value of 12% (EAS, Citation2014) but below the value of 15% that is cited to favour microbial growth (EAS, Citation2014; Glucas, Citation1982). This can be attributed to inadequate post-harvest handling practices employed prior and/or during landing site and market storage (Cockerell et al., Citation1971; Unlusayin, Erdilal, Gumuş & Gulyavuz, Citation2010; Kubiriza et al., Citation2020) used in the different sites and markets. The moisture content also implied that the shelf life of dried silver cyprinid found in landing site and market stores is reduced, and the safety of its consumers is at risk due to the possibility of mycotoxin production by toxigenic fungi.
4.1.2. Protein content
The observed difference in protein content between landing site store and market store silver cyprinid can be attributed to a varied degree of protein degradation by microbes to yield TVBN and TMA (Immaculate et al., Citation2013; Prakash et al., Citation2011; Saritha et al., Citation2012), differences in moisture content, and efficiency of drying to destroy proteolytic enzymes (Boran & Karacam, Citation2011). However, the protein content of market samples was lower than values reported in market samples in Kenya (Owaga et al., Citation2010), and that of landing site samples was lower than values reported in previous studies (Kubiriza et al., Citation2020).
4.1.3. Fat content
The fat content of market samples was lower than values reported by Owaga et al. (Citation2010) and those obtained for landing site samples. The observed differences in the fat content of silver cyprinid samples could be explained by differences in moisture content and rate of formation of lipid oxidation products such as FFA, aldehydes like TBARs (Huss, Citation1995). This is in agreement with the findings of Farid et al. (Citation2015), and Saritha et al. (Citation2012) who identified a reduction in lipid content of stored fish samples. The high-fat content of some stored fish samples could also show that fat content variations within fish species, probably due to the richness of the food for silver cyprinid from the different fishing grounds.
4.1.4. Ash content
Market samples had ash content that was within the range specified in previous market studies (Owaga et al., Citation2010). Silver cyprinid samples obtained from landing sites, and markets had varied ash content values which were below the permissible limit of 15% for dry silver cyprinid (EAS, Citation2014). The observed difference in ash content can be attributed to variation in moisture content (Bille & Shemkai, Citation2006; Kabahenda et al., Citation2011; Chukwu & Shaba, Citation2009; Owaga et al., Citation2010), pretreatment method, drying method used, adulterant level and mineral contents (Kabahenda et al., Citation2009; Ssebisubi, Citation2011). Salting of silver cyprinid in Kiyindi could have adulterated its mineral content thus increasing the ash content of fish samples (Chukwu, Citation2009; Unlusayin et al., Citation2010).
4.1.5. Iron content
The iron content of samples within and between landing sites and markets was higher than previously documented values (Kabahenda et al., Citation2011). The high iron content in market samples as compared to landing site samples could probably be due to the difference in moisture content, variation in storage duration at room temperature. At high temperatures like room temperature, heme iron is easily converted to non-heme iron (Alcock et al., Citation2006) and thus a decrease in heme-iron content of the fish (Gomez-Basauri & Regenstein, Citation1992). Generally, about 60 g of market store silver cyprinid can provide the RDA for iron among infants although this increases to 75 g for the case of landing site store samples.
4.2. Microbial quality
Total plate counts, yeast and moulds, as well as total coliforms exceeded the acceptable limits of 5, 4 and 0 log cfu/g respectively set by East Africa (EAS, Citation2014). The higher total plate count, total coliform content, and aflatoxin content of market samples than landing site samples can be attributed to adulteration during packaging, transportation, storage coupled with insect infestations and high moisture content of these samples (Cockerell et al., Citation1971; Glucas, Citation1982). The high yeast and moulds content in Silver cyprinid could suggest that its consumers were at risk of mycotoxin ingestion (Nnagbo et al., Citation2018) which could cause immunity suppression, decreased growth, malnutrition and mortality (Gong & Hall, Citation2008).
Total coliform is an indicator of hygiene standard in food handling (Buchanan & Oni, Citation2012; Samakupa et al., Citation2003). This has implications which include increased diarrhoea among children less than 2 years (Agustina et al., Citation2013) and risk of other waterborne illnesses among individuals of all age groups (Onyango et al., Citation2018). Thus, the lowest content of total coliforms in Silver cyprinid from certain locations indicated better hygiene practices among the fish handlers in the places from which the silver cyprinid was obtained.
High aflatoxin content directly relates to the observed high yeast and mould counts in the fish samples. The high variability in the aflatoxin content above acceptable 0.05 ppb for infant food and 10 ppb for adults (Codex, Citation2013) in all the markets should be a concern to the consumers, fisheries and public health authorities. This is because the consumption of fish contaminated with aflatoxins results in liver damage and eventually death (Essien et al., Citation2005).
4.2.1. Markers of protein and fat degradation
Microbial metabolism of amino acids in the protein yields total volatile basic nitrogen (TVBN) and trimethylamine (TMA) (Immaculate et al., Citation2013; Prakash et al., Citation2011; Saritha et al., Citation2012). TVBN and TMA are thus widely used to estimate fish spoilage and/or quality (Jinadasa, Citation2014)
The concentration of TVBN and TMA in market fish samples in this study were higher than those from landing site stores. This could be linked to protein degrading spoilage microbes being in higher counts in market samples which were stored for a longer duration than landing site samples. This is in concordance with Yusuf et al. (Citation2010) who reported an increased activity of spoilage bacteria to enhance breakdown of TMAO to TMA, hence the buildup of TVBN. However, TVBN is made of TMA and di-methyl amine (DMA) but DMA is not related to bacterial spoilage (Wu & Bechtel, Citation2008). Generally, TVBN and TMA were within the acceptable limits of 35 mgN/100 g, 15 mgN/100 g, respectively in fish (Huss, Citation1988), indicating that there was limited the extent of protein degradation of proteins.
Based on FFA values the silver cyprinid was food grade according to Bimbo (Citation1998). The higher FFA content in the market would suggest an influence of a longer storage duration coupled with poor storage practices that could have favoured enzymatic lipid oxidation compared to a shorter storage duration of landing site samples. In addition to this, Nguyen et al. (Citation2012) related increased lipid hydrolysis to inadequate drying of fish and the presence of microbes. However, in this study, there was no positive correlation of moisture content with FFA for both market and landing site fish samples, although the market samples with a relatively higher total plate counts had the higher FFA.
Studies of (Huss, Citation1995) and Brigitte et al. (Citation2004) indicated that hydroperoxides convert to TBARs under high temperature and UV light. However, these were not considered during this study. Moreover, oxidation of lipids can follow different pathways and at different rates (Antolovich et al., Citation2002). This could partly explain the difference in concentration of TBARs between market and landing site fish samples. Lipid oxidation products are known to reduce the palatability and nutritive value of dried fish (Wasowicz et al., Citation2004), hence the need for protection of fishery products against oxidative changes. The presence of lipid oxidation products in dried fish alongside rendering it unpalatable reduces nutritive values and can lead to oxidative damage (Wasowicz et al., Citation2004).
5. Conclusion
Silver cyprinid found at market and landing site stores was of poor quality and thus not fit for human consumption. This undermines its role as a nutrient-dense affordable animal protein source food that can be incorporated in staple-based diets to curb the prevalent malnutrition among vulnerable individuals. Thus, there is a need for policy and regulations to enforce adequate and timely post-harvest handling (at harvesting, processing, packaging and storage) of silver cyprinid destined for human consumption. This will improve the utilization of fish as food by the vulnerable individuals who have a high nutrient requirement.
Conflict of interest
None of the authors had a conflict of interest in the study and data presented in this manuscript.
Acknowledgements
The authors acknowledge the assistance of Dr Kendra Byrd for review, Semwanga Nathan Makerere University for guidance on data analysis and scientific writing.
Additional information
Funding
Notes on contributors

Julia Kigozi
Julia Kigozi, is an Agricultural Engineer, specializing in Food Process Engineering. She in her research groups specifically focusses on Post handling of crops, design and optimization of Food engineering operations, design of food processing equipment and plant Design. She has been involved in the design and construction of a fruit pulper, a batch pasteurizing equipment, vegetable/root chopping machine, Biomass Solar dryer, rice planter, continuous pasteurizer, juice packing machine, Refractance Window Dryer among others. Other areas of research have included; A study of the drying kinetics, quality and shelf stability of dried Rastrineobola Argentea (mukene fish), development of Shelf-table Fish sausage and Cracker, development of commercial products from sorghum, millet, matooke flour, Technical Inspection and Verification of Agricultural Equipment and Machineries. Julia has also worked with Agro-processors to facilitate their growth in production; specifically, in setting up their infrastructure and optimizing their process.
References
- Adebayo-Tayo, A. C., Odu, N., Michael, M. U., & Okonko, I. O. (2012). Multi-drug resistant (MDR) organisms isolated from sea-foods in Uyo, South-Southern Nigeria. Nature and Science, 10(3), 61–15. http://www.sciencepub.net
- Agustina, R., Sari, T. P., Satroamidjojo, S., Bovee-Oudenhoven, I. M., Feskens, E. J., & Kok, F. J. (2013). Association of food-hygiene practices and diarrhea prevalence among Indonesian young children from low socioeconomic urban areas. BMC Public Health, 13(977), 1-12. https://doi.org/10.1186/1471-2458-13-977
- Akande, G., & Diei-Ouadi, Y. (2010). Post-harvest losses in small-scale fisheries: Case studies in five sub-Saharan African countries (Fisheries and Aquaculture Technical Paper. No. 550). FAO.
- Alcock, B., Cabrera, O. N., Barkoula, M. N., Spoelstra, B. A., Loos, J. and Peijs, T. (2006). Low velocity impact performance of recyclable all polypropylene composites. Composites Science and Technology 66 (2006) 1724-1737 https://doi.org/10.1016/j.compositesa.2006.01.003
- Angelo, A. J. (1996). Lipid oxidation in foods. Critical Reviews in Food Science and Nutrition, 36(3), 175–224. https://doi.org/10.1080/10408399609527723
- Antolovich, M., Prenzler, P., Patsalides, E., McDonald, S., & Robards, K. (2002). Methods for testing antioxidant activity. The Analyst, 127(1), 183–198. https://doi.org/10.1039/b009171p
- Association of Official Analytical Chemists (AOAC). (1996). Association of official analytical chemists. Official methods of analysis (16th ed.). Gaithersburg.
- Association of Official Analytical Chemists (AOAC). (2005). Association of official analytical chemists. Official methods of analysis (18 ed.). Gaithersburg.
- Béné, C., Macfadyen, G., & Allison, E. H. (2007). Increasing the contribution of small-scale fisheries to poverty alleviation and food security. United Nations Food and Agriculture Organization (FAO).
- Bille, P. G., Shemkai, R. H. (2006). Process development, nutrition and sensory characteristics of spiced-smoked and sun-dried Dagaa (Rastrineobola argentea) from Lake Victoria, Tanzania. African Journal of Food, Agriculture, Nutrition and Development, 6(2), 1–12. doi:10.4314/ajfand.v6i2.71737
- Bimbo, A. (1998). Guidelines for characterizing food grade oils. International News on Fats, Oils and Related Materials, 9(5), 473–483. https://www.researchgate.net/publication/279618543
- Boran, G., & Karacam, H. (2011). Seasonal changes in proximate composition of some fish species from the black sea. Turkish Journal of Fisheries and Aquatic Sciences, 11(1), 01–05. doi:10.4194/trjfas.2011.0101
- Brigitte, M. B., Boogaard, B., & Heijenen, C. (2004). Preservation of fish and meat.http://www.fao.org/DOCREP/T0713E/T0713E00.html
- Buchanan, L. R., & Oni, R. (2012). Use of microbiological indicators for assessing hygiene controls for the manufacture of powdered infant formula. Journal of Food Protection, 75(5), 989–999. https://doi.org/10.4315/0362-028X.JFP-11-532
- Chukwu, O. (2009). Influences of Drying Methods on Nutritional Properties of Tilapia Fish (Oreochromis nilotieus). World Journal of Agricultural Sciences 5 (2): 256–258 ISSN 1817–3047
- Chukwu, O. and Shaba, I. M. (2009). Effects of Drying Methods on ProximateCompositions of Catfish (Clarias gariepinus). World Journal of Agricultural Sciences, 5(1), 114–116. ISSN 1817-3047
- Cockerell, I., Francis, B., & Halliday, D. (1971). Changes in the nutritive value of concentrate feedstuffs during storage. Proceedings of Conference of Feed Resources and improvement of Animal feeding methods in the CENTO Region countries, Tropical products Institute, (181–192). London.
- Codex. (2013). Proposed draft standard for oils.
- Connell, J. (1995). Control of fish quality (4th ed.). Fishing News Books Ltd; WileyBlackwell.
- EAS. (2014). East African standard dried fish Rastrineobola argentea-Specification DEAS 826:2014. ICS 67.120.30.
- Essien, J. P., Ekpo, M. A., & Brooks, A. A. (2005). Mycotoxigenic and proteolytic potential of moulds associated with smoked shark fish (Chlamydoselachus anguincus). Journal of Applied Sciences and Environmental Management, 9(3), 53–57. http://www.bioline.org.br/ja
- Farid, F. B., Latifa, G. A., Nahid, M. N., & Begum, M. (2015). Protective effect of brine-salt curing on physico-chemical attributes on the taki fish (Channa punctatus) and the tengra fish (Mystus tengra) at room temperature. Bangladesh Journal of Zoology, 42(2), 271–276. https://doi.org/10.3329/bjz.v42i2.23369 2 doi:10.3329/bjz.v42i2.23369
- Glucas, I. J. (1982). Present fish drying techniques in Zambia and suggested improvements. FAO. ( F.J.Zam (73/00/3 FAO)).
- Gomez-Basauri, J. V. and Regenstein, J. M. (1992). Processing and frozen storage effects on the iron content of Cod and Mackerel. Journal of Food science, 5, 1332–1336. https://doi.org/10.1111/j.1365-2621.1992.tb06850.x
- Gong, Y. T., & Hall, A. J. (2008). Aflatoxin exposure and impaired child growth in West Africa: An unexplored international public health burden. In J. Leslie, R. Bandyopadhyay, & A. Visconti (Eds.), Mycotoxins: Detection methods, management, public health and agricultural trade (pp. 53–65).
- Gopakumar, K. (2002). Textbook of fish processing technology. Indian council of agricultural research New Delhi.
- Huss, H. H. (1988). Fresh fish quality and quality changes (Fisheries Series No. 29). Sagwan Press.
- Huss, H. H. (1995). Quality and quality changes in fresh fish (FAO Fisheries Technical Paper. No. 348, p. 195). FAO.
- Ibengwe, L. (2011). Reducing post-harvest losses of the Artisanal Dagaa (Rastrineobola argentea) fishery in Lake Victoria, Tanzania: A cost and benefit analysis. Fisheries Training Programme, The United Nations University.
- Ikape, S. I., & Cheikyula, J. O. (2017). Fish spoilage in the tropics : A review. Octa Journal of Biosciences, 5(2), 34–37. https://www.researchgate.net/publication/329921962
- Immaculate, K., Sinduja, P., Velammal, A., & Patterson, J. (2013). Quality and shelf life status of salted and sun dried fishes of Tuticorin fishing villages in different seasons. International Food Research Journal, 20(4), 1855–1859.
- Jinadasa, K. (2014). Determination of quality of marine fishes based on total volatile base nitrogen test (TVB-N). Journal of Science and Nature, 12(5), 106–111. http://www.sciencepub.net/nature
- Kabahenda, M. K., Amega, R., Okalany, E., Husken, S. M., & Heck, S. (2011). Protein and micronutrient composition of low-value fish products commonly marketed in the Lake Victoria region. World Journal of Agricultural Sciences, 7(5), 521–526.
- Kabahenda, M. K., Omony, P., & Hüsken, S. M. (2009). Post-harvest handling of low-value fish products and threats to nutritional quality: A review of practices in the Lake Victoria region. Investingation in Sustainable Solutions. WorldFish Center.
- Kirk, R. S. & Sawyer, R. (1991). Pearson's composition and analysis of foods, 507–544. ISBN: 044302149X 9780443021497
- Kubiriza, G. K., Ssempijja, D., Mubiru, E., Semwanga, N., Odoli, C. O., Masette, M., Zalwango, J., & Masette, M. (2020). Oxidative stability and proximate composition of silver cyprinid (Rastrineobola argentea) used for fishmeal in East Africa. Journal of Applied Aquaculture, 1–21. https://doi.org/10.1080/10454438.2020.1727808
- LVFO. (2012). Manual for processing and marketing of small-sized pelagics, popular version.
- Malle, P. and Poumeyrol, M. 1989. A New Chemical Criterion for the Quality Control of Fish: Trimethylamine/Total Volatile Basic Nitrogen (%). Journal of Food Protection, 52(6), 419–423. doi:10.4315/0362-028X-52.6.419
- Masette, M., & Kwetegyeka, J. (2013). The effect of artisanal preservation methods on nutritional security of “ Mukene ” Rastrineobola argentea caught from Lakes Victoria and Kyoga in Uganda. Uganda Journal of Agricultural Sciences, 14(2), 95–107.
- Mbunda, A. E. (2013). The quality changes in smoked and dried fresh water sardine (Rastrineobola argentea) and marine pelagic fish (caplin) as influenced by processing methods. [ final project]. http://www.unuftp.is/static/fellows/document/arnold12prf
- Nguyen, V. M., Thorarinsdottir, K. A., Thorkelsson, G., Gudmundsdottir, A., & Arason, S. (2012). Influences of potassium ferrocyanide on lipid oxidation of salted cod (Gadus morhua) during processing, storage and rehydration. Journal of Food Chemistry, 131(4), 1322–1331. https://doi.org/10.1016/j.foodchem.2011.09.126
- Nkwoemeka, N. (2019). Mycological quality of Rastrineobola argentea (Silver cyprinid fish) sold in markets in Owerri metropolis, Nigeria.
- Nnagbo, P. A., Nkwoemeka, N. E., Obum-Nnadi, C. N., Egbule, U. C., & Umunna, C. C. (2018). Mycological quality of Rastrineobola argentea (Silver cyprinid fish) sold in markets in Owerri metropolis, Nigeria. International Journal of Innovative Science, Engineering & Technology, 5(11), 94–97.
- Ogonda, L. A., Muge, E. K., Mulaa, F. J., & Mbatia, B. N. (2014). Proximate composition of Rastrineobola argentea (Dagaa) of Lake Victoria- Kenya. African Journal of Biochemistry Research, 8(1), 1–6. https://doi.org/10.5897/AJBR2013.0720
- Okonko, I., Adejoye, O., Ogunnusi, T., Fajobi, E., & Shittu, O. (2008). Microbiological and physicochemical analysis of different water samples used for domesticpurposes in Abeokuta and Ojota, Lagos state, Nigeria. African Journal of Biotechnology, 7(5),, 617–621. http://www.academicjournals.org/AJB
- Onyango, A. E., Okoth, M. W., Kunyanga, C. N., & Aliwa, B. O. (2018). Microbiological quality and contamination level of water sources in Isiolo county in Kenya. Journal of Environmental and Public Health, 2018, 10. Article ID 2139867. https://doi.org/10.1155/2018/2139867.
- Onyuka, J. H., Kakai, R., & Ofulla, A. V. (2014). Bacterial contamination dynamics and fungal contamination levels of fish Rasterineobola argentea and Oreochromis niloticus from Lake Victoria basin of Kenya. The Journal of Food Technology. Photon, 106, 179–188. https://www.researchgate.net/publication/259573837
- Owaga, E. E., Onyango, C. A., & Njoroge, C. K. (2010). Influence of selected washing treatments and drying Temperatures on proximate composition of Dagaa (Rastrineobolaargentea), a small pelagic fish species. African Journal of Food, Agriculture, Nutrition and Development, 10(7), 1–14. https://www.ajol.info/index.php/ajfand/article/view/59031
- Pal, M., Ketema, A., Anberber, M., Mulu, S., & Dutt, Y. (2016). Microbial quality of fish and fish products. Beverage and Food World, 43(2), 46-49. https://www.researchgate.net/publication/295857928
- Pokorny, J., & Dieffenbacher, A. (1989). Determination of 2-thiobarbituric acid value: Direct method - results of a collaborative study and the standardised method. Pure and Applied Chemistry, 61(6), 1165–1170. https://doi.org/10.1351/pac198961061165
- Prakash, S., Jeyasanta, I., & Patterson, J. (2011). Microbial quality of salted and sun-dried sea foods of Tuticorin dry fish market, southeast coast of India. International Journal of Food Microbiological Research, 2(2), 188–195.
- Samakupa, P. A., Einarsson, H., & Eyþórsdóttir, A. (2003). Hygiene indicators in a fish processing establishment-a case study in a white fish processing establishment (Final Project Report). United Nations University, Fisheries Training Programme.
- Saritha, K., Immaculate, K. J., Aiyamperumal, V., & Patterson, J. (2012). Microbial and biochemical qualities of salted and sun-dried sea foods of Cuddalore, southeast coast of India. International Journal of Microbiology Research, 3(2), 138–143. http://idosi.org/ijmr/ijmr3(2)12/11.pdf
- Schormuller, J. (1968). Handbuch der Lebensmittelchemie (BandIII/2). Springer Verlag.
- Ssebisubi, M. (2011). Analysis of Small-Scale Fisheries’ Value- Chains in Uganda. Background report FAO Rome
- Ssebisubi, M. (2013). The status fishing communities in Buikwe district Uganda.
- Unlusayin, M., Erdilal, R., Gumuş, B. and Gulyavuz, H. (2010). The effects ofdifferent salting methods on extract loss from rainbow trout. Pakistan Journal of Veterinary, 30(3), 131 – 134. ISSN: 0253–8318
- USDA. (2015). Romer Labs AgraStrip Total Aflatoxin Qualitative Test Test Manual. Pp 0–6
- Wasowicz, E., Anna, G., Marzanna, H., Henryk, H. J., Jozef, K., Maria, M., Sylwia, M., Magdalena, R., Urszula, S., & Renata, Z., (2004). Oxidation of lipids in food. Polish Journal of Food and Nutrition Sciences, 13, 87–100. https://www.researchgate.net/publication/281317898
- Wu, T. H., & Bechtel, P. J. (2008). Ammonia, dimethylamine, trimethylamine, and trimethylamine oxide from raw and processed fish by-products. Journal of Aquatic Food Product Technology, 17(1), 27–38. https://doi.org/10.1080/10498850801891140
- Yusuf, A. M., Sharif, M. I., Ripon, K. A., & Faruque, O. (2010). Post mortem variation in total volatile base nitrogen and trimethylamine nitrogen between Galda (Macrobrachium rosenbergii) and Bagda (Penaeus monodon). University Journal of Zoology, Rajshahi University, 28, 7–10. http://journals.sfu.ca/bd/index.php/UJZRU ISSN 1023-6104