Abstract
Purpose: To describe angiographic, computed-tomography (CT) and pathologic features of ETHIBLOC_Reloaded as a re-designed zein-based fluid embolic agent. Materials and methods: In eight pigs, both kidneys underwent selective transarterial embolization (with complete embolization as embolization endpoint). Each group consisted of two pigs with four embolized kidneys: I-pure ETHIBLOC_Reloaded, II-ETHIBLOC_Reloaded/iodized oil mixture (1:1), III-ETHIBLOC_Reloaded/ethanol-60% mixture (8:2) and IV-Histoacryl/iodized oil mixture (1:3). One hour after embolization, CT imaging, sacrifice and kidney harvest followed. Angiographic (visibility and vascular occlusion pattern), CT (visibility) and pathologic (vascular occlusion pattern) features were compared. Results: The embolization endpoint was reached in all animals. Applying Angiography, embolic agents were definitely visible during embolization in all study groups. Vascular occlusion occurred from distal (arcuate and interlobar arteries) to proximal (renal artery), whereby the most distal levels were reached in II and III. Applying CT imaging, embolic agents were definitely visible in hilar and intraparenchymal arteries in all groups. Pathology proved occlusion of segmental, interlobar and arcuate arteries in all groups, and additionally occlusion of interlobular arteries, pre-glomerular arterioles and glomerular capillaries in I, II and III. Conclusion: ETHIBLOC_Reloaded is a promising re-designed zein-based embolic agent that can be used safely and effectively for transarterial embolization of the pig kidney.
Public Interest Statement
Transarterial embolization is a minimal-invasive image-guided ultra-precise treatment for the occlusion of pathologic vessels performed by Interventional Radiologists. Different life- threatening conditions including acute bleeding and vascular malformation can be treated by applying transarterial embolization. Irrespective of the anatomical region, current drawbacks of embolization materials are (I) limited control during embolization potentially resulting in suboptimal acute embolization results, (II) artifacts after embolization resulting in unclear findings during imaging follow-up as well as (III) mid-term and long-term treatment failure such as recanalization with the need of repeated treatment. With the introduction of ETHIBLOC_Reloaded as a new ready for use liquid embolization material, transarterial embolization procedures may be improved due to its significantly improved X-ray visibility and favorable occlusion characteristics.
Competing Interests
The authors declare no competing interest.
1. Introduction
In transarterial embolization, every embolic agent has specific characteristics regarding indication, application, vascular occlusion and recanalization pattern (Sommer et al., Citation2010). For fluid embolic agents, precipitation and polymerization are the physical principles underlying the process of vascular occlusion, with original Ethibloc (Ethicon, Norderstedt, Germany) and cyanoacrylate (for example, Histoacryl; N-butyl cyanoacrylate; B. Braun, Melsungen, Germany) as the most studied materials (Mironov, Citation1990; Rassweiler, Kauffmann, & Rohrbach, Citation1980; Razavi & Murphy, Citation2007; Richter, Rassweiler, Kauffmann, Wenz, & Crawford, Citation1984; Richter, Rohrbach, Kauffmann, & Raßweiler, Citation1981; Vanlangenhove, Everaert, Van Maele, & Defreyne, Citation2014; Woo et al., Citation2013).
Cyanoacrylate is regularly used for embolization of varicoceles, bronchial arteries, gastroduodenal ulcer bleeding, type II endoleaks and pre-operative portal vein embolization (Bellemann et al., Citation2012; Krämer et al., Citation2000; Mironov, Citation1990; Razavi & Murphy, Citation2007; Vanlangenhove et al., Citation2014; Woo et al., Citation2013).
Although original Ethibloc was clinically established, it has been withdrawn from the commercial market in 2008 since sterility concerns of the commercial packaging process (Sommer et al., Citation2017). For original Ethibloc, excellent outcomes had been reported for different indications: transarterial nephrectomy, aneurysmal bone cysts, arteriovenous, lymphatic and venous malformations, portal vein embolization, hemorrhage, and pancreatic duct fistula (Buck, Rassweiler, Leibbrand, Miller, & Eisenberger, Citation1986; Buecker, Keulers, & Guenther, Citation1997; Emran et al., Citation2006; George et al., Citation2009; Herbreteau, Citation2007; Krämer et al., Citation2000; Radeleff et al., Citation2008; Rassweiler et al., Citation2008). High reliability, safety and efficacy were the major clinical advantages (Mironov, Citation1990; Rassweiler et al., Citation1980, Citation2008; Razavi & Murphy, Citation2007; Vanlangenhove et al., Citation2014). Unfortunately, due to the additional costs of a new certification process, a new product is still not available.
Original Ethibloc consisted of zein (a prolamine protein found in corn) as occlusion material, ethanol as solvent, oleum papaveris as softener and sodium amidotrizoate as X-ray contrast material (Bücheler, Hupe, Klosterhalfen, Altenähr, & Erbe, Citation1978; Gebhardt & Stolte, Citation1978). Since suboptimal X-ray visibility, different users prepared individual mixtures of original Ethibloc and iodized oil (Lipiodol Ultra-Fluide; Guerbet, Roissy, France) with the intention to improve X-ray visibility (Buck et al., Citation1986; Buecker et al., Citation1997; Emran et al., Citation2006; George et al., Citation2009; Herbreteau, Citation2007; Krämer et al., Citation2000; Mironov, Citation1990; Radeleff et al., Citation2008; Rassweiler et al., Citation1980, Citation2008; Richter et al., Citation1981, Citation1984). The major limitations of these handmade mixtures were decreased product stability and unpredictable precipitation behavior resulting in non-standardized embolization (Rassweiler et al., Citation2008; Richter et al., Citation1981, Citation1984).
In this study, Ethibloc was re-designed with the rationale to provide a fluid embolic agent with appropriate viscosity for transmicrocatheter application, adequate X-ray visibility under human scale angiography as well as controllable and reproducible precipitation/vascular occlusion characteristics. For this new material, hereinafter referred to as ETHIBLOC_Reloaded, oleum papaveris was completely replaced by iodized oil. Since this modification was implemented in the standardized manufacturing process, a ready for use embolic agent with optimal X-ray visibility should result. In this first in vivo study, the purpose was to evaluate its angiographic, computed-tomography (CT) and pathologic features in an in vivo pig kidney study. The hypothesis was defined as follows: ETHIBLOC_Reloaded can be used safely and effectively for transarterial embolization of the pig kidney.
2. Materials and methods
The ethics committee approved this study. All experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals.
2.1. Groups
Pure ETHIBLOC_Reloaded was used for embolization in four kidneys of two pigs (I-pure ETHIBLOC_Reloaded). In another eight kidneys of four pigs, either pure ETHIBLOC_Reloaded in mixture (1:1) with iodized oil (II-ETHIBLOC_Reloaded/iodized oil mixture) (four kidneys in two pigs) or pure ETHIBLOC_Reloaded in mixture (8:2) with ethanol-60% (III-ETHIBLOC_Reloaded/ethanol-60% mixture) (four kidneys in two pigs) was used for embolization. The rationale to use different emulsions of ETHIBLOC_Reloaded was to create different viscosities (potentially affecting the precipitation process and consequently the vascular occlusion pattern) and different X-ray visibility (potentially affecting the control during embolization). For the original Ethibloc, Richter et al. recommended a viscosity of 120–220 mPa*s for capillary embolization (Richter et al., Citation1984). Since the viscosities - determined by applying a rotational viscometer (Searle system) for dynamic viscosities—were 200, 107 and 100 mPa*s at 37°C in I, II and III, respectively, the recommended viscosities for capillary embolization could be achieved (Figure (a) resulting in a viscous fluid embolic agent (Figure (b) and (c)).
Figure 1. Material characteristics. (a) Viscosity Chart—the viscosities were 373, 132 and 130 mPa*s at 20°C as well as 200, 107 and 100 mPa*s at 37°C for I-Pure ETHIBLOC_Reloaded, II-ETHIBLOC_Reloaded/iodized oil mixture (1:1) and III-ETHIBLOC_Reloaded/ethanol-60% mixture (8:2) as well as 16.4 mPa*s at 20°C and 9.9 mPa*s at 37°C for IV-Histoacryl/iodized oil mixture (1:3), respectively; (b) and (c) I-Pure ETHIBLOC_Reloaded—homogeneous zein-based embolic agent with the typical yellow aspect and a viscous consistency.
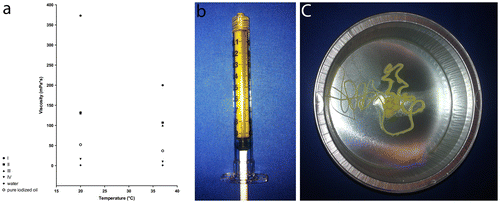
As control, Histoacryl was used in another four kidneys of two pigs. Histoacryl as medical glue is an established liquid embolic agent and commonly used in clinical practice (Mironov, Citation1990; Razavi & Murphy, Citation2007; Vanlangenhove et al., Citation2014; Woo et al., Citation2013). According to good clinical practice, Histoacryl in mixture (1:3) with iodized oil (IV-Histoacryl/iodized oil mixture) was used resulting in a viscosity of 9.9 mPa*s at 37°C (Figure (a).
2.2. Preparation of the embolic agents
On the day of embolization, pure ETHIBLOC_Reloaded was warmed in a heating cabinet at 37°C for 30 min. In I, the warmed pure ETHIBLOC_Reloaded was stirred, filled into a 5 ml Luer-Lock syringe and connected to the microcatheter for embolization (I-pure ETHIBLOC_Reloaded). In II and III, either iodized oil or ethanol-60% was added to and mixed with the warmed pure ETHIBLOC_Reloaded (II-ETHIBLOC_Reloaded/iodized oil mixture (1:1) and III-ETHIBLOC_Reloaded/ethanol-60% mixture (8:2)). In IV, iodized oil was added to and mixed with Histoacryl (IV-Histoacryl/iodized oil mixture (1:3) resulted. In I, III and IV, the embolic agents remained stable for several minutes. In II, the emulsion began to partition into two phases immediately at rest. To obtain the best homogeneity for all embolic agents, re-mixing right before injection was performed.
2.3. Animal procedures
Eight female landrace pigs (body weight of 33–37 kg) were used. Anesthesia was started with intravenous injection of ketamine (10 mg/kg; Ketamin, Medistar, Hannover, Germany), azaperone (6 mg/kg; Stresnil, Janssen Animal Health, Beerse, Belgium) and midazolam (0.4 mg/kg; Dormicum, Roche, Basel, Switzerland). Pigs were intubated, and anesthesia was maintained with isoflurane (Isofluran CP, CP-Pharma, Burgdorf, Germany). A 4F introducer sheath was inserted in the femoral artery. Two radiologists (CMS and US with more than 10 years experience in interventional radiology) performed all embolization procedures. Under fluoroscopy-guidance (Polystar TOP, Siemens Medical Solutions, Forchheim, Germany), the right renal artery was catheterized with a 4F selective catheter. Selective angiography (2 images/s; digital subtraction angiography (DSA) and non-subtracted angiography (NSA)) using 5 ml iodinated contrast material (iomeprol, Imeron 300; Bracco, Konstanz, Germany) was performed. A 2.7 F coaxial microcatheter (Progreat, Terumo, Tokyo, Japan) was placed in the right renal artery.
Embolization was performed by using the prepared 5 ml Luer-Lock syringes connected to the microcatheter. For optimal documentation, embolization was performed under angiography-guidance (2 images/s; DSA and NSA). The embolization endpoint was complete embolization of the kidney. 3 ml glucose 40% was injected before and after embolization according to standard technique (Woo et al., Citation2013). After removal of the microcatheter, selective angiography via the 4F catheter (2 images/s; DSA and NSA) was performed using 5 ml iodinated contrast material. Embolization of the left kidney followed using the same technique.
One hour after embolization, CT imaging of the abdomen was performed without intravenous contrast material (Somatom Definition Flash; Siemens Medical Solutions, Forchheim, Germany). The scanning parameters included a tube voltage of 120 kVp, a reference current-time product of 240 mAs (CARE Dose 4D®), a pitch of 0.55 and a collimation of 64 × 0.6 mm. Images were reconstructed as multi-planar images in the axial plane with a slice thickness of 1 mm and an overlap of 0.7 mm with a soft tissue kernel (I3–30) and a bone kernel (I3–70) (Safire; Somatom Definition Flash; Siemens Medical Solutions, Forchheim, Germany).
Subsequently, animals were sacrificed with an intravenous injection of 40 ml potassium chloride 7.5%, kidneys harvested and preserved in formalin 4%.
2.4. Pathology
Each kidney was laminated (5 mm thick sections) either along the long or the short axis. Two paraffin-embedded specimens from the upper and lower segments (including cortex and medulla) were prepared. From each paraffin-embedded specimen, three sections with a thickness of 4 μm were stained according to standard protocols, one with hematoxylin and eosin (HE), one with Acid Fuchsin Orange G (AFOG), and one with Elastica van Gieson (EvG).
2.5. Study goals
2.5.1. Angiography
The control during embolization was described. Thereby, resistance during injection was determined by applying a semi-quantitative five-point Likert score (1-very low, 2-low, 3-intermediate, 4-high, 5-very high). The precision to reach the embolization endpoint was determined by applying a semi-quantitative five-point Likert score (1-very low, 2-low, 3-intermediate, 4-high, 5-very high). The volumes to reach the embolization endpoint and procedure-related complications were recorded.
Angiographies during embolization (DSA and NSA) (without additional contrast material) were used to describe the visibility of the embolic agents during embolization. Thereby, a semi-quantitative three-point Likert score (1-definitely not visible, 2-probably visible, 3-definitely visible) was applied (Sommer et al., Citation2013).
Angiographies after embolization (NSA) (without additional contrast material) were used to describe the visibility of the embolic agents. For the angiographic visibility after embolization, six different arterial levels (renal artery, polar arteries, segmental arteries, interlobar arteries, arcuate arteries and interlobular arteries) were analyzed: for the renal artery, polar arteries and segmental arteries by applying a semi-quantitative three-point Likert score (1-definitely not visible, 2-probably visible, 3-definitely visible), as well as for interlobar arteries, arcuate arteries and interlobular arteries by applying a semi-quantitative five-point Likert score (1-never visible, 2-seldomly visible, 3-regularly visible, 4-often visible, 5-always visible).
Angiographies (DSA with 5 ml iodinated contrast material) after embolization were used to describe the vascular occlusion pattern. Each arterial level was analyzed with respect to the visibility of the injected iodinated contrast material: for the renal artery, polar arteries and segmental arteries by applying a semi-quantitative three-point Likert score (1-definitely not visible, 2-probably visible, 3-definitely visible) as well as for interlobar arteries, arcuate arteries and interlobular arteries by applying a semi-quantitative five-point Likert score (1-never visible, 2-seldomly visible, 3-regularly visible, 4-often visible, 5-always visible).
2.5.2. CT imaging features
CT images were used to semi-quantitatively and quantitatively assess the visibility of all embolic agents.
The semi-quantitative visibility of the embolic agents was analyzed separately for hilum and parenchyma by applying a semi-quantitative three-point Likert score (1-definitely not visible, 2-probably visible, 3-definitely visible) (Sommer et al., Citation2013).
The quantitative visibility of the embolic agents was analyzed by applying density measurements (HU, Hounsfield units) with regions-of-interest (ROIs) of 20–30 mm2 (Sommer et al., Citation2013). Those density measurements were performed separately for the upper, middle and lower segments of all kidneys (for the brightest areas as well as for the darkest areas within the parenchyma).
All image analyses were carried out using a picture archiving and communication system (GE Centricity 4.1, GE Healthcare, Barrington, USA).
2.5.3. Pathology
For evaluation of the histological vascular occlusion pattern, the level of vascular occlusion was determined for each section. Arterial levels were classified as previously described (Bilbao et al., Citation2008, Citation2009): polar and/or segmental arteries, interlobar arteries, arcuate arteries, interlobular arteries, pre-glomerular arterioles and glomerular capillaries.
2.6. Statistics
Prism software (Version 6.00, GraphPad Software, LaJolla, USA) was used for the statistical procedures. Semi-quantitative and quantitative data were presented as median and 25 and 75% percentiles (if not otherwise specified). To evaluate the statistical differences for the semi-quantitative data (between the four different groups), the Kruskal Wallis test was applied. To evaluate the statistical differences for the procedure-related complications, the χ2 test for trend was applied (the contingency table included four rows (groups) and two columns (embolization of a kidney with complication and embolization of a kidney without complication)). To evaluate the statistical differences for the quantitative data between the four different groups, the Kruskal Wallis test was applied. p = 0.05 was regarded as the level of statistical significance.
3. Results
The embolization endpoint was reached in all animals.
3.1. Angiography
3.1.1. Control during embolization
Resistance during injection was 4.5 (4.0;5.0) in I, 4.0 (3.3;4.0) in II, 3.5 (3.0;4.0) in III, and 4.0 (4.0;4.8) in IV (n.s.). Precision to reach the embolization endpoint was 5.0 (4.3;5.0) in I, 4.0 (3.0;5.0) in II, 4.0 (3.3;4.8) in III, and 4.0 (3.0;5.0) in IV (n.s.). The volume to reach the embolization endpoint was 3.8 ml (3.1;4.2) in I, 4.1 ml (3.4;4.8) in II, 4.1 ml (3.9;4.4) in III, and 3.0 ml (2.7;3.3) in IV with significant differences (p = 0.04). No complications occurred in I and II, and one complication occurred in III and IV (n.s.). In both cases, embolic agent was dislocated to the origin of the renal artery, in III during embolization since too fast injection and in IV during microcatheter removal after embolization since sticking.
3.1.2. Angiographic visibility during and after embolization
For DSA and NSA, angiographic visibility during embolization was 3.0 (3.0;3.0) in all groups (n.s., respectively). Angiographic visibility after embolization was comparable for renal artery, polar arteries and segmental arteries (Table ; Figure (a)–(d)). For interlobar, arcuate and interlobular arteries, however, angiographic visibility after embolization was significantly different between the groups, with the best visibility in II and the worst visibility in IV (Table ; Figure (a)–(d)).
Table 1. Angiographic visibility after embolization
Figure 2. Angiography. (a) DSA with 5 ml iodinated contrast material before embolization; (b) NSA without additional iodinated contrast material after embolization: I-Pure ETHIBLOC_Reloaded; (c) DSA with 5 ml iodinated contrast material after embolization: III-ETHIBLOC_Reloaded/ethanol-60% mixture (8:2); (d) DSA with 5 ml iodinated contrast material after embolization: IV-Histoacryl/iodized oil mixture (1:3); (e) Chart for the angiographic vascular occlusion pattern after embolization.
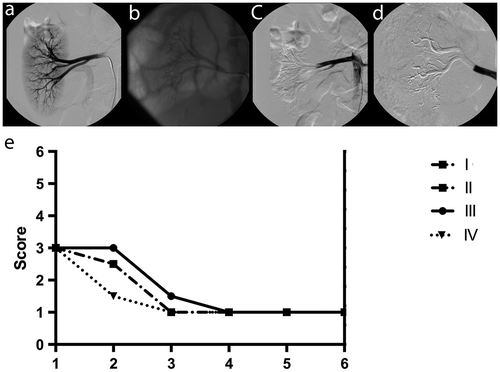
3.1.3. Angiographic vascular occlusion pattern
Detailed data are illustrated (Figure (e)). For interlobar arteries, arcuate arteries, and interlobular arteries, the angiographic vascular occlusion pattern was 1.0 (1.0;1.0) in all groups (n.s., respectively). For renal artery, the angiographic vascular occlusion pattern was 3.0 (3.0;3.0) in all groups (n.s., respectively). For polar arteries and segmental arteries, the angiographic vascular occlusion pattern was between 2.5 (2.0;3.0) and 3.0 (3.0;3.0) as well as 1.0 (1.0;1.0) and 1.5 (1.0;2.0) without significant differences (n.s., respectively).
3.2. CT imaging features
Semi-quantitative CT visibility for hilum soft tissue kernel I3–30 was 2.0 (2.0;2.0) in all four groups (n.s.) (Figure (a)–(d)). Clear differentiation between the excreted iodinated contrast material within the pelvic system and the embolic agent within arteries of the hilum was not possible. Semi-quantitative CT visibility for hilum bone kernel I3–70, parenchyma soft tissue kernel I3–30 and parenchyma bone kernel I3–70 was 3.0 (3.0;3.0), 3.0 (3.0;3.0) and 3.0 (3.0;3.0) in all groups (n.s., respectively).
Figure 3. CT imaging features. (a) Axial plane, I3–30f kernel, without intravenous contrast material, right kidney - I-Pure ETHIBLOC_Reloaded; (b) Axial plane, I3–70f kernel, without intravenous contrast material, left kidney—II-ETHIBLOC_Reloaded/iodized oil mixture (1:1); (c) Axial plane, I3–30f kernel, without intravenous contrast material, left kidney—III-ETHIBLOC_Reloaded/ethanol-60% mixture (8:2); (d) Axial plane, I3–70f kernel, without intravenous contrast material, right kidney—IV-Histoacryl/iodized oil mixture (1:3).
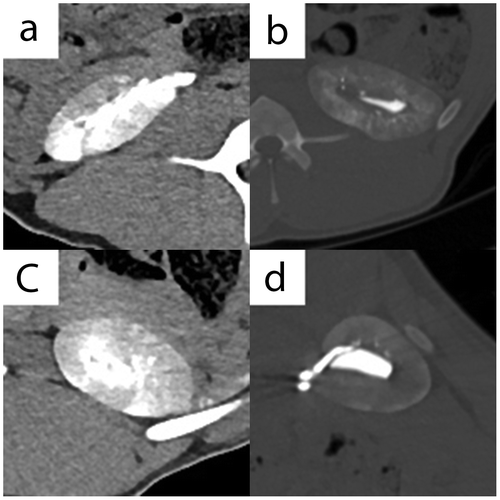
Quantitative CT visibility for the brightest areas within the parenchyma showed the highest numbers in II, followed by IV, and then I and III, with significant differences (Table ; Figure (a)–(d)). Quantitative CT visibility for the darkest areas within the parenchyma showed the highest numbers in II, followed by IV, and then I and III, with significant differences (Table ; Figure (a)–(d)).
Table 2. CT visibility
3.3. Pathology
In all groups, embolic agents were detected in segmental, interlobar and arcuate arteries (Figure (a)–(e)). Additionally interlobular arteries, pre-glomerular arterioles and glomerular capillaries were occluded in I, II and III. Since all embolic agents were acid-fuchsin negative in the AFOG staining, relevant fibrin and thrombus components could be excluded. In the majority of cases, and independent of the arterial level, vascular casting was observed in I, II and III.
Figure 4. Pathology. (a) Gross pathology - I-Pure ETHIBLOC_Reloaded Homogeneous cast within interlobar (black asterisk) and arcuate/interlobular arteries (white asterisk); (b) Histopathology - EvG staining - I-Pure ETHIBLOC_Reloaded The EvG positive embolic agent shows almost complete occlusion of a segmental artery (solid black arrow); (c) Histopathology - AFOG staining - IV-Histoacryl/iodized oil mixture (1:3). The acid-fuchsin negative embolic agent shows only partial occlusion of a segmental artery (solid black arrow); (d) Histopathology - EvG staining - II-ETHIBLOC_Reloaded/iodized oil mixture (1:1). The embolic agent is inked black with almost complete occlusion of an interlobular artery (empty black arrow) and a glomerular capillary (solid black arrow); (e) Histopathology - EvG staining - III-ETHIBLOC_Reloaded/ethanol-60% mixture (8:2). The embolic agent is inked black with almost complete occlusion of a pre-glomerular arteriole (empty black arrow).

4. Discussion
ETHIBLOC_Reloaded as a re-designed zein-based fluid embolic agent proved safety and efficacy for transarterial embolization of the pig kidney. The precision to reach the embolization endpoint was “high” or “very high” although the resistance during microcatheter injection was “intermediate” to “very high”. Adequate X-ray visibility was another feature of ETHIBLOC_Reloaded. With ETHIBLOC_Reloaded, either as ready for use product or as handmade emulsion after mixture with iodized oil or ethanol-60%, a combination of capillary, pre-capillary and central embolization can be obtained. Compared with Histoacryl as control, both X-ray visibility and vascular occlusion pattern are superior for ETHIBLOC_Reloaded. Our results confirm the hypothesis that ETHIBLOC_Reloaded can be used safely and effectively for transarterial embolization of the pig kidney.
In the original Ethibloc, the hydrophobic occlusion material zein is dissolved in ethanol. After contact with blood, zein precipitates and occludes the target vessel (Kauffmann & Richter, Citation1986; Kauffmann, Richter, Rohrbach, & Wenz, Citation1989; Rassweiler et al., Citation1980; Richter et al., Citation1981, Citation1984). To better control this effect, a pre-injection of glucose is mandatory. Glucose delays the precipitation of original Ethibloc, which is caused by osmotic effects (for example, attachment of water molecules to the glucose and deceleration of the dissolution of ethanol) (Mironov, Citation1990). After embolization, an additional injection of glucose is required to flush the catheter and to push the original Ethibloc deeper into the arteries (Mironov, Citation1990). Since the precipitation of original Ethibloc is affected by the ethanol component, it is likely that pure ETHIBLOC_Reloaded would show comparable precipitation characteristics as the pure original Ethibloc. The rationale to use the ionic contrast material sodium amidotrizoate in the original Ethibloc was X-ray visibility, but in any case the X-ray visibility was still not optimal. Theoretically, an increase in the portion of sodium amidotrizoate would improve the X-ray visibility. However, only the pure original Ethibloc with a sodium amidotrizoate content of 162 mg/ml resulted in a soft viscous material that is feasible for transmicrocatheter application. A further increase of sodium amidotrizoate would lead to a lower pH value resulting in an inferior tertiary structure and consecutively uncontrollable embolization. Richter et al. (Citation1984) published two options for contrast-enhanced Ethibloc. For option No. 1, original Ethibloc was mixed manually with iodized oil (3:1). For option No. 2, iodized oil replaced oleum papaveris in equal parts during the standardized manufacturing process (resulting in the so-called Ethibloc-N). With ETHIBLOC_Reloaded, the original idea of Ethibloc-N was revived in the form of a ready for use product. Although the chemical components were the same for both materials, ETHIBLOC_Reloaded is the result of a fully automated production line—or with other words of a standardized manufacturing process—whereas Ethibloc-N is the result of a hand-made mixture of chemists. Although option No. 1 and option No. 2 lead to a clear increase of the X-ray visibility (for example, calculated radiodensities of 210 mg iodine/ml for the original Ethibloc/iodized oil mixture and 190 mg iodine/ml for Ethibloc-N [compared with a calculated radiodensitiy of 120 mg iodine/ml for pure original Ethibloc]), the handmade mixture had two relevant drawbacks: deceleration of precipitation time and decrease of viscosity. By contrast, Ethibloc-N maintained the same viscosity as the pure original Ethibloc. The authors concluded that the substituted iodized ester had the same softening function but does not affect negatively product stability, pH level and precipitation characteristics.
Pure ETHIBLOC_Reloaded shows an X-ray visibility that is effective at controlling the embolization endpoint and a viscosity and precipitation time that are effective at reaching occlusion at the level of pre-glomerular arterioles and glomerular capillaries.
As for original Ethibloc, handmade mixture of ETHIBLOC_Reloaded with iodized oil increases X-ray visibility but decreased viscosity and product stability. The handmade mixture of ETHIBLOC_Reloaded with ethanol-60% results in a stable embolic agent, and the decreased viscosity allows easier microcatheter injection while still maintaining X-ray visibility and control during embolization.
In clinical practice, Histoacryl is mixed with iodized oil in different ratios (1:1 to 1:6) in order to improve the X-ray visibility but also to adjust its time to polymerization (Razavi & Murphy, Citation2007; Vanlangenhove et al., Citation2014). In this context, it is noteworthy that although the viscosity of the Histoacryl/iodized oil mixture is clearly lower compared with ETHIBLOC_Reloaded in its different emulsions, it must not be concluded that the arterial penetration depth is higher for Histoacryl. This is explained by the complex and basically different principles of vascular occlusion: precipitation versus polymerization. Because Histoacryl polymerizes after contact with ionic fluids (for example, blood), glucose control is required also (Bellemann et al., Citation2012; Razavi & Murphy, Citation2007; Vanlangenhove et al., Citation2014). To avoid critical issues such as catheter occlusion and sticking, only experienced operators should use Histoacryl (Mine et al., Citation2013).
Limitations of this study include the non-survival design (for example, no data for recanalization, inflammation and necrosis) and the embolization technique (for example, no data for balloon-assistance and segmental embolization). Furthermore, other clinically established fluid embolic agents were not used as controls.
In conclusion, ETHIBLOC_Reloaded is a promising re-designed zein-based embolic agent. Either as ready for use product or as handmade emulsion after mixture with iodized oil or ethanol-60%, it can be used safely and effectively for a combination of capillary, pre-capillary and central embolization of the pig kidney. Further controlled trials are warranted in order to describe long-term outcome and clinical success.
Additional information
Funding
Notes on contributors
Christof M. Sommer
Priv. -Doz. Dr. med. Christof M. Sommer is a board- certified radiologist with a clinical and scientific focus on interventional oncology, emergency radiology and endovascular management of vascular malformations, peripheral arterial occlusive disease and aortic disease. After graduating from medical school (Ruperto Carola University Heidelberg, Heidelberg, Germany), he received a research fellowship in vascular and interventional radiology at the New York Presbyterian/Weill Cornell Med Center, New York, USA, and completed his doctoral thesis. During specialist training in Heidelberg (University Hospital Heidelberg, Radiologic Clinic, Clinic of Diagnostic and Interventional Radiology, Heidelberg, Germany) and Stuttgart (Stuttgart Clinics, Center of Radiology, Clinic of Diagnostic and Interventional Radiology, Stuttgart, Germany), Dr. Sommer was awarded the Werner Porstmann Price from the German Roentgen Society and the Venia Legendi in radiology at the Ruperto Carola University Heidelberg. Currently, Dr. Sommer is attending interventional radiologist in Heidelberg and Stuttgart.
References
- Bellemann, N., Stampfl, U., Sommer, C. M., Kauczor, H. U., Schemmer, P., & Radeleff, B. A. (2012). Portal vein embolization using a histoacryl/lipiodol mixture before right liver resection. Digestive Surgery, 29, 236–242.10.1159/000339748
- Bilbao, J. I., de Luis, E., García de Jalón, J. A., de Martino, A., Lozano, M. D., de la Cuesta, A. M., Sangro, B. (2008). Comparative study of four different spherical embolic particles in an animal model: A morphologic and histologic evaluation. Journal of Vascular and Interventional Radiology, 19, 1625–1638.10.1016/j.jvir.2008.07.014
- Bilbao, J. I., de Martino, A., de Luis, E., Díaz-Dorronsoro, L., Alonso-Burgos, A., Martínez de la Cuesta, A., … De Jalón, J. A. G. (2009). Biocompatibility, inflammatory response, and recannalization characteristics of nonradioactive resin microspheres: Histological findings. CardioVascular and Interventional Radiology, 32, 727–736.10.1007/s00270-009-9592-9
- Bücheler, E., Hupe, W., Klosterhalfen, H., Altenähr, E., & Erbe, W. (1978). Neue Substanz zur therapeutischen Embolisation von Nierentumoren. [A new substance for the therapeutic embolisation of renal tumours] RöFo - Fortschritte auf dem Gebiet der Röntgenstrahlen und der bildgebenden Verfahren, 128, 599–603.10.1055/s-0029-1230912
- Buck, J., Rassweiler, J., Leibbrand, M., Miller, K., & Eisenberger, F. (1986). Die persistierende Varikozele nach hoher Ligatur der Vena spermatica interna. [Persistent varicocele following high ligation of the internal spermatic vein. Phlebography and vaso-occlusion with ethibloc] RöFo - Fortschritte auf dem Gebiet der Röntgenstrahlen und der bildgebenden Verfahren, 144, 263–267.10.1055/s-2008-1048785
- Buecker, A., Keulers, P., & Guenther, R. W. (1997). Successful closure and embolization of a fistula between the pancreatic duct and a pseudocyst using ethibloc. CardioVascular and Interventional Radiology, 20, 394–396.10.1007/s002709900176
- Emran, M. A., Dubois, J., Laberge, L., Al-Jazaeri, A., Bütter, A., & Yazbeck, S. (2006). Alcoholic solution of zein (Ethibloc) sclerotherapy for treatment of lymphangiomas in children. Journal of Pediatric Surgery, 41, 975–979.10.1016/j.jpedsurg.2006.01.019
- Gebhardt, C., & Stolte, M. (1978). Occlusion of pancreatic duct system by injection of a fast-solidifying amino acid solution (author’s transl). Langenbecks Archives of Surgery, 346, 149–166.10.1007/BF01261238
- George, H. L., Unnikrishnan, P. N., Garg, N. K., Sampath, J. S., Bass, A., & Bruce, C. E. (2009). Long-term follow-up of Ethibloc injection in aneurysmal bone cysts. Journal of Pediatric Orthopaedics B, 18, 375–380.10.1097/BPB.0b013e32832f724c
- Herbreteau, D. (2007). Indications et limites des techniques d'embolisation dans les malformations vasculaires pédiatriques [Pediatric vascular malformations: Embolization]. Archives de Pédiatrie, 14, 712–714.10.1016/j.arcped.2007.02.041
- Kauffmann, G. W., & Richter, G. M. (1986). Palliative capillary embolization in renal carcinoma. Annales de Radiologie, 29, 205–207.
- Kauffmann, G. W., Richter, G. M., Rohrbach, R., & Wenz, W. (1989). Prolonged survival following palliative renal tumor embolization by capillary occlusion. Cardiovascular and Interventional Radiology, 12, 22–28.10.1007/BF02577121
- Krämer, S. C., Görich, J., Rilinger, N., Siech, M., Aschoff, A. J., Vogel, J., Brambs, H.-J. (2000). Embolization for gastrointestinal hemorrhages. European Radiology, 10, 802–805.10.1007/s003300051007
- Mine, T., Murata, S., Nakazawa, K., Onozawa, S., Ueda, T., Miyauchi, M., … Kumita, S. (2013). Glue embolization for gastroduodenal ulcer bleeding: Contribution to hemodynamics and healing process. Acta Radiologica, 54, 934–938.10.1177/0284185113484644
- Mironov, A. (1990). Embolization of meningiomas using liquid embolizing agents. RöFo - Fortschritte auf dem Gebiet der Röntgenstrahlen und der bildgebenden Verfahren, 153, 327–334.10.1055/s-2008-1033386
- Radeleff, B., Schawo, S., Hoffmann, K., Schemmer, P., Noeldge, G., Kauffmann, G. W., … Richter, G. M. (2008). Efficacy and safety of percutaneous transhepatic portal embolization before right liver resection using an ethibloc/lipiodol mixture: A single-center experience. Digestive Surgery, 25, 52–59.10.1159/000118795
- Rassweiler, J., Kauffmann, G. W., & Rohrbach, R. (1980). Kapillare Embolisation. RöFo - Fortschritte auf dem Gebiet der Röntgenstrahlen und der bildgebenden Verfahren [Capillary embolization. Part I: Occlusion of the entire arterial system of the rat kidney] RöFo - Fortschritte auf dem Gebiet der Röntgenstrahlen und der bildgebenden Verfahren, 133, 644–653.10.1055/s-2008-1056808
- Rassweiler, J., Prager, P., Haferkamp, A., Alken, P., Kauffmann, G. W., & Richter, G. (2008). Transarterial nephrectomy: The current status of experimental and clinical studies. Journal of Endourology, 22, 767–782.10.1089/end.2007.9826
- Razavi, M. K., & Murphy, K. (2007). Embolization of bronchial arteries with N-Butyl cyanoacrylate for management of massive hemoptysis: A technical review. Techniques in Vascular and Interventional Radiology, 10, 276–282.10.1053/j.tvir.2008.03.006
- Richter, G., Rohrbach, R., Kauffmann, G. W., & Raßweiler, J. (1981). Capillary embolization. Part II. Occlusion of the entire arterial system of experimentally induced renal tumors. RöFo - Fortschritte auf dem Gebiet der Röntgenstrahlen und der bildgebenden Verfahren, 135, 85–97.10.1055/s-2008-1056836
- Richter, G., Rassweiler, J., Kauffmann, G. W., Wenz, W., & Crawford, D. B. (1984). Experimental study of the effectiveness of capillary embolization using contrast-enhanced Ethibloc®. Investigative Radiology, 19, 36–44.10.1097/00004424-198401000-00009
- Sommer, C. M., Pallwein-Prettner, L., Vollherbst, D. F., Seidel, R., Rieder, C., Radeleff, B. A., … Pereira, P. L. (2017). Transarterial embolization (TAE) as add-on to percutaneous radiofrequency ablation (RFA) for the treatment of renal tumors: Review of the literature, overview of state-of-the-art embolization materials and further perspective of advanced image-guided tumor ablation. European Journal of Radiology, 86, 143–162.10.1016/j.ejrad.2016.10.024
- Sommer, C. M., Stampfl, U., Bellemann, N., Holzschuh, M., Kueller, A., Bluemmel, J., … Radeleff, B. A. (2013). Multimodal visibility (radiography, computed tomography, and magnetic resonance imaging) of microspheres for transarterial embolization tested in porcine kidneys. Investigative Radiology, 48, 213–222.
- Sommer, C. M., Stampfl, U., Bellemann, N., Ramsauer, S., Loenard, B. M., Haferkamp, A., … Radeleff, B. A. (2010). Patients with life-threatening arterial renal hemorrhage: CT angiography and catheter angiography with subsequent superselective embolization. CardioVascular and Interventional Radiology, 33, 498–508.10.1007/s00270-009-9787-0
- Vanlangenhove, P., Everaert, K., Van Maele, G., & Defreyne, L. (2014). Tolerance of glue embolization under local anesthesia in varicoceles: A comparative study of two different cyanoacrylates. European Journal of Radiology, 83, 559–563.10.1016/j.ejrad.2013.11.018
- Woo, S., Yoon, C. J., Chung, J. W., Kang, S. G., Jae, H. J., Kim, H. C., … Woo, Y. N. (2013). Bronchial artery embolization to control hemoptysis: Comparison of N-Butyl-2-Cyanoacrylate and polyvinyl alcohol particles. Radiology, 269, 594–602.10.1148/radiol.13130046
