Abstract
Introduction: Lanthanum carbonate (LC) and sevelamer hydrochloride (SH) are non-calcium-based phosphate binders (NCPB), used to manage hyperphosphatemia in patients with chronic kidney disease. We compared the efficacy of LC and SH in lowering serum phosphate level in patients on haemodialysis or continuous ambulatory peritoneal dialysis. Methods: Treatment profiles of a group of dialysis patients on NCPB were retrospectively analyzed between 2010 and 2014 for a mean duration of one year. The treatment group included patients (n = 28) who were initially on SH and switched to LC because of uncontrolled hyperphosphataemia. Patients receiving ≥12 months SH treatment were included in the control group (n = 10). Results: There was a significant within patient fall in serum phosphate from a mean of 2.4 ± 0.5 mmol/L after 3–6 months on SH to 1.7 ± 0.4 mmol/L following 3–6 months on LC (p < 0.001). These within patient changes differed significantly from those observed in the control group (interaction p = 0.003). Mean phosphate binder pill burden also fell significantly from 5.6 ± 2.3 tablets to 2.6 ± 0.8 tablets daily (p < 0.001). A significant within patient increase in serum bicarbonate level from 23.2 ± 3.5 to 24.7 ± 2.7 mmol/L (p = 0.020) was observed in the treated patients, reflecting a resolution of mild acidosis associated with the hydrochloride salt of sevelamer which was not evident in the control group (interaction p = 0.022). Conclusion: This study demonstrates the efficacy of LC in reducing the serum phosphate level, pill burden, and improving bicarbonate level in dialysis patients.
Public Interest Statement
Renal Failure is a major public-health problem in the world and renal failure patients can suffer from mineral metabolism. Among these patients the cardiovascular disease is the commonest cause of death. Higher levels of serum phosphate are associated with bad outcomes in dialysis patients, so different types of phosphate binders are used to keep phosphate level near normal range. Phosphate binders bind dietary phosphate when they are taken with food. There is calcium and non-calcium based phosphate binders. Use of calcium-based phosphate binders can result in rise of serum calcium, which is associated with cardiovascular mortality in renal failure. The calcium free phosphate-binders such as sevelamer and lanthanum have been reported to reduce phosphate level without increasing serum calcium.
Competing Interests
The authors declare no competing interests.
1. Introduction
Chronic kidney disease (CKD) is complicated with hyperphosphatemia and renal bone disease and is a frequent phenomenon in dialysis patients (Tonelli, Pannu, & Manns, Citation2010). High phosphate level increases the risk of secondary hyperparathyroidism, renal bone disease, and vascular calcification (Block et al., Citation2004; Ganesh, Stack, Levin, Hulbert-Shearon, & Port, Citation2001; Kestenbaum et al., Citation2005; Melamed et al., Citation2006; Tentori et al., Citation2008). There is also an association between increased phosphate level, cardiovascular, and all-cause mortality in dialysis patients (Block, Hulbert-Shearon, Levin, & Port, Citation1998; Young et al., Citation2004). Despite dietary phosphate restriction and adequate dialysis, 90% of dialysis patients still need oral phosphate binders (Hutchison, Citation2009; Isakova et al., Citation2009; Tonelli et al., Citation2010).
Sevelamer hydrochloride (SH) is a non-calcium-based phosphate binders (NCPB) that does not increase serum calcium level. However, recent meta-analyzes failed to establish the comparative superiority of SH over calcium-based phosphate binders (Jamal, Fitchett, Lok, Mendelssohn, & Tsuyuki, Citation2009; Navaneethan, Palmer, Craig, Elder, & Strippoli, Citation2009). Moreover, this agent presents two major limitations: a significant incidence of gastrointestinal side effects and a high phosphate binder pill burden, leading to either the need to discontinue the drug, or nonadherence to the prescribe dose (Tomasello, Dhupar, & Sherman, Citation2004). The limitations of current regimens for uncontrolled hyperphosphataemia underscore the need for a safe and an efficacious calcium- and aluminum-free alternatives with reduced tablet load. Lanthanum carbonate (LC) is a NCPB with therapeutic potency similar to aluminum hydroxide but a more favorable safety profile. Several randomized control trials (RCT) have established LC to be a useful and well tolerated agent for phosphate control with low tablet burden in CKD patients on dialysis (Al-Baaj, Speake, & Hutchison, Citation2005; Joy, Finn, & Group LAMS, Citation2003; Shigematsu & Lanthanum Carbonate G, Citation2008a, Citation2008b).
There are a few small, short duration studies showing the efficacy of SH and LC to reduce phosphate binder pill burden and improve serum bicarbonate levels (Filiopoulos et al., Citation2011; Kasai, Sato, Murata, & Kinoshita, Citation2012). Another study revealed that a combination of LC and SH helped to reduce serum phosphate and pill burden in dialysis patients (Arenas et al., Citation2010). The current study is one of the few studies to compare directly the effectiveness of LC and SH for hyperphosphataemia in dialysis patients.
2. Materials and methods
2.1. Objectives
To compare the efficacy of LC and SH in terms of their effects on serum phosphate, calcium, bicarbonate and parathyroid hormone in dialysis patients.
2.2. Inclusion criteria
Patients on dialysis for at least 3 months were included in this study. The treatment group included patients who were on SH for at least 6 months with uncontrolled hyperphosphataemia and were subsequently switched to LC. Patients who were not switched to LC and received SH for at least 12 months were included in the control group.
2.3. Exclusion criteria
Patients with any of following criteria were excluded: (1) parathyroidectomy; (2) pregnancy or lactation; (3) malignancy; (4) previous gastrointestinal (GI) surgery or ongoing GI dysfunction, including peptic ulcer, inflammatory bowel diseases, or GI bleeding in the past 6 months.
2.4. Study design
This was a retrospective cohort study that assessed the effects of LC. The data were collected at baseline (the beginning of the 6 month SH phase) and every 3 subsequent months throughout a 12 month period for each patient.
2.5. Primary outcome
The primary outcome of study was to examine the efficacy of LC in comparison to SH in controlling serum phosphate.
2.6. Data analysis and statistics
Medical record abstraction provided patient data on age, gender, mode of dialysis, vascular access, comorbidities, primary cause of CKD, drug treatment and the reason for changing phosphate binder and any side effects. Laboratory data included serum calcium, corrected calcium, serum phosphate, parathyroid hormone (PTH), 25-OH vitamin D, alkaline phosphatase, total cholesterol, LDL cholesterol and Kt/V.
IBM SPSS version 23 was used to analyze the data. Two-tailed tests with a significance level of 5% were used throughout. Continuous variables were used as mean ± SD and categorical variables as proportions. Patient characteristics by treatment group (SH-SH or SH-LC) are shown in . Two-sample t tests were used to test for differences at baseline between the treatment groups. displays the mean and SD for each of the biochemical parameters observed during 3–6 months and during 9–12 months of treatment along with the within patient change over time by treatment group and p-value of the associated paired t test. Repeated measures analysis of variance was used to test for interaction between the effects of time and treatment group on each biochemical parameter. The resulting interaction p-value shown in essentially tests whether the within patient change in the parameter differs significantly by treatment group.
Table 1. Baseline characteristics of patients in treatment and control groups
Table 2. Within patient changes of biochemical parameters observed in control group (SH to SH) and treatment group (SH to LC)
3. Results
3.1. Patients
There were 38 adult patients with an age range of 20–82 years (57.4 ± 14.5 years) from Western Sydney Local Health District (WSLHD) who were on haemodialysis (60.5%) and peritoneal dialysis (39.5%) for ≥3 months. None of them changed dialysis modality during the study. The patients were divided into treatment (n = 28) and control (n = 10) groups (). The main reasons for changing phosphate binder in the treatment group from SH to LC were uncontrolled hyperphosphataemia and pill burden. Patients used 800 mg tablet of SH and 1,000 mg of LC in this study group. Diabetes and glomerulonephritis were the common causes of CKD in both groups. Mean duration of dialysis at the commencement of a patient’s 12 month study period was 3.8 years in the treatment group (SH-LC) and 2.2 years in the control group (SH-SH) ().
There were no serious adverse events found in either group except that two patients in the treatment group developed GI intolerance whilst on SH and one patient in the control group had constipation.
3.2. Serum phosphate level
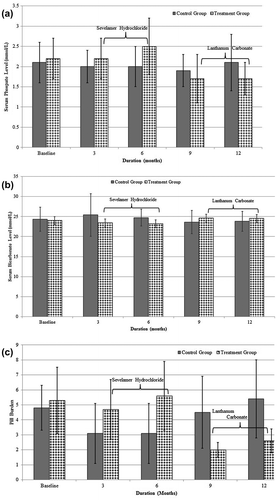
Mean serum phosphate level significantly reduced in the treatment group from 2.37 ± 0.53 while on SH to 1.71 ± 0.44 mmol/L after switching over to LC (p < 0.001), whereas it remained almost constant in the control group during 12 months of SH treatment ((a)). The reduction in phosphate level within patients over time observed in the SH-LC treatment group differed significantly (p = 0.003) from that observed in the SH control group ().
Figure 1. Changes in biochemical parameters in dialysis patients during treatment with phosphate binders i.e. SH and LC: (a) a significant reduction in serum phosphate levels was observed in LC treated patients (p < 0.001); (b) an improvement in bicarbonate levels was observed after switching to LC (p = 0.020); (c) pill burden significantly reduced after switching over to LC (p < 0.00001), (SH one tablet = 800 mg, LH one tablet = 1,000 mg).
3.3. Serum bicarbonate level
Overall SH treatment resulted in a reduction of the mean serum bicarbonate level in both groups ((b)). In controls, it fell from 25.05 ± 3.68 to 23.7 ± 2.03 mmol/L. In the treatment group, a fall from 24.0 ± 3.4 to 23.2 ± 3.54 mmol/L was initially observed while on SH, but a switch to LC lead a significant increase in serum bicarbonate level from 23.2 ± 3.5 to 24.7 ± 2.7 mmol/L (p = 0.020), reflecting a resolution of mild acidosis associated with the hydrochloride salt of sevelamer which was not evident in the control group (interaction p = 0.022) ().
3.4. Phosphate binder pill burden and other biochemical parameters
Phosphate binder therapy and dosage titration at baseline, three, six, nine and twelve months in the treatment and control groups is described in (c). In the treatment group, the mean dose was 4.7 ± 2.0 tablets/day of SH for three months rising to 5.6 ± 2.3 tablets/day at 6 months without any reduction of the mean phosphate level, as shown in the Supplementary Table. A switch to LC significantly reduced the pill burden at nine months (2.0 ± 0.5 tablets/day) and twelve months (2.6 ± 0.8 tablets/day), respectively (p < 0.00001). In the control group, the mean dose of SH was 3.1 ± 2.0 tablets/day at 3 months, which gradually increased to 5.4 ± 2.6 tablets/day, however it did not reduce serum phosphate level (Supplementary Table).
SH treatment improved mean cholesterol level and mean low density lipoprotein (LDL) level in both control and treatment groups but LC did not lower the levels of either (Supplementary Table).
4. Discussion
Whereas the ideal target level of serum phosphate is not known, the initial goal of therapy should be to reduce the level until it approaches the normal range (Kidney Disease: Improving Global Outcomes CKDMBDWG, Citation2009), knowing that normal serum phosphate level will be unachievable in many patients. The effectiveness of calcium-based phosphate binders and SH in controlling phosphate level is well proven in many clinical studies. The high pill burden owing to worse toxic effects including digestive intolerance is a disadvantage for many patients (Arenas et al., Citation2010; Filiopoulos et al., Citation2011; Salusky, Citation2006). For this reason, poor compliance to the recommended medication is a factor in reducing the efficacy of these drugs, leading to poor clinical outcomes. LC has been shown as an effective drug with a low incidence of side effects, and a reduced pill burden in dose titration studies (Hutchison, Speake, & Al-Baaj, Citation2004; Joy et al., Citation2003). Therefore the use of LC as a first line agent in NCPB therapy may offer a particular advantage in patients who need better phosphate control.
This is a retrospective study with a cumulative follow-up of one year comparing the efficacy of SH and LC in haemodialysis and peritoneal dialysis patients. Whereas previous studies have provided convincing evidence about the use of LC as an effective drug in controlling phosphate level in combination therapy (Arenas et al., Citation2010; Filiopoulos et al., Citation2011; Kasai et al., Citation2012; Winnie Chan et al., Citation2010). This study further extends knowledge about the use of LC to control hyperphosphataemia. A switch from SH to LC resulted in better phosphate control and a significant reduction in phosphate binder pill burden (p < 0.001). Patients in the treatment group were more frequently on haemodialysis and were older; therefore one may speculate that this group poorly tolerated a high pill burden resulting in non-compliance. The reduced pill burden with LC may have contributed to better compliance and resulted in improved phosphate control. The patient’s adherence to LC and significant reduction in pill burden raises the hope of long-term beneficial effects. Along with the resolution of hyperphosphataemia an increase in serum bicarbonate level was also observed after the switch to LC (p = 0.02) which probably reflects a correction of mild acidosis associated with the hydrochloride salt of Sevelamer. Similar findings were also observed by Winnie Chan et al. (Citation2010) in their study of hemodialysis patients treated with LC for a period of 3 months. There was no significant change in parathyroid hormone, 25-OH vitamin D, alkaline phosphatase and serum calcium levels in this study, as also evident in the previous LC studies (Arenas et al., Citation2010; Filiopoulos et al., Citation2011; Winnie Chan et al., Citation2010). Whether sustained phosphate control would suppress the parathyroid hormone level remains to be investigated.
A few of the patients developed GI intolerance and constipation while on SH treatment. An improvement was seen with change to LC. There are a few head to head studies comparing the performance of SH against LC. Substitution of SH with LC improves constipation symptoms in haemodialysis patients (Suzuki, Ichie, Hayashi, Sugiura, & Sugiyama, Citation2015). Kasai et al. (Citation2012) reported similar efficacy in the control of serum phosphate in haemodialysis patients in their randomized crossover study, with SH causing more constipation. Sprague et al. (Citation2009) found a significant difference in phosphate control in favor of LC in their crossover study.
There are a few limitations of this study: it is a relatively small retrospective study. We were not able to reliably collect data on compliance (pill count), diet, vitamin D dosage, timing of blood tests and timing of ingestion of phosphate binders which could have affected the interpretations of our results. These issues may be addressed in future prospective clinical trials, including large groups to examine the effects of LC as a first line phosphate binder in dialysis patients.
In conclusion, this study demonstrates that LC is effective in reducing serum phosphate level in dialysis patients, particularly those who have uncontrolled hyperphsophataemia with SH. There is an added advantage of reduced pill burden which may encourage compliance.
Table__28Suppl_29.docx
Download MS Word (20 KB)Additional information
Funding
Notes on contributors
Asrar Khan
Asrar Khan has an excellent track record of obtaining professional qualifications (MBBS, IMM (General Medicine), FCPS Nephrology and FRACP Nephrology. He has substantial number of research work publications in International medical journals in NDT, KI and Singapore Medical Journal. He had presented posters in various international nephrology conferences. He always take initiative to present lectures and case presentations. He attended many local and international Nephrology conferences and workshops. He is very keen to attend Webinars and reviews journals to update the knowledge and recent advances. He has special interest in renal bone disease and phosphate binders.
References
- Al-Baaj, F., Speake, M., & Hutchison, A. J. (2005). Control of serum phosphate by oral lanthanum carbonate in patients undergoing haemodialysis and continuous ambulatory peritoneal dialysis in a short-term, placebo-controlled study. Nephrology Dialysis Transplantation, 20, 775–782. doi:10.1093/ndt/gfh693
- Arenas, M. D., Rebollo, P., Malek, T., Moledous, A., Gil, M. T., Alvarez-Ude, F., … Cotilla, E. (2010). A comparative study of 2 new phosphate binders (sevelamer and lanthanum carbonate) in routine clinical practice. Journal of Nephrology, 23, 683–692.
- Block, G. A., Hulbert-Shearon, T. E., Levin, N. W., & Port, F. K. (1998). Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: A national study. American Journal of Kidney Diseases, 31, 607–617.10.1053/ajkd.1998.v31.pm9531176
- Block, G. A., Klassen, P. S., Lazarus, J. M., Ofsthun, N., Lowrie, E. G., & Chertow, G. M. (2004). Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. Journal of the American Society of Nephrology, 15, 2208–2218. doi:10.1097/01.ASN.0000133041.27682.A2
- Filiopoulos, V., Koutis, I., Trompouki, S., Hadjiyannakos, D., Lazarou, D., & Vlassopoulos, D. (2011). Lanthanum carbonate versus sevelamer hydrochloride: Improvement of metabolic acidosis and hyperkalemia in hemodialysis patients. Therapeutic Apheresis and Dialysis, 15, 20–27. doi:10.1111/j.1744-9987.2010.00868.x
- Ganesh, S. K., Stack, A. G., Levin, N. W., Hulbert-Shearon, T., & Port, F. K. (2001). Association of elevated serum PO(4), Ca x PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. Journal of the American Society of Nephrology, 12, 2131–2138.
- Hutchison, A. J. (2009). Oral phosphate binders. Kidney International, 75, 906–914. doi:10.1038/ki.2009.60
- Hutchison, A. J., Speake, M., & Al-Baaj, F. (2004). Reducing high phosphate levels in patients with chronic renal failure undergoing dialysis: A 4-week, dose-finding, open-label study with lanthanum carbonate. Nephrology Dialysis Transplantation, 19, 1902–1906. doi:10.1093/ndt/gfh282
- Isakova, T., Gutierrez, O. M., Chang, Y., Shah, A., Tamez, H., Smith, K., … Wolf, M. (2009). Phosphorus binders and survival on hemodialysis. Journal of the American Society of Nephrology, 20, 388–396. doi:10.1681/ASN.2008060609
- Jamal, S. A., Fitchett, D., Lok, C. E., Mendelssohn, D. C., & Tsuyuki, R. T. (2009). The effects of calcium-based versus non-calcium-based phosphate binders on mortality among patients with chronic kidney disease: A meta-analysis. Nephrology Dialysis Transplantation, 24, 3168–3174. doi:10.1093/ndt/gfp350
- Joy, M. S., Finn, W. F., & Group LAMS (2003). Randomized, double-blind, placebo-controlled, dose-titration, phase III study assessing the efficacy and tolerability of lanthanum carbonate: A new phosphate binder for the treatment of hyperphosphatemia. American Journal of Kidney Diseases, 42, 96–107.10.1016/S0272-6386(03)00554-7
- Kasai, S., Sato, K., Murata, Y., & Kinoshita, Y. (2012). Randomized crossover study of the efficacy and safety of sevelamer hydrochloride and lanthanum carbonate in japanese patients undergoing hemodialysis. Therapeutic Apheresis and Dialysis, 16, 341–349. doi:10.1111/j.1744-9987.2012.01071.x
- Kestenbaum, B., Sampson, J. N., Rudser, K. D., Patterson, D. J., Seliger, S. L., Young, B., … Andress, D. L. (2005). Serum phosphate levels and mortality risk among people with chronic kidney disease. Journal of the American Society of Nephrology, 16, 520–528. doi:10.1681/ASN.2004070602
- Kidney Disease: Improving Global Outcomes CKDMBDWG. (2009). KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney international. Supplement, 113, 1–130. doi:10.1038/ki.2009.188
- Melamed, M. L., Eustace, J. A., Plantinga, L., Jaar, B. G., Fink, N. E., Coresh, J., … Powe, N. R. (2006). Changes in serum calcium, phosphate, and PTH and the risk of death in incident dialysis patients: A longitudinal study. Kidney International, 70, 351–357. doi:10.1038/sj.ki.5001542
- Navaneethan, S. D., Palmer, S. C., Craig, J. C., Elder, G. J., & Strippoli, G. F. (2009). Benefits and harms of phosphate binders in CKD: A systematic review of randomized controlled trials. American Journal of Kidney Diseases, 54, 619–637. doi:10.1053/j.ajkd.2009.06.004
- Salusky, I. B. (2006). A new era in phosphate binder therapy: What are the options? Kidney International, 70, 10–15. doi:10.1038/sj.ki.5001997
- Shigematsu, T., & Lanthanum Carbonate G. (2008a). Multicenter prospective randomized, double-blind comparative study between lanthanum carbonate and calcium carbonate as phosphate binders in Japanese hemodialysis patients with hyperphosphatemia. Clinical Nephrology, 70, 404–410.10.5414/CNP70404
- Shigematsu, T., & Lanthanum Carbonate Research G. (2008b). Lanthanum carbonate effectively controls serum phosphate without affecting serum calcium levels in patients undergoing hemodialysis. Therapeutic Apheresis and Dialysis, 12, 55–61. doi:10.1111/j.1744-9987.2007.00541.x
- Sprague, S. M., Ross, E. A., Nath, S. D., Zhang, P., Pratt, R. D., & Krause, R. (2009). Lanthanum carbonate vs. sevelamer hydrochloride for the reduction of serum phosphorus in hemodialysis patients: A crossover study. Clinical Nephrology, 72, 252–258.10.5414/CNP72252
- Suzuki, D., Ichie, T., Hayashi, H., Sugiura, Y., & Sugiyama, T. (2015). Gastrointestinal symptoms after the substitution of sevelamer hydrochloride with lanthanum carbonate in Japanese patients undergoing hemodialysis. Pharmazie, 70, 522–526.
- Tentori, F., Blayney, M. J., Albert, J. M., Gillespie, B. W., Kerr, P. G., Bommer, J., … Port, F. K. (2008). Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: The dialysis outcomes and practice patterns study (DOPPS). American Journal of Kidney Diseases, 52, 519–530. doi:10.1053/j.ajkd.2008.03.020
- Tomasello, S., Dhupar, S., & Sherman, R. A. (2004). Phosphate binders, K/DOQI guidelines, and compliance: The unfortunate reality. Dialysis & Transplantation, 33, 236–240.
- Tonelli, M., Pannu, N., & Manns, B. (2010). Oral phosphate binders in patients with kidney failure. New England Journal of Medicine, 362, 1312–1324. doi:10.1056/NEJMra0912522
- Winnie Chan, W. L., Rounsley, K., Chapman, E., Collings, K., Dale, C., De Waal, S., … Borrows, R. (2010). Lanthanum carbonate is an effective hypophosphatemic agent for hemodialysis patients intolerant of other phosphate binders. Journal of Renal Nutrition, 20, 270–277. doi:10.1053/j.jrn.2009.10.009
- Young, E. W., Akiba, T., Albert, J. M., McCarthy, J. T., Kerr, P. G., Mendelssohn, D. C., & Jadoul, M. (2004). Magnitude and impact of abnormal mineral metabolism in hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS). American Journal of Kidney Diseases, 44, 34–38.10.1016/S0272-6386(04)01103-5
