?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In this work, we report antibacterial activity of 3,6-di(pyridin-2-yl)-1,2,4,5-tetrazine (pytz) capped Pd(0) nanoparticles (TzPdNPs). The TzPdNPs were characterized by transmission electron microscopy (TEM) and powder X-ray diffraction techniques (PXRD). The antibacterial properties of TzPdNPs were studied using Gram-positive B. subtilis and Gram-negative E. coli by the plate count method. The antimicrobial activity of this TzPdNPs shows that the microbial growth has been fully inhibited only for Gram-positive B. subtilis bacteria. Morphological changes obtained by transmission electron microscope observation show that TzPdNPs can cause leakage and chaos of intracellular contents.
Public Interest Statement
Infectious diseases remain among the top five causes of mortality in countries of all socioeconomic classes worldwide, resulting in 9.2 million deaths in 2013 (about 17% of all deaths). At any given time, an estimated 1.4 million people are affected by health care-associated infections (HAIs) in developed countries, around 5–10% of patients in hospitals get HAIs, but the risk is 200–2,000% higher in undeveloped countries. Therefore, antibacterial agents play a significant role in combating microbial infections. However, due to the wide use of antibiotics and the emergence of antibiotic-resistant bacterial strains, conventional antibiotics are becoming less effective, resulting in poor treatment efficacy and significantly increased cost of health care. Each year, in the United States alone, more than 2 million people with antibiotic-resistant infections are reported, leading to at least 23,000 deaths. Therefore, there is continuous demand to discover new antimicrobial agents to control microbial infections.
Competing interests
The authors declare no competing interests.
1. Introduction
As a result of increasing microbial resistance to multiple antimicrobial agents and development of resistant strains, there is an increasing demand for novel antimicrobial materials with superior performance for disinfection applications. In this regard, nanoparticles are particularly effective, due to a high surface-to-volume ratio, which provides a large active surface in the contact with micro-organisms. It has been demonstrated that nanoparticles (NPs) have a wide variety of unique applications, including cell targeting (Manolova et al., Citation2008; Weissleder, Kelly, Sun, Shtatland, & Josephson, Citation2005), intravenous nucleic acid delivery (Dobson, Citation2006; Li & Szoka, Citation2007; Neuberger, Schöpf, Hofmann, Hofmann, & von Rechenberg, Citation2005), environmental remediation (Kamat & Meisel, Citation2003; Tratnyek & Johnson, Citation2006; Zhang et al., Citation2010), and catalysis (Witham et al., Citation2010). The antimicrobial function of metal ions and NPs has also long been recognized and exploited industrially for several purposes, including amendments to textiles and cosmetics, food processing, water treatment, and so on (Ilić et al., Citation2009; Mueller & Nowack, Citation2008). Commonly used antimicrobial nanocomposite materials include metal ions or NPs (silver (Tamboli et al., Citation2012; Vukoje et al., Citation2014), copper (Cady et al., Citation2011; Cioffi et al., Citation2005; Liang et al., Citation2012), gold (Boomi & Prabu, Citation2013; Mei et al., Citation2014), platinum (Ma et al., Citation2012), metal oxide (titanium dioxide (Zhang & Chen, Citation2009), zinc oxide (Liang et al., Citation2012), copper oxide (Ojas, Bhagat, Gopalakrishnan, & Arunachalam, Citation2008)), and organically modified nanoclay (Rhim, Hong, Park, & Ng, Citation2006), to name a few.
So far, the antimicrobial property of silver, copper, gold, and platinum NPs has been investigated thoroughly; however, potential of palladium nanoparticles (PdNPs) as antimicrobial agent remains a relatively undeveloped area. Pd is one of the most widely used transition metals for carbon–carbon and carbon–heteroatom cross-coupling reactions such as the Suzuki–Miyaura reaction (Citation1995), Heck reaction (Heck, Citation1968a, Citation1968b, Citation1968c, Citation1968d; Heck & Nolley, Citation1972), Kumada reaction (Jana, Pathak, & Sigman, Citation2011), Sonogashira reaction (Sonogashira, Citation2002), Negishi reaction (Astruc, Citation2007), Stille reaction (Milstein & Stille, Citation1978), and Buchwald–Hartwig reaction (Widenhoefer & Buchwald, Citation1996). Pd has also been used as a catalyst to manufacture pharmaceuticals (Malleron, Fiaud, & Legros, Citation2000), degrade harmful environmental pollutants (Nutt, Hughes, & Wong, Citation2005), and as sensors for the detection of various analytes (Chang et al., Citation2008; Favier, Walter, Zach, Benter, & Penner, Citation2001; Yu et al., Citation2005). Additionally, Pd and Pd2+ ions also play fundamental roles in several biotechnological processes (Baccar, Adams, Abdelghani, & Obare, Citation2013). Baccar et al. developed a non-enzymatic biosensor using various sizes of PdNPs to detect hydrogen peroxide in milk (Citation2013). The anti-tumor, -viral, -malarial, -fungal and -bacterial activities of various Pd(II) complexes of N, O, S donor ligands, and drugs are long known (Baccar et al., Citation2013; Garoufis, Hadjikakou, & Hadjikakou, Citation2009). Size-dependent antimicrobial effects of PdNPs have also been reported recently (Adams, Walker, Obare, Docherty, & van Raaij, Citation2014; Garoufis, Hadjikakou, & Hadjiliadis, Citation2005).
In the present study, we report the activity of 3,6-di-(pyridin-2-yl)-1,2,4,5-tetrazine (pytz) capped PdNPs (TzPdNPs) against bacterial growth. To investigate the antibacterial activity of TzPdNPs, the viability of Gram-positive Bacillus subtilis was investigated in the presence of TzPdNPs.
2. Experimental
2.1. Materials and methods
The synthesis of 3,6-di(pyridin-2-yl)-1,2,4,5-tetrazine (pytz) capped Pd(0) nanoparticles (TzPdNPs) was carried out according to the method reported earlier (Das, Samanta, Ray, & Biswas, Citation2015). The antimicrobial activities were investigated against bacterial strains Gram-positive Bacillus subtilis (MTCC 441) and Gram-negative Escherichia coli (MTCC 2939).
2.2. Instrumentation
Powder X-ray diffraction (PXRD) patterns were obtained on a Philips PW 1140 parallel beam X-ray diffractometer with Bragg–Bretano focusing geometry and monochromatic CuKα radiation (λ = 1.540598 Å). Transmission electron micrographs (TEM) images were collected using JEOL JEM-2100 microscope working at 200 kV. TEM samples were prepared by sonicating an aliquot of the sample in ethanol for 15 min and a single drop of this suspension was drop-casted onto carbon-coated 300 mesh copper grids. Grids were allowed to air-dry prior to imaging.
2.3. Antimicrobial test for TzPdNPs
The antibacterial activities of TzPdNPs were measured by serial dilution method. The 50 mg of TzPdNPs was suspended in 6 mL of Muller-Hinton broth and inoculated with 1 mL of 18-h stock culture of Bacillus subtilis. Aliquots of each sample were diluted and 200 μL were plated on sterile Petri dishes, covered with Muller-Hinton agar. The plates were incubated for 20 h at 37°C. After the incubation period, the colony-forming units (CFU) of each plate were determined. Antimicrobial activity of pytz was also tested by the same method to compare its activity with TzPdNPs.
2.4. Minimum inhibitory concentration (MIC)
Bacillus subtilis was cultured in Muller-Hinton broth at 37°C on a shaker incubator at 200 rpm for 6 h. Then the concentration of micro-organism of 1 × 108 CFU mL−1 dilution was fixed at 0.1, optical density at 600 nm with addition of medium and then again diluted to 1 × 106 CFU mL−1 with medium. We mixed bacterial suspension (1 × 106 CFU mL−1, 100 μL) with the solutions of TzPdNPs with different concentrations in medium and the final volume of the solutions was adjusted to 10 mL with medium. The solutions were shaken at 37°C on a shaker incubator at 200 rpm for 24 h. The bacterial viability was determined by measuring optical density at 600 nm (OD600 nm). Simultaneously, aliquots of each solution were diluted and plated on Muller-Hinton agar. The plates were incubated at 37°C for 20 h and colonies were counted and compared to those on control plates to calculate changes in the cell growth inhibition. The percentage of cell growth reduction (R, %) was calculated using the following equation:(1)
(1)
where C0 is the number of CFU from the control sample and C is the number of CFU from treated samples. Each concentration was prepared and measured in triplicate, and all experiments were repeated at least thrice in parallel.
2.5. Antibacterial growth kinetics
Bacterial suspension (~1 × 107 CFU mL−1) was inoculated with different concentrations (63−250 μg mL−1) of TzPdNPs in Muller-Hinton broth. The mixtures were shaken at 37°C on a shaker incubator at 200 rpm for 0–36 h. The initial time of addition of the bacteria was taken as zero. The growth curves resulted from bacteria only, and TzPdNPs incubated with bacteria were plotted against time. Aliquots were drawn from each of the mixtures at definite time intervals and diluted further. Afterward, 200 μL of the final solutions were plated on Muller-Hinton agar plates immediately. The plates were incubated at 37°C for 24 h, and bacterial colonies were counted. A plot of colony-forming unit (CFU) in logarithmic scale versus time (log10 CFU mL−1 vs time) was then plotted to determine the bactericidal kinetics.
2.6. Characterization of treated bacteria by TEM
The size and morphology of TzPdNPs treated and untreated bacteria were examined by TEM. Prior to microscopy analysis, bacterial samples were prepared using procedures described previously in literature (Hao, Jayawardana, Chen, & Yan, Citation2015). Bacillus subtilis was inoculated for 10 h in Muller-Hinton broth at 37°C and at 200 rpm until an OD600 of 0.5 was attained. The bacteria cell suspension (30 mL) was then harvested, centrifuged, and redispersed in pH 7.4 PBS buffer. TzPdNPs (300 μg mL−1) were added to bacteria and the mixture was incubated at 37°C for 10 h while shaking at 200 rpm. The mixture was then centrifuged at 2,000 rcf for 8 min, and the supernatant was discarded. A drop of bacteria cell suspension was placed onto a Cu grid followed by overnight drying.
3. Results and discussion
3.1. Synthesis and characterization of the TzPdNPs
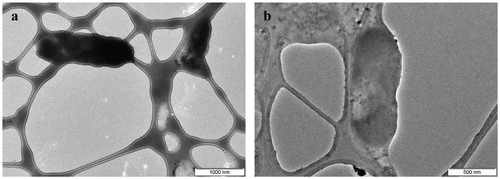
Synthesis of 3,6-di(pyridin-2-yl)-1,2,4,5-s-tetrazine (pytz) capped Pd(0) nanoparticles (TzPdNPs) was achieved by reacting aqueous solution of Na2PdCl4 with 3,6-di(pyridin-2-yl)-1,4-dihydro-1,2,4,5-tetrazine (H2pytz) in ethanol under sonication. The crystallinity and purity of TzPdNPs were examined (Figure (a)) by the PXRD technique. The XRD pattern of TzPdNPs shows the inter planar d-spacing of XRD peaks correspond to (111), (200), (220), and (311) planes of Pd(0) with fcc structure (JCPDS No. 01–1310), without any impurity phase. The morphology and size distribution of the prepared nanoparticles were elucidated from the TEM. Figure (b) exhibits morphological images of the prepared palladium nanoparticles. The shape of the particles is quasi-spherical and the size of the particles varies from 1.5 to 3.5 nm and the average size observed was 2.5 ± 0.2 nm (Figure (c)). It can also be noted that, despite the small size, the particles are separated from one another and no significant aggregation is observed.
3.2. Antibacterial activity
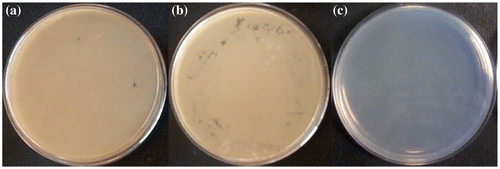
The antibacterial activities of these TzPdNPs nanoparticles have been evaluated toward Gram-positive B. Subtilis and Gram-negative E. coli. TzPdNPs (50 mg) were dispersed in 6 mL of Mueller-Hinton Broth and inoculated with 1 mL of freshly cultured bacteria. After an incubation time of 18 h, sample was diluted and plated on Mueller-Hinton agar plates. The plates were incubated for 20 h. Colonies were counted and recorded for dilutions containing between 30 and 300 colonies. The Mueller-Hinton agar plates with the B. subtilis exhibited no growth of bacterial colonies for 109–107 CFU mL−1 concentrations of bacteria, indicating excellent antibacterial activity against Gram-positive bacteria (Figure ). Antibacterial activities of TzPdNPs were then compared with pytz and a control solution was prepared with the bacteria but without any nanoparticles. Solutions of bacteria with TzPdNPs, pytz, and without any nanoparticles were diluted to105 CFU mL−1 after 18 h incubation and plated on Mueller-Hinton agar plates. As shown in Figure , after 20 h of incubation, dense bacterial colonies were observed on the control and pytz Mueller-Hinton agar B. subtilis (Figures (a) and (b)). However, the Mueller-Hinton agar plates with the TzPdNPs exhibited no growth of bacterial colonies, indicating again excellent antibacterial activity of TzPdNPs against B. subtilis (Figure (c)).
Figure 2. Antibacterial activities of TzPdNPs with (a) 109 (b) 108 (c) 107 CFU mL−1 Bacillus subtilis.
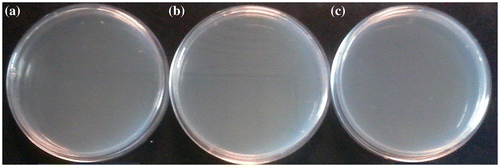
Figure 3. Photographs of colonies of B. subtilis (a) control, (b) after treatment with pytz and (c) treated with TzPdNPs.
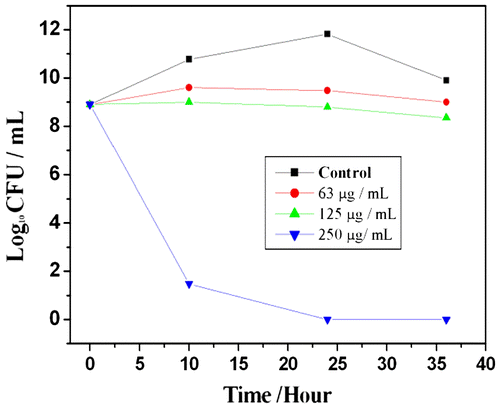
MIC values of TzPdNPs were evaluated by growth inhibition of B. subtilis by varying the concentration of TzPdNPs and measuring their OD600 nm after 24 h. The original bacterial concentration was 1 × 106 CFU mL−1. Aliquots of each sample were diluted and plated on Mueller-Hinton agar, incubated for 24 h, and colonies were counted. The MIC of TzPdNPs to inhibit proliferation of B. subtilis was determined to be 62.5 μg mL−1. Percentage of the cell growth reduction was calculated to be 95% for B. subtilis after 24 h of incubation. The effect of TzPdNPs nanoparticles on the growth kinetics of B. subtilis in liquid media was studied (Figure ) further. Freshly cultured bacteria were added with various concentrations of TzPdNPs solution; the optical density at 600 nm (OD600 nm) based on the turbidity of the cell suspension was measured to monitor bacterial growth. Each solution was also diluted, plated on Mueller-Hinton agar, incubated for 24 h, and bacterial colonies were counted. The growth of both the bacteria was inhibited as the concentration of TzPdNPs increased, but the rates of bacterial growth inhibition were different. The growth of B. subtilis started to decrease when the concentration of TzPdNPs was 62.5 μg mL−1 and at a concentration of 250 μg mL−1, the growth can be completely inhibited (Figure ). It can also be observed that no colony was formed for bacteria in the plate until 36 h.
Next, we explored antibacterial mechanisms of TzPdNPs. The exact mechanisms of NPs toxicity against bacteria are not understood completely. Generally, NPs are able to bind to the membrane of bacteria by electrostatic interaction and disrupt the integrity of the bacterial membrane (Thill et al., Citation2006). Nanotoxicity is generally triggered by the induction of oxidative stress by formation of reactive oxygen species (ROS), following the administration of NPs (Nel et al., Citation2009; Soenen, Rivera-Gil, Montenegro, Parak, & De Smedt, Citation2011). On the other hand, rather different mechanism has been proposed particularly for silver nanoparticles. Previous studies suggest that silver nanoparticles (AgNPs) release Ag+ ions which interact strongly with thiol groups of intracellular enzymes and proteins, leading to degeneration (Agnihotri, Mukherji, & Mukherji, Citation2013; Kim, Lee, Cha, Kim, & Kang, Citation2007; Xiu, Zhang, Puppala, Colvin, & Alvarez, Citation2012). The activities of enzymes and proteins are closely related to physiological functions of bacteria (Eckhardt et al., Citation2013; Xiu et al., Citation2012). Therefore, it may be suggested that Ag+ could affect the physiological functions of intracellular enzymes and proteins, leading to inhibition of activity and cellular damage.
To clarify the antibacterial mechanism of the action of TzPdNPs, we investigated ultrastructural damage of B. subtilis before and after incubation with TzPdNPs, examining TEM images. Untreated bacteria exhibited a smooth surface and distinct nucleoid structures (Figure (a)). In contrast, the bacterial cell surface became rough after incubation with TzPdNPs for 12 h. Moreover, numerous blebs were formed with cytoplasmic release (Figure (b)). The penetration of positively charged nanoparticles into negatively charged bacterial cytomembranes via electrostatic interactions accelerates cell division, leading to the formation of blebs. Literature reports have shown that electrostatic attraction between negatively charged bacterial cells and positively charged nanoparticles is crucial for the activity of nanoparticles as bactericidal materials (Hamouda & Baker, Citation2000; Stoimenov, Klinger, Marchin, & Klabunde, Citation2002). In addition, pytz molecules on nanoparticle probably provide a lipophilic segment compatible with the lipid bilayer of the bacterial cytoplasmic membrane (Beney & Gervais, Citation2001) and can cause decreased membrane fluidity and disrupt the cytoplasmic membrane (Silvestro, Weiser, & Axelsen, Citation2000). These two factors act together to damage the bacterial cytomembrane, resulting in leakage of intracellular content. It is also possible that the nanoparticles interact with the intracellular enzymes and proteins to inhibit their activity. Therefore, positively charged nanoparticles increase the permeability of the cytoplasmic bacterial membrane due to the presence of external pytz. Subsequently, these nanoparticles penetrate the cells and strongly associate with intracellular enzymes and proteins, resulting in cell death.
4. Conclusions
Synthesis of 3,6-di(pyridin-2-yl)-1,2,4,5-tetrazine (pytz) capped PdNPs was achieved by a simple method. Synthesized TzPdNPs have been characterized by powder X-ray diffraction and transmission electron microscope techniques. A systematic evaluation of the antibacterial activity of the 3,6-di(pyridin-2-yl)-1,2,4,5-tetrazine (pytz) capped Pd(0) nanoparticles (TzPdNPs) was carried out and showed excellent bactericidal properties against Gram positive B. subtilis bacteria. TzPdNPs showed effective antimicrobial behavior, which caused 100% mortality against B. subtilis bacterial strains within 24 h. This material reported here can be used for different applications in biomaterials.
Funding
The authors received no direct funding for this research.
Acknowledgment
We are thankful to Dr P. Biswas, Department of Chemistry, Indian Institute of Engineering Science and Technology, for helpful suggestions during work and for preparation of the manuscript.
Additional information
Notes on contributors
Sutapa Joardar
Sutapa Joardar (SJ) is currently working as an assistant professor in the Department of Biotechnology, Neotia Institute of Technology, Management and Science, West Bengal, India. The focus of her research is application of nanostructured materials as antimicrobial agents. She is particularly interested about the antimicrobial properties of metallic nanoparticles immobilized on carrier such as zeolites, silicates, and polymer. Paramita Bhattacharjee (PB) is currently working as an assistant professor in the Department of Food Technology & Bio-Chemical Engineering, Jadavpur University, West Bengal, India. The focus of her research is supercritical fluid extraction, microencapsulation, gamma irradiation technology, technology of fats and oils, and technology of herbs and spices.
References
- Adams, C. P., Walker, K. A., Obare, S. O., Docherty, K. M., & van Raaij, M. J. (2014). Size-dependent antimicrobial effects of novel palladium nanoparticles. PLoS ONE, 9, e85981.10.1371/journal.pone.0085981
- Agnihotri, S., Mukherji, S., & Mukherji, S. (2013). Immobilized silver nanoparticles enhance contact killing and show highest efficacy: Elucidation of the mechanism of bactericidal action of silver. Nanoscale, 5, 7328–7340.10.1039/c3nr00024a
- Astruc, D. (2007). Palladium nanoparticles as efficient green homogeneous and heterogeneous carbon−carbon coupling precatalysts: A unifying view. Inorganic Chemistry, 46, 1884–1894.10.1021/ic062183h
- Baccar, H., Adams, C. P., Abdelghani, A., & Obare, S. O. (2013). Chronoamperometric-based detection of hydrogen peroxide using palladium nanoparticles. International Journal of Nanotechnology, 10, 563–576.10.1504/IJNT.2013.053525
- Beney, L., & Gervais, P. (2001). Influence of the fluidity of the membrane on the response of microorganisms to environmental stresses. Applied Microbiology and Biotechnology, 57, 34–42.
- Boomi, P., & Prabu, H. G. (2013). Synthesis, characterization and antibacterial analysis of polyaniline/Au–Pd nanocomposite. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 429, 51–59.10.1016/j.colsurfa.2013.03.053
- Cady, N. C., Behnke, J. L., & Strickland, A. D. (2011). Copper-based nanostructured coatings on natural cellulose: Nanocomposites exhibiting rapid and efficient inhibition of a multi-drug resistant wound pathogen, A. baumannii, and mammalian cell biocompatibility in vitro. Advanced Functional Materials, 21, 2506–2514.10.1002/adfm.201100123
- Chang, Z., Fan, H., Zhao, K., Chen, M., He, P., & Fang, Y. (2008). Electrochemical DNA biosensors based on palladium nanoparticles combined with carbon nanotubes. Electroanalysis, 20, 131–136.10.1002/(ISSN)1521-4109
- Cioffi, N., Torsi, L., Ditaranto, N., Tantillo, G., Ghibelli, L., Sabbatini, L., … Traversa, E. (2005). copper nanoparticle/polymer composites with antifungal and bacteriostatic properties. Chemistry of Materials, 17, 5255–5262.10.1021/cm0505244
- Das, S., Samanta, S., Ray, S., & Biswas, P. (2015). 3,6-Di(pyridin-2-yl)-1,2,4,5-tetrazine capped Pd(0) nanoparticles: A catalyst for copper-free Sonogashira coupling of aryl halides in aqueous medium. RSC Advances, 5, 75263–75267.10.1039/C5RA13252E
- Dobson, J. (2006). Magnetic nanoparticles for drug delivery. Drug Development Research, 67, 55–60.10.1002/(ISSN)1098-2299
- Eckhardt, S., Brunetto, P. S., Gagnon, J., Priebe, M., Giese, B., & Fromm, K. M. (2013). Nanobio silver: Its interactions with peptides and bacteria, and its uses in medicine. Chemical Reviews, 113, 4708–4754.10.1021/cr300288v
- Favier, F., Walter, E. C., Zach, M. P., Benter, T., & Penner, R. M. (2001). Hydrogen sensors and switches from electrodeposited palladium mesowire arrays. Science, 293, 2227–2231.10.1126/science.1063189
- Garoufis, A., Hadjikakou, S. K., & Hadjiliadis, N. (2005). In M. Gielen & E. R. T. Tiekink (Eds.), Metals in medicine, Palladium (Pd), in metallotherapeutic drugs and metal-based diagnostic agents: The use of metals in medicine (pp. 399–415). John Wiley & Sons.
- Garoufis, A., Hadjikakou, S. K., & Hadjikakou, N. (2009). Palladium coordination compounds as anti-viral, anti-fungal, anti-microbial and anti-tumor agents. Coordination Chemistry Reviews, 253, 1384–1397.10.1016/j.ccr.2008.09.011
- Hamouda, T., & Baker, Jr., J. R. (2000). Antimicrobial mechanism of action of surfactant lipid preparations in enteric Gram-negative bacilli. Journal of Applied Microbiology, 89, 397–403.10.1046/j.1365-2672.2000.01127.x
- Hao, N., Jayawardana, K. W., Chen, X., & Yan, M. (2015). One-step synthesis of amine-functionalized hollow mesoporous silica nanoparticles as efficient antibacterial and anticancer materials. ACS Applied Materials & Interfaces, 7, 1040–1045.10.1021/am508219g
- Heck, R. F. (1968a). The arylation of allylic alcohols with organopalladium compounds. A new synthesis of 3-aryl aldehydes and ketones. Journal of the American Chemical Society, 90, 5526–5531.10.1021/ja01022a035
- Heck, R. F. (1968b). Allylation of aromatic compounds with organopalladium salts. Journal of the American Chemical Society, 90, 5531–5534.10.1021/ja01022a036
- Heck, R. F. (1968c). The palladium-catalyzed arylation of enol esters, ethers, and halides. A new synthesis of 2-aryl aldehydes and ketones. Journal of the American Chemical Society, 90, 5535–5538.10.1021/ja01022a037
- Heck, R. F. (1968d). The addition of alkyl- and arylpalladium chlorides to conjugated dienes. Journal of the American Chemical Society, 90, 5542–5546.
- Heck, R. F., & Nolley, J. P. (1972). Palladium-catalyzed vinylic hydrogen substitution reactions with aryl, benzyl, and styryl halides. The Journal of Organic Chemistry, 37, 2320–2322.10.1021/jo00979a024
- Ilić, V., Šaponjić, Z., Vodnik, V., Potkonjak, B., Jovančić, P., Nedeljković, J., & Radetić, M. (2009). The influence of silver content on antimicrobial activity and color of cotton fabrics functionalized with Ag nanoparticles. Carbohydrate Polymers, 78, 564–569.
- Jana, R., Pathak, T. P., & Sigman, M. S. (2011). Advances in transition metal (Pd,Ni,Fe)-catalyzed cross-coupling reactions using alkyl-organometallics as reaction partners. Chemical Reviews, 111, 1417–1492.10.1021/cr100327p
- Kamat, P. V., & Meisel, D. (2003). Nanoscience opportunities in environmental remediation. Comptes Rendus Chimie, 6, 999–1007.10.1016/j.crci.2003.06.005
- Kim, Y. H., Lee, D. K., Cha, H. G., Kim, C. W., & Kang, Y. S. (2007). Synthesis and characterization of antibacterial Ag−SiO2 nanocomposite. The Journal of Physical Chemistry C, 111, 3629–3635.10.1021/jp068302w
- Li, W., & Szoka, Jr., F. C. (2007). Lipid-based nanoparticles for nucleic acid delivery. Pharmaceutical Research, 24, 438–449.10.1007/s11095-006-9180-5
- Liang, X., Sun, M., Li, L., Qiao, R., Chen, K., Xiao, Q., & Xu, F. (2012). Preparation and antibacterial activities of polyaniline/Cu0.05Zn0.95O nanocomposites. Dalton Transactions, 41, 2804–2811.10.1039/c2dt11823h
- Ma, S., Izutani, N., Imazato, S., Chen, J. H., Kiba, W., Yoshikawa, R., … Ebisu, S. (2012). Assessment of bactericidal effects of quaternary ammonium-based antibacterial monomers in combination with colloidal platinum nanoparticles. Dental Materials Journal, 31, 150–156.10.4012/dmj.2011-180
- Malleron, J. L., Fiaud, J. C., & Legros, J. Y. (2000). Handbook of palladium-catalyzed organic reactions. London: Academic Press.
- Manolova, V., Flace, A., Bauer, M., Schwarz, K., Saudan, P., & Bachmann, M. F. (2008). Nanoparticles target distinct dendritic cell populations according to their size. European Journal of Immunology, 38, 1404–1413.10.1002/(ISSN)1521-4141
- Mei, L., Zhang, X., Wang, Y., Zhang, W., Lu, Z., Luo, Y., … Li, C. (2014). Multivalent polymer–Au nanocomposites with cationic surfaces displaying enhanced antimicrobial activity. Polymer Chemistry, 5, 3038–3044.10.1039/c3py01578e
- Milstein, D., & Stille, J. K. (1978). A general, selective, and facile method for ketone synthesis from acid chlorides and organotin compounds catalyzed by palladium. Journal of the American Chemical Society, 100, 3636–3638.10.1021/ja00479a077
- Miyaura, N., & Suzuki, A. (1995). Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chemical Reviews, 95, 2457–2483.10.1021/cr00039a007
- Mueller, N. C., & Nowack, B. (2008). Exposure modeling of engineered nanoparticles in the environment. Environmental Science & Technology, 42, 4447–4453.10.1021/es7029637
- Nel, A. E., Mädler, L., Velegol, D., Xia, T., Hoek, E. M. V., Somasundaran, P., … Thompson, M. (2009). Understanding biophysicochemical interactions at the nano-bio interface. Nature Materials, 8, 543–557.10.1038/nmat2442
- Neuberger, T., Schöpf, B., Hofmann, H., Hofmann, M., & von Rechenberg, B. (2005). Superparamagnetic nanoparticles for biomedical applications: Possibilities and limitations of a new drug delivery system. Journal of Magnetism and Magnetic Materials, 293, 483–496.10.1016/j.jmmm.2005.01.064
- Nutt, M. O., Hughes, J. B., & Wong, M. S. (2005). Designing Pd-on-Au bimetallic nanoparticle catalysts for trichloroethene hydrodechlorination. Environmental Science & Technology, 39, 1346–1353.10.1021/es048560b
- Ojas, M., Bhagat, M., Gopalakrishnan, C., & Arunachalam, K. D. (2008). Ultrafine dispersed CuO nanoparticles and their antibacterial activity. Journal of Experimental Nanoscience, 3, 185–193.
- Rhim, J.-W., Hong, S.-I., Park, H.-M., & Ng, P. K. W. (2006). Preparation and characterization of chitosan-based nanocomposite films with antimicrobial activity. Journal of Agricultural and Food Chemistry, 54, 5814–5822.10.1021/jf060658h
- Silvestro, L., Weiser, J. N., & Axelsen, P. H. (2000). Antibacterial and antimembrane activities of Cecropin A in Escherichia coli. Antimicrobial Agents and Chemotherapy, 44, 602–607.
- Soenen, S. J., Rivera-Gil, P., Montenegro, J.-M., Parak, W. J., & De Smedt, S. C. (2011). Cellular toxicity of inorganic nanoparticles: Common aspects and guidelines for improved nanotoxicity evaluation. Nano Today, 6, 446–465.10.1016/j.nantod.2011.08.001
- Sonogashira, K. J. (2002). Development of Pd–Cu catalyzed cross-coupling of terminal acetylenes with sp2-carbon halides. Journal of Organometallic Chemistry, 653, 46–49.10.1016/S0022-328X(02)01158-0
- Stoimenov, P. K., Klinger, R. L., Marchin, G. L., & Klabunde, K. J. (2002). Metal oxide nanoparticles as bactericidal agents. Langmuir, 18, 6679–6686.10.1021/la0202374
- Tamboli, M. S., Kulkarni, M. V., Patil, R. H., Gade, W. N., Navale, S., & Kale, B. B. (2012). Nanowires of silver–polyaniline nanocomposite synthesized via in situ polymerization and its novel functionality as an antibacterial agent. Colloids and Surfaces B: Biointerfaces, 92, 35–41.10.1016/j.colsurfb.2011.11.006
- Thill, A., Zeyons, O., Spalla, O., Chauvat, F., Rose, J., Auffan, M., & Flank, A. M. (2006). Cytotoxicity of CeO2 nanoparticles for Escherichia coli. Physico-chemical insight of the cytotoxicity mechanism. Environmental Science & Technology, 40, 6151–6156.10.1021/es060999b
- Tratnyek, P. G., & Johnson, R. L. (2006). Nanotechnologies for environmental cleanup. Nano Today, 1, 44–48.10.1016/S1748-0132(06)70048-2
- Vukoje, I. D., Džunuzović, E. S., Vodnik, V. V., Dimitrijević, S., Ahrenkiel, S. P., & Nedeljković, J. M. (2014). Synthesis, characterization, and antimicrobial activity of poly(GMA-co-EGDMA) polymer decorated with silver nanoparticles. Journal of Materials Science, 49, 6838–6844.10.1007/s10853-014-8386-x
- Weissleder, R., Kelly, K., Sun, E. Y., Shtatland, T., & Josephson, L. (2005). Cell-specific targeting of nanoparticles by multivalent attachment of small molecules. Nature Biotechnology, 23, 1418–1423.10.1038/nbt1159
- Widenhoefer, R. A., & Buchwald, S. L. (1996). Formation of palladium bis(amine) complexes from reaction of amine with palladium tris(o-tolyl)phosphine mono(amine) complexes. Organometallics, 15, 3534–3542.10.1021/om9603169
- Witham, C. A., Huang, W., Tsung, C. K., Kuhn, J. N., Somorjai, G. A., & Toste, F. D. (2010). Converting homogeneous to heterogeneous in electrophilic catalysis using monodisperse metal nanoparticles. Nature Chemistry, 2, 36–41.10.1038/nchem.468
- Xiu, Z.-M., Zhang, Q.-B., Puppala, H. L., Colvin, V. L., & Alvarez, P. J. J. (2012). Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Letters, 12, 4271–4275.10.1021/nl301934w
- Yu, S., Welp, U., Hua, L. Z., Rydh, A., Kwok, W. K., & Wang, H. H. (2005). Fabrication of palladium nanotubes and their application in hydrogen sensing. Chemistry of Materials, 17, 3445–3450.10.1021/cm048191i
- Zhang, H., & Chen, G. (2009). Potent antibacterial activities of Ag/TiO2 nanocomposite powders synthesized by a one-pot sol−gel method. Environmental Science & Technology, 43, 2905–2910.10.1021/es803450f
- Zhang, D., Wei, S., Kaila, C., Su, X., Wu, J., Karki, A. B., … Guo, Z. (2010). Carbon-stabilized iron nanoparticles for environmental remediation. Nanoscale, 2, 917–919.