Abstract
Tanneries that employ chrome tanning are indicted for discharging their effluents contaminated with health hazard chromium species necessitating monitoring their levels. The levels of chromium species (total chromium, hexavalent chromium, and trivalent chromium) in the discharged effluent of Bahir Dar tannery, Ethiopia, were determined using the inductively coupled plasma optical emission spectrometry, ultra-violet spectrometry and by difference, respectively. The level of total chromium in the discharged tannery effluent sample collected before and after treatment was 82.40 ± 0.20 ppm and 2.96 ± 0.06 ppm, respectively showing that the total chromium concentration in the tannery waste discharged directly to the Blue Nile river water system is above the national permitted limit (2 ppm). Moreover, the concentration of the carcinogenic chromium species (hexavalent chromium) detected in the tannery effluent sample collected before and after treatment was 0.18 ± 0.01 and 0.08 ± 0.002 ppm, respectively indicating that the discharged tannery effluent adds the carcinogenic chromium species to the Blue Nile River in a level above the recommended level by WHO guidelines. The result necessitates the assessment of the level of chromium species in the discharged effluent of all the tanneries in the country.
PUBLIC INTEREST STATEMENT
Trace metals are known to be carcinogenic and hence cause health problems. Chrome tanning that uses chromium in the form of Chromium(III) sulfate has long been regarded as the most efficient and effective tanning in most tanneries in the world including Ethiopia. Two health aspects of chrome tanning are as follows:
● Although the chromium in the form used (Cr(III)) is essential, it still induces health problems when present above the permissible level.
● There is the possibility of formation of the most carcinogenic form of chromium (Cr(VI)) in the course of tanning process that might be attributed to possible oxidation processes between the various chemicals used under acidic medium.
Although tanneries are obligated to discharge treated effluents, research reports revealed high load of trivalent, hexavalent, and hence total chromium. Therefore, it is crucial to develop a method to monitor the level of the different forms of chromium in the discharged effluent.
Competing Interests
The authors declare no competing interests.
1. Introduction
Effective management of water resources and control of pollution are becoming increasingly important for sustainable development and human welfare. Inorganic pollutants such as mercury, cadmium, lead, copper, chromium, and nickel discharged from industries get into human food chain leading to diseases such as skin allergies, dermatitis and ulcerations, cirrhosis of liver, and skin cancer (Satyajit & Sukalyan, Citation2012). Among these, chromium is a naturally occurring element present in water, sediments, rocks, soils, plants, biota, animals, and volcanic emissions under various chemical, physical, and morphological forms (Siraj et al., Citation2012).
Chromium exists in several valence states: 0–VI oxidation states. Only two of them, the Cr(III) and Cr(VI), are however stable enough to occur in the environment (Ducros, Citation1992; Shriver, Atkins, & Langford, Citation1994), which differ from one another in charge, physicochemical properties as well as chemical and biological activities. Hexavalent chromium in the form of water-soluble complex anions in surface water undergoes reduction to Cr(III) possessing a much shorter lifetime (Callahan, Slimak, Bagel, & Grevatt, Citation1979).
In a solution state, Cr(VI) may exist in three different ionic forms: hydro chromate (HCrO4−), chromate (CrO42−), and dichromate (Cr2O72−). Although the chromate ion predominates in both basic and neutral media, the hydrochromium predominates in solutions of lower pHs indicating the pH dependence of the proportion (Dhungana & Yadav, Citation2009; EPA, Citation1984).
Chromium in the form of Cr(III) is an important microelement for plants and animals for the maintenance of glucose and metabolism of lipids and proteins. It is an essential nutrient for human health with recommended level of 50–200 µg per day for adults (Zhang & Li, Citation1987). As deficiency of trivalent chromium may exacerbate diabetes symptoms and heart conditions (Barceloux, Citation1999), an amount extremely higher that the required is also reported to be toxic (Krejpcio, Citation2001). On the contrary, hexavalent chromium is known for its carcinogenicity and its extreme toxicity at a level above 0.57 mg/kg-day (Zhang & Li, Citation1987). It causes allergic and asthmatic reactions, is carcinogenic, and is 1,000 times as toxic as trivalent chromium and in general impairs health effects including diarrhea, stomach and intestinal bleedings, cramps, and liver and kidney damage (Barceloux, Citation1999). The toxicological impact of chromium (VI) originates from the action of itself as an oxidizing agent, formation of free radicals during the reduction of Cr(VI) to Cr(III) occurring inside the cell, and its ability to pass through cellular membranes many orders of magnitude faster than does the Cr(III) (Ricardo et al., Citation2009).
Hides and skins have the ability to absorb tanning acid and other chemical substances that prevent them from decaying, make them resistant to wetting, and keep them supple and durable (Abdel-Shafy, Hegemann, & Genschow, Citation1995). Tanning is essentially the reaction of collagen fibers in the hide with tannins, chromium, alum, or other chemical agents. The common tanning agents used in most leather industries are trivalent chromium in the form of basic chromium sulfate (BCS) and vegetable tannins extracted from specific tree barks. Alum, formaldehyde, glutar aldehyde, and other vegetable extracts are also some of the tanning agents (Abdel-Shafy et al., Citation1995).
Chrome-tanned leather tends to be softer and more flexible than vegetable-tanned leather, has higher thermal stability, is very stable in water, and takes less time to produce than vegetable-tanned leather making chrome tanning of choice (Akcay, Oguz, & Karapire, Citation2003).
The amount of chromium salt added for tanning being about 8% of the leather weight, only a small portion of the added chromium (60%–70%) is fixed to the leather and hence significant amount is discharged inducing environmental pollution (Esmaeili et al., Citation2005).
High levels of even the trivalent chromium in the discharged tannery effluents supplemented by the dynamic interconversion between Cr(III) and Cr(VI) in aqueous environments may lead to a health risk (Zhang, Citation2000). Because of the opposing (essentiality and carcinogenicity) effects of chromium compounds, speciation and determination of each species plays an important role in biochemical and toxicological investigations.
Ethiopia being the second in the World in livestock with a fast growing economy, the number of Industries in general and Tanneries in particular is growing drastically. The Ethiopian government is still promoting for further industrialization which on the other hand may pollute the environment by discharging untreated effluents. Hence, monitoring the level of each potential pollutant including the chromium species in the discharged industrial effluents (tannery effluents) is indispensable.
Spectroscopic techniques are the most reported techniques used for the speciation and determination of chromium in real samples like discharged tannery effluents (Abraha, Gebrekidan, & Afework, Citation2009; Addis, Citation2006; Balogh, Maga, Hargitai-Toth, & Andruch, Citation2000; Dayananda & Revanasiddappa, Citation2007; Fabiyi & Donnio, Citation2007; Li, Tai, & Gu, Citation2001; Revanasiddappa & Kiran Kumar, Citation2002, Citation2003; Telepcakova, Andruch, & Balogh, Citation2005; Zaitoun, Citation2005).
In this study, two spectroscopic techniques, the ICP-OES for the determination of total chromium and UV–Vis based on 1,5-diphenylcarbazide (DPC) as a complexing agent for the determination of hexavalent chromium in the discharged tannery effluent of Bahir Dar tannery, which to the best of our knowledge is not reported, were employed. The trivalent chromium concentration was estimated by simple difference between the ICP-OES result UV–Vis spectrometric result.
2. Experimental
2.1. Description of the study area
In this study, tannery effluent samples were collected from “Bahir Dar tannery” located in Bahir Dar city, Ethiopia. The tannery is located to the South-East of Bahir Dar city and West of Blue Nile (Abay) River. Two sampling sites, one just before the treatment plant and the other immediately after the treatment plant, were selected as sampling sites that enabled us to assess the level of chromium species discharged into the treatment plant and the Abay river.
Composite samples were collected daily for three consecutive days from each sampling site (before and after treatment) using 250 mL polyethylene plastic bottles. The collected samples were immediately acidified by adding 2 mL of concentrated HNO3 into each 250 mL sample.
2.2. Chemicals and apparatus
In this study, nitric acid (70%), perchloric acid (70%), hydrochloric acid (37%), sodium hydroxide, potassium mono hydrogen phosphate, potassium di-hydrogen phosphate, potassium dichromate, 1,5-DPC, and acetone all from Fluka were used. Distilled deionized water (DDW) was used throughout the study. All the reagents were of analytical grade that they were used without prior treatment.
UV–Visible double beam spectrophotometer (LAMBDA 35, PerkinElmer) and inductively plasma optical emission spectrometer (ULTIMA 2, HORIBA scientific) were used for the determination of the hexavalent and total chromium levels in the discharged tannery effluents collected from the selected sampling sites, respectively. A refrigerator (FR 1082) and digital balance (PW 124) with a precision ±0.0001were also used to preserve the samples and weigh the mass of chemicals, respectively.
2.3. Analysis of the samples
2.3.1. Sample pre-treatment
The collected samples were digested with a 1:1 volume ratio mixture of HNO3 (70%) and HClO4 (70%) acid following a modified procedure (Awotoye, Adewole, Salami, & Ohiembor, Citation2009). Briefly, after homogenizing the three samples collected at each sampling site in a 1 L polyethylene plastic bottle, 50 mL portion of the composite sample was placed in a 250 mL beaker. After adding 10 mL mixture of HNO3 and HClO4, the beaker was covered with watch glass and then the sample was digested using a heating mantle for 1 h at 120°C. The sample was then cooled, diluted to 100 mL with deionized water, and preserved in a refrigerator for the total chromium determination using the ICP-OES.
Similarly, a procedure reported elsewhere (Tsunenobu, Shiro, Hideo, & Yasuharu, Citation1977) was used for the determination of hexavalent chromium. Briefly, 25 mL homogenized discharged effluent sample collected from each sampling site was first filtered using a filter paper. To the filtrated aliquot in a 100 mL volumetric flask, 1 mL of DPC reagent was added and aged for about 10 min until pink color is developed. The complex was then diluted to the 100 mL mark using pH 2 phosphate buffer solution (PBS). The resulting solutions from the two sampling sites were preserved in a refrigerator until analyzed by UV–Vis spectroscopy.
2.4. Procedure
2.4.1. Preparation of buffer solution
PBS of pH 2 was prepared by mixing appropriate volumes of equi-molar (1 M) potassium di-hydrogen phosphate and potassium mono hydrogen phosphate in DDW. The pH was adjusted with 0.2 M HCl and 0.2 M NaOH.
2.4.2. Preparation of stock and working chromium solutions for ICP-OES analysis
A 1,000 ppm standard stock solution of hexavalent chromium was prepared by dissolving 711.5 mg potassium dichromate in 250 mL of DDW. Working standard solutions of 0.00, 0.2, 0.8, 3.2, and 12.8 ppm Cr(VI) solutions were prepared from an intermediate stock solution (100 ppm) through serial dilution and were analyzed using ICP-OES under optimized operating conditions shown in Table .
Table 1. The operating conditions of ICP-OES
2.4.3. Preparation of working Cr(VI) and DPC solutions for UV–Vis analysis
A series of four working standard solutions containing 0.01, 0.05, 0.25, and 1.25 ppm of hexavalent chromium in pH 2 PBS including a blank solution were prepared from the stock solution. Furthermore, a 0.25 g of DPC, which is a common complexing agent for chromium (VI), was dissolved in 5 mL acetone and shacked until complete dissolution (Hua, Chan, Wu, & Wu, Citation2009). The DPC in acetone was further diluted to 100 mL with DDW. A freshly prepared 1 mL DPC solution was then added to each working standard and a pink color was immediately developed except the blank solution. The blank and standard solutions were then analyzed using the UV–Visible spectrophotometer at a wave length of 540 nm.
3. Results and discussion
3.1. Determination of total chromium using ICP-OES
ICP-OES was used for the determination of the total chromium concentration at the two selected sites of Bahir Dar tannery. Figure presents the ICP-OES calibration curve for standard chromium concentrations. As can be observed from the figure, a linear dependence of the ICP-OES emission intensity (I) on the total concentration of chromium (CrT) was observed in the concentration range 0.2–12.8 ppm with a linear regression equation, correlation coefficient, and method limit of detection (MLD = 3*s where s = standard deviation of the blank) of I = 898 + 33,523CrT (ppm), 0.99947, and 6.35*10−5 ppm, respectively.
Figure 1. Plot of ICP-OES emission intensity versus total chromium concentration (CT = 0.0, 0.2, 0.8, 3.2, 6.4, and 12.8 ppm).
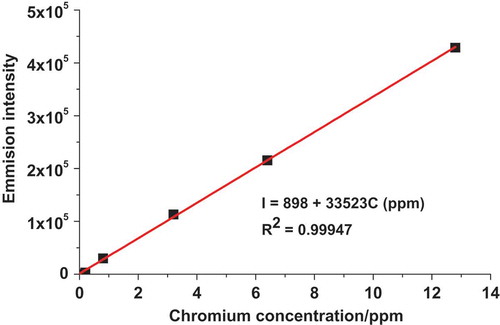
The emission intensity of total chromium in the real samples collected from the two sampling sites was measured using the ICP-OES under the same conditions as for the calibration curve. Table presents the summary of the total chromium concentration detected in the discharged tannery effluents collected from the two sampling sites of Bahir Dar tannery.
Table 2. Summary of the average total chromium concentration (CrT) in samples collected from two sites around Bahir Dar tannery using the ICP-OES
As can be seen from the table, the total chromium concentration detected in the discharged tannery effluent before and after the treatment plant was found to be 82.40 and 2.96 ppm, respectively. A total chromium concentration of 2.96 ppm in a discharged tannery effluent after treatment plant indicated that the chromium load is above the national permissible level (2 ppm). This also indicated that the treatment method employed in the studied tannery is not efficient enough to keep the total concentration level in the discharged effluent under the national and of course international permissible levels.
3.2. Determination of hexavalent chromium in discharged tannery effluent using UV–Vis spectrophotometer
In this study, the chromium (VI) concentration at different sites of Bahir Dar tannery was determined by UV–Vis spectrophotometry. A linear dependence of absorbance (A) on the concentration of Cr(VI) was obtained in the concentration range 0.01–1.25 ppm with a linear regression equation, correlation coefficient, and method limit of detection of A = 0.01 + 0.64 C(VI) (ppm), 0.99888, and 9.4*10−6 ppm, respectively.
Figure presents the plot of UV–Vis spectrophotometric absorbance as a function of standard hexavalent chromium concentration in the range 0.00–1.25 ppm.
Figure 2. Plot of UV–Vis absorbance versus chromium (VI) concentration (0.00, 0.01, 0.05, 0.25, 0.75, and 1.25 ppm).
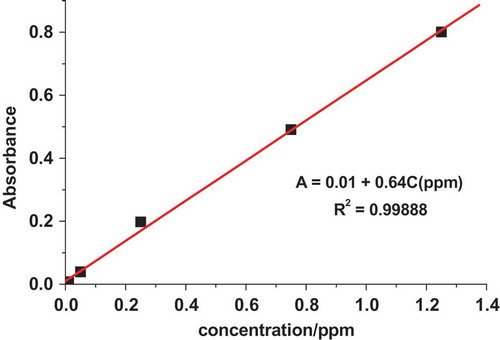
The absorbance of Cr(VI) in the real samples collected from the two sampling sites was measured using the UV–Vis spectrophotometer under the same conditions as for the analyses of the standard solutions. Table presents summary of the Cr(VI) concentration in the samples collected from the two sampling sites. The level of Cr(VI) in the discharged waste water of Bahir Dar tannery was found to be 0.18 ppm before treatment and 0.08 ppm after treatment.
Table 3. Summary of the average Cr(VI) concentration in samples collected from the two sampling sites in Bahir Dar tannery
Although the hexavalent chromium concentration in the sample collected right after the treatment plant is under the national permissible level, it is still above the permissible level by the WHO guideline (WHO, Citation1996). Presence of hexavalent chromium in the discharged effluent at an amount higher than the allowed limit may lead to Nile River pollution and hence pollution of the people using the river water for different purposes.
3.3. Determination of trivalent chromium in the discharged tannery effluent
As the total chromium concentration (CrT) in the discharged tannery effluent determined using the ICP-OES represents that the sum of the hexavalent and trivalent species and the hexavalent chromium is determined using the UV–Vis spectrometry, it is reasonable to take the difference as a good estimate of the trivalent chromium concentration ((Cr(III) = CrT—Cr(VI)). A cumulative error may lead to sort of variation that could only be alleviated by the simultaneous determination of both chromium species. The level of Cr(III) calculated at the two sampling sites by difference method is summarized in Table .
Table 4. Summary of the average Cr(III) concentration in the discharged effluent of Bahir Dar tannery collected from the two sites
Table 5. The maximum allowed concentration of chromium species that could be discharged to surface water after treatment according to different countries and WHO guidelines
As can be seen from Table , the level of trivalent chromium at each sampling site is nearly the level of total chromium at the respective site. As the mechanism by which trivalent chromium is converted to the carcinogenic form (hexavalent chromium) is not fully investigated, high level of trivalent chromium in the discharged industrial effluent that certainly contain various chemicals may lead to an ultimately increased concentration of the hexavalent chromium in the water system around the factory.
3.4. Comparison of the chromium levels in the present study with their values in different guidelines
It is important to compare the results obtained from the analysis of chromium in Bahir Dar tannery waste water with the values sited in other countries. This comparison helps one to realize the level of each chromium species in the target tannery and evaluate the divergence from selected guidelines.
4. Conclusion and recommendations
The total chromium and hexavalent chromium content in a discharged effluent of Bahir Dar tannery collected at two sampling sites (before and after treatment) were determined using ICP-OES and UV–Vis spectrophotometry, respectively. Furthermore, the level of trivalent chromium was estimated by the difference of the two methods.
The levels of the total chromium, hexavalent chromium, and trivalent chromium species in the discharged tannery effluent collected before the treatment sampling site were 82.40, 0.18, and 82.22 ppm, respectively indicating the significant load of chromium species necessitating an efficient treatment plant. Furthermore, the study showed that the discharged tannery effluent sampled right after the treatment plant contained total, hexavalent, and trivalent chromium species in the level of 2.96, 0.08, and 2.88 ppm, respectively. The total chromium concentration in the tannery effluent discharged to the water system around the factory is above the permitted level of many countries including Ethiopia while the hexavalent chromium is above the WHO guideline (0.05–0.06 ppm). Higher levels of chromium species in the discharged effluent than the respective permitted limits are indications of poor efficiency of the treatment plant.
If the present mode of disposal of the tannery effluent is continued, it is likely that the surface and ground water of an extensive area around the factory, including the Nile River, would become highly saline and contaminated with toxic metals like the hexavalent chromium. This in turn may induce health problems to the society consuming vegetables grown around the factory. Thus, the factory management must seek for an alternative tanning materials or improve the efficiency of their treatment plant before health problems associated with the chromium they discharged are pronounced.
Acknowledgments
The authors greatly acknowledge the Chemistry Department of Bahir Dar University for providing us the necessary chemicals. School of Chemical and Food Engineering of Bahir Dar University is also acknowledged for accessing the ICP-OES and UV–Vis instruments. Finally, our appreciation goes to the Bahir Dar tannery for permitting us to collect effluent samples.
Additional information
Funding
Notes on contributors
Meareg Amare
I am Meareg Amare (Dr) (the corresponding author), working as analytical chemistry professor at Bahir Dar University, Department of Chemistry. Our group is mainly engaged in both basic and applied research all on environmental issues.
Although most of our reported works are on electrochemical method development using modified electrodes and their application for monitoring the environment, we still have works on assessing the level of selected heavy metals (carcinogenic) in discharged industrial effluent in general and tanneries in particular. In this regard, we are working in collaboration of national and regional environmental protection offices.
References
- Abdel-Shafy, H. I., Hegemann, W., & Genschow, E. (1995). Fate of heavy metals in the leather tanning industrial wastewater using anaerobic process. Journal of Environmental Management and Health, 6(2), 28–33.
- Abraha, G., Gebrekidan, G., & Afework, M. (2009). Environmental impacts of Sheba Tannery (Ethiopia) effluents on the surrounding water bodies. Bulletin of Chemical Society of Ethiopia, 23(2), 269–274.
- Addis, M. (2006). Determination of chromium (III) and chromium (VI) in the tannery effluents of Awash and Addis Ababa leather industries (M.Sc. Thesis). Addis Ababa University, Ethiopia.
- Akcay, H., Oguz, A., & Karapire, C. (2003). Study of heavy metal pollution and speciation in Buyak Menderes and Gediz river sediments. Water Research, 37(4), 813–822.
- Awotoye, O. O., Adewole, M. B., Salami, A. O., & Ohiembor, M. O. (2009). Arbuscular mycorrhiza contribution to the growth performance and heavy metal uptake of Helianthus annuus LINN in pot culture. African Journal of Environmental Science and Technology, 3(6), 157–163.
- Balogh, I. S., Maga, I. M., Hargitai-Toth, A., & Andruch, V. (2000). Spectrophotometric study of the complexation and extraction of chromium (VI) with cyanine dyes. Talanta, 53(3), 543–549.
- Barceloux, D. G. (1999). Chromium. Journal of Toxicology: Clinical Toxicology, 37, 173–194.
- Callahan, M. A., Slimak, M. W., Bagel, N., & Grevatt, P. C. (1979). Water-related environmental fate of 129 priority pollutants, vol. II. U.S. EPA, office of water planning and standards, office of water and waste management. Washington, DC. EPA/440/4-79-029.
- Dayananda, B. P., & Revanasiddappa, H. D. (2007). Spectrophotometric determination of chromium by oxidation of prochlorperazine dimaleate. Oxidation Communications, 30(1), 197–205.
- Dhungana, T. P., & Yadav, P. N. (2009). Determination of chromium in tannery effluent and study of adsorption of Cr(VI) on sawdust and charcoal from sugarcane bagasses. Journal of Nepal Chemical Society, 23, 93–101.
- Ducros, V. (1992). Chromium metabolism. A literature review. Biological Trace Element Research, 32, 65–77.
- EEPA. (2003). Standards for industrial pollution control in Ethiopia, Part Three: Standards for Industrial effluents. ESIS project - US/ETH/99/068/ETHIOPIA, EPA/UNIDO, Addis Ababa, 46p.
- EPA. (1984). Health assessment document for chromium. Environmental criteria and assessment office, research triangle park, NC. p1. EPA/600/8-83-014F. NTIS PB 85-115905 Available from National Technical Information Service: Springfield, VA.
- Esmaeili, A., Nia, M., & Vazirineja, R. (2005). Chromium (III) removal and recovery from tannery wastewater by precipitation process. American Journal of Applied Sciences, 2(10), 1471–1473. doi:10.3844/ajassp.2005.1471.1473
- Fabiyi, F. A. S., & Donnio, A. Z. (2007). Use of variamine blue as a chromogenic reagent for rapid spectrophotometric determination of nano amount of chromium. Synthesis and Reactivity in Inorganic, Metal-Organic, and Nano-Metal Chemistry, 37(10), 809–812.
- Hossain, M. A., Kumita, M., Michigami, Y., Islam, T. S., & Mori, S. (2005). Rapid speciation analysis of Cr(VI) and Cr(III) by reversed-phase high-performance liquid chromatography with UV detection. Journal of Chromatographic Science, 43(2), 98–103.
- Hua, L., Chan, Y. C., Wu, Y. P., & Wu, B. Y. (2009). The determination of hexavalent chromium (Cr6+) in electronic and electrical components and products to comply with RoHS regulations. Journal of Hazardous Materials, 163, 1360–1368. doi:10.1016/j.jhazmat.2008.07.150
- Jakov, B. (2005). Costs of tannery waste treatment. 15th Session of the UNIDO Leather and Leather Products Industry Panel, Leon, Mexico.
- Krejpcio, Z. (2001). Essentiality of chromium for human nutrition and health. Polish Journal of Environmental Studies, 10(6), 399–404.
- Li, W., Tai, C., & Gu, X. (2001). Spectrophotometric determination of chromium(VI) with Cr(VI)-o-Cl-PF–TDPC ternary complex after preconcentration on an organic solvent-soluble membrane filter. International Journal of Environmental Analytical Chemistry, 81, 127–135. doi:10.1080/03067310108044350
- Quevauviller, P., Marrier, E. A., & Griepink, B. (1995). Quality assurance for environmental analysis. Techniques and Instrumentation in Analytical Chemistry, 17, 1–25.
- Revanasiddappa, H. D., & Kiran Kumar, T. N. (2002). Rapid spectrophotometric determination of chromium with trifluoperazine hydrochloride. Chemical Analysis (Warsaw), 47, 311–313.
- Revanasiddappa, H. D., & Kiran Kumar, T. N. (2003). Highly sensitive spectrophotometric determination of chromium using leuco xylene cyanol FF. Talanta, 60, 1–8. doi:10.1016/S0039-9140(02)00567-2
- Ricardo, S., Manuel, C. C., Maria Ines, C. M., Sergio de Souza, H. J., Fernanda, V. M. P., Lılian, I. D. S., … Ricardo, E. S. (2009). Simultaneous speciation of chromium by spectrophotometry and multicomponent analysis. Chemical Speciation and Bioavailability, 21(3), 153–160. doi:10.3184/095422909X466095
- Satyajit, R., & Sukalyan, D. (2012) Assessment of water quality around mine sites. (Thesis). Department of mining engineering, National institute of Technology, Rourkela, India.
- Shriver, D. F., Atkins, P. W., & Langford, C. H. (1994). Inorganic chemistry (2nd ed.). Oxford: Oxford University Press.
- Siraj, S., Islam, M., Das, P. C., Masum, S., Jahan, I. A., Ahsan, A., & Shajahan, M. D. (2012). Removal of chromium from tannery effluent using chitosan-charcoal composite. Journal of the Bangladish Chemical Society, 25(1), 53–61.
- Telepcakova, M., Andruch, V., & Balogh, I. S. (2005). Indirect extraction-spectrophotometric determination of chromium. Chemical Papers, 59(2), 109–112.
- Tsunenobu, S., Shiro, G., Hideo, Y., & Yasuharu, N. (1977). Spectrophotometric determination of chromium (III) and chromium (VI) in sea water. Bulletin of the Institute of Chemical Research, Kyoto University, 55(5), 429–440.
- WHO. (1996). Chromium in drinking-water. Geneva: Author.
- Zaitoun, M. A. (2005). Spectrophotometric determination of chromium (VI) using cyclam as a reagent. International Journal of Environmental Chemistry, 85, 399–407. doi:10.1080/03067310500075913
- Zhang, H. (2000). Light and Iron(III)-induced oxidation of chromium(III) in the presence of organic acids and manganese(II) in simulated atmospheric water. Atmospheric Environment, 34(10), 1633–1640. doi:10.1016/S1352-2310(99)00384-2
- Zhang, J., & Li, X. (1987). Chromium pollution of soil and water in Jinzhou. Journal of Chines Preventive Medicine, 21, 262–264.
