Abstract
Food fermentations in West Africa are still conducted spontaneously because of the lack of knowledge, unavailability of adequate technologies and the lack of production facilities. This study aimed at using maize flour as carrier material to develop a multifunctional freeze-dried starter culture with a mixture of lactic acid bacteria (LAB) and yeast strains. The maize flour carrier was tested for microorganisms’ freeze-drying, water activity (aw) as well as microbial viability. Two commercial carrier materials, i.e. soluble starch and skimmed milk, were used for comparison. After microbial freeze-drying, similar aw values (aw = 0.05–0.06) were obtained with the maize flour and the commercial carriers, i.e. skimmed milk and soluble starch. A slight increase of aw (0.07–0.08) was observed with the maize flour after 15 and 45 days storage at 4°C. For the three carrier materials, similar microbial log reduction (1.78–1.35 units with Lactobacillus fermentum Lf and 0.73–0.67 units with Saccharomyces cerevisiae Sc) was recorded just after freeze-drying and remained constant after 15 and 45 days storage at 4°C. Further, a defined freeze-dried starter culture was developed using the maize flour carrier as for a mixed culture of LAB and yeast strains. The defined freeze-dried culture showed stable fermentation activity during storage. The results indicated that the maize flour-based carrier could be suitable for microbial freeze-drying. Therefore, installing starter culture production facilities in West Africa to control cereal fermentation is possible. It will contribute to upgrading the sector of cereal food in this part of the world.
PUBLIC INTEREST STATEMENT
This research was performed to investigate the suitability of maize flour as low-cost carrier materiel for starter culture preparation in West Africa. The study compared the maize flour with commercial carriers such as soluble starch and skimmed milk and the result showed that the maize flour has the comparative utility. The public interest of this research is that it provides consistent knowledge that could contribute to develop easily available consumable for starter culture production which remains common challenge for food fermentation in West Africa. The use of such starter culture for food fermentation will confer profound benefits such as consistent sensory quality, improved hygiene and safety, resulting in fermented food products with high nutritional quality.
1. Introduction
Developing production facilities of suitable starter culture is common challenge for food fermentation in West Africa. Defined starter cultures are pure cultures of microorganisms which are propagated in microbial media or carrier materials and generally dried, as e.g., freeze-dried and spray-dried, to produce easy-to-use ingredients that are stable and flexible for applications in food fermentations (Huang et al., Citation2017; Vogel et al., Citation2011). The use of starter culture for food fermentation confers profound benefits such as improved hygiene, safety and quality control resulting in fermented food products with high nutritional and organoleptic quality (Holzapfel, Citation1997). The use of microbial starter culture in Africa is not yet common because of lack of knowledge, unavailability of adequate technologies and production facilities.
The viability and stability of a defined starter culture are key challenges and depend on the carrier and the desiccation technique (Marcial-Coba et al., Citation2019). Though freeze-drying is expensive, it offers long-term preservation due to the satisfactory microbial survival rates (Marcial-Coba et al., Citation2019). However, selection of suitable carrier material is essential for successful freeze-drying and end use of the defined starter culture. Skimmed milk and soluble starch are generally used as carriers for microbial freeze-drying (Bolla et al., Citation2011; Kavitake et al., Citation2018). However, the use of an easy-to-access flour such as maize flour as carrier could reduce the production cost of the defined starter culture and increase the success of its application in cereal fermentations which are popular in West Africa. In this part of the world, fermented cereal products are numerous and contribute significantly to people nutrition (Nout, Citation2009). Most of the cereal fermented foods are processed from doughs used as multipurpose intermediate ingredients for the preparation of several types of meal (Houngbédji, Padonou, et al., Citation2019). The most popular cereal doughs known in Benin and Togo include mawè, ogi; with similar mawè-like and ogi-like products existing in other parts of West African countries (Hounhouigan et al., Citation1994). Mawè and similar products are used for the preparation of a variety of West African traditional cooked dishes, including paste (e.g. makumè, akassa, banku and come), porridge (e.g., koko and aklui), beverages (akpan), steam-cooked bread (ablo), fritters (massa, pâté), and couscous (Yèkè-yèkè) (Houngbédji et al., Citation2018; Hounhouigan et al., Citation1993).
This study aims at using maize flour (unfermented and autoclaved dehulled maize flour) as carrier to prepare a defined freeze-dried starter culture of LAB and yeast strains. Further, the defined starter culture was used in fermentation trials to assess its ability to acidify maize dough for mawè production and enhance free amino acid content of mawè.
2. Materials and methods
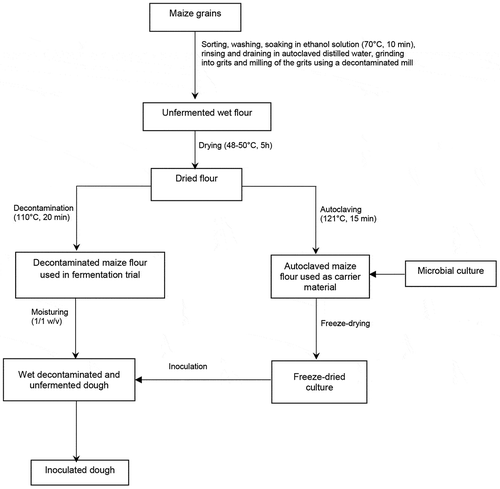
2.1. Preparation and use of maize flour
A maize variety adapted for flour production (small grain and white colour) was purchased in Abomey-Calavi market (southern Benin, 6°27′north and 2°21′east) for the preparation of a non-fermented and dehulled maize flour. Sorted and wet cleaned maize grains were soaked in ethanol solution (70%, v/v) for 10 min and rinsed with sterilized distilled water to reduce the microbial load of the raw material. The decontaminated maize grains were ground into gritz using a decontaminated abrasive mill. The obtained gritz were immediately milled into unfermented dough (pH = 6.5 ± 0.45, water content = 41 ± 1.8%). The unfermented wet dough was dried at 45–50 °C for 4–5 h. One share of the resulting flour was used as carrier material for freeze-drying and the second share used in fermentation trials as summarized in .
2.2. Microbial strains and carrier material
Microbial strains used in this study included three strains of lactic acid bacteria namely one of Lactobacillus fermentum (Lf), one of Pediococcus acidilactici (Pa) and one of Weissella confusa (Wc) as well as three yeast strains namely one strain of Saccharomyces cerevisiae (Sc), one of Kluyveromyces marxianus (Km) and one of Pichia kudriavzevii (Pk), as shown in . The strains were isolated from spontaneously fermented mawè doughs and stored at −80 °C in MRS broth (CM0361 Oxoid, pH 5.6) for LAB and MYGP broth [3 g of yeast extract (Difco), 3 g of malt extract (Difco), 5 g of bactopeptone (Difco) and 10 g of glucose per litre of distilled water, pH adjusted to 5.6 using HCl, 1 N] for yeast, containing 20% (v/v) of glycerol (Houngbédji et al., Citation2018).
Table 1. Microbial strains included in the study
The maize flour prepared as described above was autoclaved at 121 °C for 15 min to eliminate the entire endogenous flora. The suitability of this maize flour for use as carrier material during microbial cells freeze-drying was tested in comparison to two commercial carrier materials, i.e. soluble starch powder (Sigma-Aldrich) and skimmed milk powder (Sigma-Aldrich).
2.3. Microbial cell propagation
Each strain was streaked onto MRS agar (for LAB) or MYGP agar (for yeasts) and incubated at 30 °C for 48 h. A single colony was picked and transferred in a tube containing 10 mL of MRS or MYGP broth and cultivated at 30 °C for 24 h. The resulting culture was transferred to 1000 mL of appropriate medium, and incubated at 30 °C for 20 h. The 20 h culture was then centrifuged (12,000 x g, 5 min) and the pellet washed once in 1% saline peptone (1% NaCl, w/w; and 5 g/L of bactopeptone) and redistributed in a volume of 1% saline peptone to yield a concentration of 1013 cells/mL for LAB and 1011 cells/mL for yeast.
2.4. Preparation of blends of microbial cell suspensions – carriers for freeze-drying experiments
First, the blends of microbial cell suspension-carrier material were prepared for S. cerevisiae (Sc) and L. fermentum (Lf) singly, using each of the three carrier materials (maize flour, soluble starch and skimmed milk). This first step was to demonstrate the comparative utility of maize flour. Second, the mixture of the 6 microbial strains presented in was incorporated in maize flour. Then, 1 mL of each of the propagated cell cultures prepared above was centrifuged and cells were harvested and re-suspended in 60 mL of distilled sterile water to obtain either a single culture of Lf and Sc strains, or a mixed culture of the 6 strains. Each of the resulting cell suspension was added to 20 mL of 25% sucrose solution and 20 g of the carrier to yield 100 mL cells-carrier suspension with 1011 cells/mL for each LAB and 109 cells/mL for each yeast.
2.5. Freeze-drying procedure
One millilitre of microbial cells and carrier suspensions were distributed in glass vials (Injektionsflasche 6 mL, Dedecke GmbH, Königswinter). The freeze-drying experiments were conducted using an Alpha 1–2 LDplus freeze drier (Martin-Christ, Germany) following manufacturer instructions. Right after freeze-drying, microbial viability was checked. Moreover, during storage at 4 °C, viability was assessed at 15 and 45 days.
2.6. Water activity and viability of freeze-dried cultures
Water activity and microbial viability after freeze-drying were evaluated on Lf and Sc for which all the three carriers were experimented. Water activity (aw) was measured at 26 °C using a water activity meter (Hygrolab, Rotronic AG, Switzerland). The microbial viability was determined by plate counting. Results obtained with maize flour used as carrier were compared with those of soluble starch and skimmed milk.
2.7. Fermentation trials using freeze-dried cultures
Maize flour was used as a carrier to freeze-dry mixed culture made of all 3 LAB (Lf, Pa, Wc) and 3 yeasts (Sc, Km, Pk) included in the study. The ability of 3 days, 15 days and 45 days stored freeze-dried mixed cultures to ferment mawè was evaluated in comparison to a fresh mixed culture. The dried mawè prepared above was decontaminated by autoclaving at 110 °C for 20 min, which decreased the endogenous flora to <1 log cfu/g. For the inoculation of the decontaminated mawè, 100 µL of the fresh culture of microbial suspension obtained before freeze-drying and 1–1.5 g of the freeze-dried culture were diluted each in separate 48.5–49.0 mL distilled sterile water, added with 50 g decontaminated mawè flour to obtain two separate 100 g inoculated mawè (with 54–58 % moisture content), at counts of 3 × 8 and 3 × 6 log 10 CFU/g for LAB and yeasts, respectively. The inoculated mawè was left for fermentation at 30 °C. Samples were taken out at the onset of the fermentation, 6, 24, 48 and 72 h for pH monitoring and plate counting as well as free amino acid content analysing only for 48 h samples.
2.8. pH and titratable acidity determination, moisture content, particle size, plate counting and amino acid quantification during the fermentation
The pH was determined using an InoLab digital pH meter (3505 pH meter, JENWAY) on homogenized mixture of 10 g sample and 20 mL of distilled water. Subsequently, the suspension was added with 70 mL distilled water and titration performed using 0.1 N NaOH; phenolphthalein (1% w/v) was used as indicator and results were expressed as % lactic acid of mawè (dry basis). The moisture content was determined for 5 g of sample calculated based on the weight before and after 72 h of oven drying according to the American Association of Cereal Chemists method 44–15A (American Association of Cereal Chemists, Citation2000). For particle size determination, 100 g of 24 h flour was shaken for 15 min through screen sieves, on an Endecotts test sieve shaker (Endecotts, London, UK). Thereafter, the mass of sample retained on each sieve was recorded. Plate counting was performed by serially diluting 0.5 g of the fermented mawè in 4.5 mL 1% (w/v) saline peptone, pH 5.6 and 100 μL spread plated on MRS agar for LAB and MYGP agar supplemented with 1% chloramphenicol for yeasts, and incubated at 30 °C for 48–72 h. The amino acids were measured on dried oil-extracted of 28 h fermented mawè, inoculated with the 3 days stored freeze-dried culture, following Waters PICO•TAG method (Bidlingmeyer, Citation1984) using HPLC System components designed by Waters Corporation (Waters, Citation1984). The overnight extraction of the total amino acids (TAA; encompassing complex and free amino acids) and free amino acids (FAA) of the dried oil-extracted samples were treated with 6 M HCl at 110° C (for TAA) and 0.1 M at room temperature (for FAA). Derivatization of the extracted amino acids was performed with phenylisothiocyanate, producing phenylthiocarbamyl amino acids. The amino acid derivatives were separated by reversed-phase HPLC, and detected by absorption spectrophotometry (Waters, Citation1984).
2.9. Statistical analysis
Experiments were conducted in triplicate and means and standard deviations were calculated. Data were subjected to one-way analysis of variance (ANOVA) and means were separated by least significant difference test of Fisher (P < 0.05) using STATISTICA v7.1 software.
3. Results
3.1. Characteristics of the maize flour carrier in comparison with the commercial carriers
shows physicochemical characteristics of the carrier materials. The moisture content of maize flour carrier (5.0 ± 1.2 %) was similar to the one of skimmed milk carrier (8.1 ± 1.0 %), while the soluble starch showed significant high (P < 0.05) moisture content (14.7 ± 0.2). Water activity (Aw) of maize flour carrier (0.24 ± 0.02) was similar to the one of soluble starch (0.25 ± 0.03), while the skimmed milk carrier showed significant high (P < 0.05) Aw value (0.38 ± 0.01). The pH value was similar for the three carrier materials (pH = 6.2–6.3). However, titratible acidity (TTA) was significantly different (P < 0.05) with the highest value recorded for soluble starch (TTA = 14.1 ± 0.0 % of lactic acid) followed by maize flour carrier (TTA = 9.0 ± 0.7 % of lactic acid), while significant low value recorded for skimmed milk (TTA = 2.3 ± 0.2 % of lactic acid). The particle size profile was similar for skimmed milk and soluble starch and significantly different from the one of the maize flour carrier (P < 0.05). High proportion of fine particle (size < 0.18 mm) was recorded for skimmed milk (86.2 ± 0.1 %) and soluble starch (88.7 ± 0.0 %), while less than 1 % fine particle recorded for the maize flour characterized by higher particle size i.e. 60.5 ± 0.6 % particle with size raging between 0.18 and 0.63 mm, and 32.4 ± 0.1 % particle raging between 0.63 and 1.25 mm.
Table 2. Physicochemical characteristics of the carrier materials. Values presented correspond to means ± sd of three individual measures. Means with the same letters within a line do not differ significantly (p > 0.05)
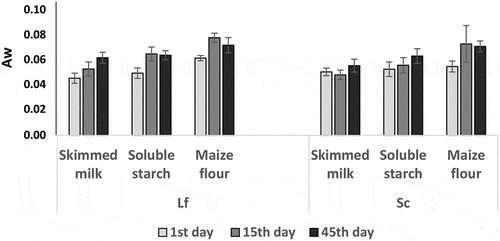
3.2. Water activity of freeze-dried cultures
shows the water activity (aw) of freeze-dried cultures of Lf or Sc single strains just after freeze-drying (1st day) and during storage at 4° C, i.e. 15th and 45th days later. For skimmed milk-based freeze-dried cultures of both strains Lf and Sc, aw was 0.04–0.05 just after freeze-dying and remained stable until 15th (aw = 0.05) and 45th days (aw = 0.05–0.06). The same trend was observed for soluble starch-based freeze-dried cultures. The use of maize flour as carrier significantly increased aw compared to freeze-dried cultures in skimmed milk and soluble starch (P ≤ 0.05), just after freeze-drying (0.06) and during storage at 4° C (0.07–0.08 at 15 days and 0.07 at 45 days).
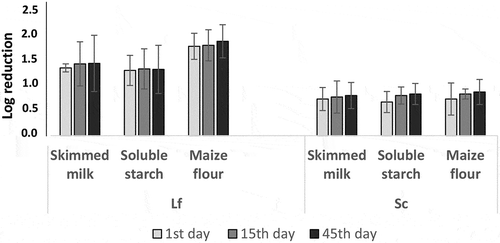
3.3. Microbial log reduction after freeze-drying process and during storage at 4° C
shows microbial log reduction of the freeze-dried cultures of single strains of Lf and Sc just after freeze-drying (1st day) and at 15th and 45th days of storage at 4° C. For Lf strain, the log reduction was similar with skimmed milk (1.35 ± 0.08 units) and soluble starch (1.30 ± 0.30 units) just after freeze-drying, while significant higher reduction of Lf (1.78 ± 0.23 units) was seen with maize flour on the 1st day of storage. The log reduction of Lf did not change significantly during storage after 15 and 45 days at 4° C for the three carrier materials. Considering the strain Sc, the log reduction was similar with the three carrier materials just after freeze-drying, i.e. 0.73 ± 0.23 units for skimmed milk, 0.67 ± 0.21 units for soluble starch and 0.73 ± 0.32 units for maize flour. The log reduction of Sc did not change significantly during storage after 15 and 45 days at 4° C for the three carrier materials.
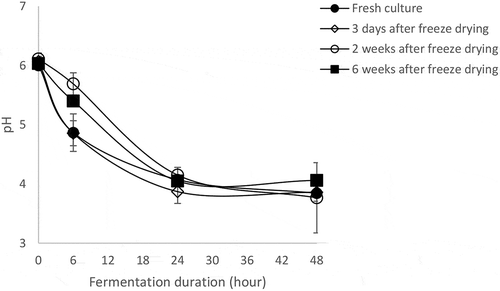
3.4. Acidification ability of the freeze-dried mixed culture after different storage duration
The ability of the mixed freeze-dried culture to acidify mawè after 3 days, 15 days and 45 days of storage at 4° C was recorded (). A same but fresh microbial-mixed culture was used as control. At the initial stage (0–6 h), pH decrease was faster for the fresh culture and the 3 days stored freeze-dried culture compared with the 15 and 45 days stored freeze-dried cultures. However, the pH dropped to the same values (3.77–4.06) for the four microbial cultures after 48 h of the fermentation.
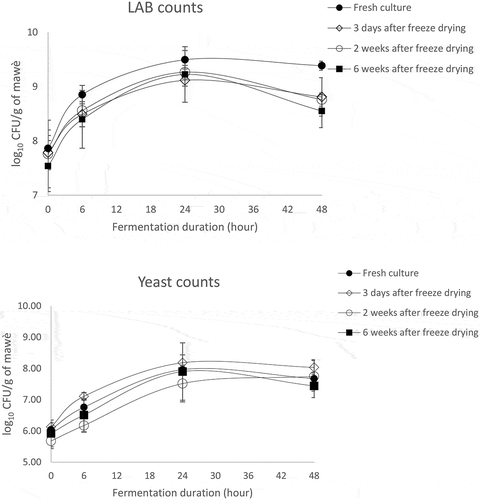
3.5. Log changes during fermentation of mawè with the fresh and the freeze-dried cultures
Concomitantly with the pH analysis, LAB and yeasts counts were recorded during the fermentation of mawè with the freeze-dried mixed culture after 3 days, 15 days and 45 days of storage at 4° C, a fresh microbial-mixed culture being used as control (). LAB count increased from 7.54–7.78 log10cfu/g at the onset, to 9.12–9.49 log10cfu/g at 24 hours of the fermentation for the four microbial cultures, i.e. the fresh culture and the 3, 15 and 45 days stored freeze-dried cultures. Thereafter, the LAB count remained similar (9.39 ± 0.03 log10cfu/g) for the fresh culture, but slightly decreased to 8.55–8.76 log10cfu/g for 3 days, 15 and 45 days stored cultures. For the four microbial cultures, yeasts count increased from 5.68 to 6.13 log10cfu/g at the onset to 7.52–8.18 log10cfu/g at 24 hours, and thereafter slightly decreased to 7.44–8.03 log10cfu/g at 48 h.
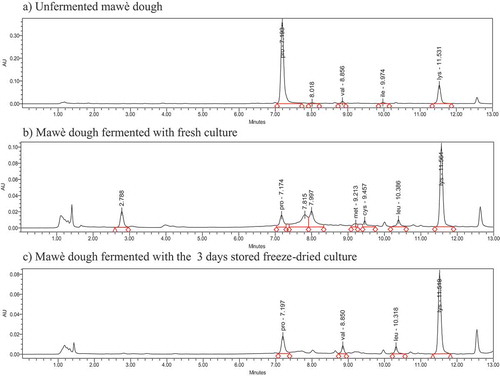
3.6. Free amino acid profile of mawè doughs fermented with the fresh and the freeze-dried cultures
presents examples of chromatograms of the free amino acid profile of non-fermented mawè used as negative control ()) as well as mawè doughs fermented with the fresh culture, i.e. positive control ()) and the 3 days stored freeze-dried microbial cultures ()). Only free proline (0.75 mg/g), valine (0.2 mg/g), and lysine (0.12 mg/g) were detected in the non-fermented mawè. The amino acid profile of the mawè doughs changed significantly after fermentation with both fresh and freeze-dried cultures. The mawè dough fermented with fresh culture had proline 0.05 mg/g, methionine 0.19 mg/g, cysteine 0.82 mg/g, leucine 0.07 mg/g and lysine 0.14 mg/g; while per g of mawè dough fermented with freeze-dried culture stored for 3 days, 0.39 mg proline, 0.01 mg of leucine 0.01, 0.12 mg of lysine and 0.02 mg of valine were recorded.
4. Discussion
Water activity (aw) is a key factor in maintaining microbial cell viability in dried products used as carrier material for probiotics or starter cultures (Marcial-Coba et al., Citation2019; Vesterlund et al., Citation2012). Lower aw values were seen for skimmed milk and soluble starch (aw = 0.04–0.05) just after freeze-dying (1st day) compared to the maize flour (aw = 0.06) with slight increase to 0.07–0.08 at 15th day and 0.07 at 45th day. Though aw of the maize flour starter seemed slightly higher compared with skimmed milk and soluble starch starters, this aw value of maize flour starter is low enough to allow stable microbial viability during storage. It was previously reported that survival of microorganisms in freeze-dried culture would be stable at aw between 0.07 and 0.1 (Higl et al., Citation2007; Vesterlund et al., Citation2012) for long-term storage. Using the three carriers materials tested in this study, log reduction of L. fermentum Lf strain (1.30–1.78 units) was higher compared with the one of S. cerevisiae Sc strain (0.67–0.73 units). Bolla et al. (Citation2011) reported comparative survival rate of yeasts and LAB just after freeze-drying. Interestingly, the microbial log reduction was similar for the three carrier materials just after freeze-drying and during storage even though the aw value of maize-based carrier was slightly higher compared to those of skimmed milk and soluble starch. The results demonstrated that maize flour could be successfully used as carrier material for bacteria and yeast starter culture freeze-drying with a successful storage at 4° C for at least 45 days.
The challenge with dried microbial cultures in the food industry is not only to maintain cell viability but also to ensure that the viable cells are metabolically active and could provide significant functional properties for food fermentations or probiotic benefits (Bolla et al., Citation2011; Mantzourani et al., Citation2019; Ogunremi et al., Citation2017). In cereal fermentation, the dried culture must be able to grow in the cereal substrate, acidify the dough and enhance the safety and nutritional characteristic of the fermented products (Brandt, Citation2014; Ogunremi et al., Citation2017). In this study, it was observed that after 48 h fermentation with the freeze-dried cultures (3, 15 and 45 days storage), the resulted fermented mawè doughs had similar microbial load and pH values as recorded for the fermented mawè obtained with the fresh culture. These results demonstrate satisfactory acidification activity of the starter culture. Acidification activity in the defined starter culture is important as the microorganisms of the starter could inhibit adventitious microbes and pathogens due to organic acids production during the fermentation (Hounhouigan et al., Citation1994; Houngbédji, Johansen, et al., Citation2019). However, the rate of pH decrease was slower between 0 and 24 hours of fermentation with the 15 and 45 days storage starter cultures compared with the 3-day storage and the fresh cultures. Furthermore, except methionine, cysteine and valine, both mawè doughs, i.e. the one fermented with the fresh culture and the one fermented with freeze-dried culture showed similar-free amino acid profile. The results demonstrated that the developed freeze-dried culture had similar metabolic activities compared with the fresh culture.
To our knowledge, this study is one of the first investigating carrier material development using cereal flour and freeze-dried starter culture assessment with the mixture of yeast and LAB strains for safer and nutritious cereal fermentation in West Africa. The results obtained are valuable to upgrade cereal fermentation sector in West Africa. However, further experimentation may be needed to demonstrate the ability of the freeze-dried culture to inhibit the growth of pathogens during fermentation.
Acknowledgements
The authors acknowledged the International Foundation for Science (IFS) for funding all the experiments described in this study. The International Committee on Food Microbiology and Hygiene (ICFMH) is acknowledged for providing mobility grants (travel and allowance) to spend two months times at the Département Technologie Alimentaire/IRSAT/CNRST Ouagadougou, Burkina Faso, where amino acid analyses were performed.
Disclosure statement
Authors declare that they have no conflict of interest.
Additional information
Funding
Notes on contributors
Marcel Houngbédji
Marcel Houngbédji is research assistant at the Laboratoire de Sciences des Aliments, Faculté des Sciences Agronomiques, Université d’Abomey-Calavi, Cotonou, Benin (LSA/FSA, UAC, Benin). His research interest is food fermentation, antibiotic resistance, and upgrading traditional foods by improving traditional processing methods or developing new processing method for new product innovation. Clarisse Sidbewende Compaoré is a researcher at Département Technologie Alimentaire (DTA/IRSAT/CNRST), Ouagadougou, Burkina Faso, Yann Eméric Madodé is Associate Professor at LSA/FSA, UAC, Benin. Sègla Wilfrid Padonou is Associate Professor at the École des Sciences et Technologies de Conservation et de Transformation des Produits Agricoles, Université Nationale d’Agriculture, Porto-Novo, Bénin. Djidjoho Joseph Hounhouigan is full Professor at LSA/FSA, UAC, Benin.
References
- American Association of Cereal Chemists. (2000). AACC method 44–15A; Approved methods of the AACC (10th ed.).
- Bidlingmeyer, B. C. (1984). Rapid analysis of amino acids using Pre-column derivatization. Journal of Chromatography B: Biomedical Sciences and Applications, 336, 93–11. https://doi.org/10.1016/S0378-4347(00)85133-6
- Bolla, P. A., Serradell, M. A., Urraza, P. J., & Antoni, G. L. (2011). Effect of freeze-drying on viability and in vitro probiotic properties of a mixture of lactic acid bacteria and yeasts isolated from kefir. Journal of Dairy Research, 78(1), 15–22. https://doi.org/10.1017/S0022029910000610
- Brandt, M. J. (2014). Starter cultures for cereal based foods. Food Microbiology, 37, 41–43. https://doi.org/10.1016/j.fm.2013.06.007
- Higl, B., Kurtmann, L., Carlsen, C. U., Ratjen, J., Forst, P., Skibsted, L. H., Kulozik, U., & Risbo, J. (2007). Impact of water activity, temperature, and physical state on the storage stability of Lactobacillus paracasei ssp. paracasei freeze-dried in a lactose matrix. Biotechnology Progress, 23(4), 794–800. https://doi.org/10.1002/bp070089d
- Holzapfel, W. H. (1997). Use of starter cultures in fermentation on a household scale. Food Control, 8(5–6), 241–258. https://doi.org/10.1016/S0956-7135(97)00017-0
- Houngbédji, M., Johansen, P., Padonou, S. W., Akissoé, N., Arneborg, N., Nielsen, D. S., Hounhouigan, D. J., & Jespersen, L. (2018). Occurrence of lactic acid bacteria and yeasts at species and strain levels during spontaneous fermentation of mawè, a cereal dough produced in West Africa. Food Microbiology, 76, 267–278. https://doi.org/10.1016/j.fm.2018.06.005
- Houngbédji, M., Johansen, P., Padonou, S. W., Hounhouigan, D. J., Siegumfeldt, H., & Jespersen, L. (2019). Effects of intrinsic microbial stress factors on viability and physiological condition of yeasts isolated from spontaneously fermented cereal doughs. International Journal of Food Microbiology, 304, 75–88. https://doi.org/10.1016/j.ijfoodmicro.2019.05.018
- Houngbédji, M., Padonou, S. W., d’Auchamp, A. M., Akissoé, N., Mengu, M. D., Jespersen, L., & Hounhouigan, D. J. (2019). Improving food value chains for cereal doughs in West Africa: Case study of mawè in Benin. Food Chain, 8(1), 1–21. https://doi.org/10.3362/2046-1887.18-00013
- Hounhouigan, D. J, Nout, M. J. R., Nago, C. M., Houben, J. H., Rombouts, F. M. (1993). Composition and microbiological and physical attributes of mawè, a fermented maize dough from Benin. International Journal Food Science Technology, 28, 513–517.
- Hounhouigan, D. J., Nout, M. J. R., Nago, C. M., Houben, J. H., & Rombouts, F. M. (1994). Microbiological changes in mawè during natural fermentation. World Journal of Microbiology & Biotechnology, 10(4), 410–413. https://doi.org/10.1007/BF00144462
- Huang, S., Vignolles, M. L., Chen, X. D., Loir, Y. L., Jan, G., Schuck, P., & Jeantet, R. (2017). Spray drying of probiotics and other food-grade bacteria: A review. Trends in Food Science & Technology, 63, 1–17. https://doi.org/10.1016/j.tifs.2017.02.007
- Kavitake, D., Kandasamy, S., Devi, P. B., & Shetty, P. H. (2018). Recent developments on encapsulation of lactic acid bacteria as potential starter culture in fermented foods – A review. Food Bioscience, 21, 34–44. https://doi.org/10.1016/j.fbio.2017.11.003
- Kurtmann, L., Carlsen, C. U., Skibted, L. H., & Risbo, J. (2009). Water activity-temperature state diagrams of freeze-dried Lactobacillus acidophilus (La-5): Influence of physical state on bacterial survival during storage. Biotechnology Progress, 25(1), 265–270. https://doi.org/10.1002/btpr.96
- Mantzourani, I., Terpou, A., Alexopoulos, A., Bezirtzoglou, E., & Plessas, S. (2019). Assessment of ready-to-use freeze-dried immobilized biocatalysts as innovative starter cultures in sourdough bread making. Foods, 8(1), 40. https://doi.org/10.3390/foods8010040
- Marcial-Coba, M. S., Knøchel, S., & Nielsen, D. S. (2019). Low-moisture food matrices as probiotic carriers. FEMS Microbiology Letters, 366(2), 1–11.
- Nout, M. J. R. (2009). Rich nutrition from the poorest – Cereal fermentations in Africa and Asia. Food Microbiology, 26(7), 685–692. https://doi.org/10.1016/j.fm.2009.07.002
- Ogunremi, O. R., Banwo, K., & Sanni, A. I. (2017). Starter-culture to improve the quality of cereal-based fermented foods: Trends in selection and application. Current Opinion in Food Science, 13, 38–43. https://doi.org/10.1016/j.cofs.2017.02.003
- Vesterlund, S., Salminen, K., & Salminen, S. (2012). Water activity in dry foods containing live probiotic bacteria should be carefully considered: A case study with Lactobacillus rhamnosus GG in flaxseed. International Journal of Food Microbiology, 157(2), 319–321. https://doi.org/10.1016/j.ijfoodmicro.2012.05.016
- Vogel, R. F., Hammes, W. P., Habermeyer, M., Engel, K. K. D., & Eisenbrand, G. (2011). Microbial food cultures – Opinion of the senate commission on food safety (SKLM) of the German research foundation (DFG). Molecular Nutrition & Food Research, 55(4), 654–662. https://doi.org/10.1002/mnfr.201100010
- Waters, C. (1984). PICO•TAG amino acid analysis system operator’s manual. Waters Corporation.