Abstract
Despite growing scientific evidence that essential oils possess important therapeutic benefits, research on their biological activities in complex human disease models is scarce. To enhance understanding in this regard, we analyzed the biological activities of an essential oil blend (EOB) in validated human cocultures with or without tumor cells. These disease models allow for measurement of changes in protein biomarkers induced by EOB treatment. This EOB is primarily composed of essential oils from frankincense resin, sweet orange peel, litsea fruit, thyme plant oil, clove bud, summer savory plant, and niaouli leaf. EOB showed significant effects on levels of important biomarkers related to inflammation, immune response, tissue remodeling, and tumor biology. In tumor cocultures, EOB treatment resulted in elevated inflammation- and immune-related biomarkers, including soluble interleukin (sIL)-17A, sIL-2, sIL-6, vascular cell adhesion molecule-1 (VCAM-1), cluster of differentiation (CD)40, CD69, soluble granzyme B (sGranB), and soluble interferon-gamma (sIFN-γ). However, several of these same biomarkers were decreased in EOB-treated nontumor cell cocultures, suggesting that EOB exhibits tumor-specific immune enhancement. In conclusion, EOB may potentially impact human cells through anti-inflammatory activities, immune enhancing functions, and modulation of wound healing.
Public Interest Statement
Essential oils have become more popular globally for health reasons. Our study examined the effects of an essential oil blend (EOB) on several human cell systems that mimic different diseases. These effects of the EOB were determined by measuring levels of biomarkers that are linked to inflammation, immune function, wound healing, and cancer biology. We found that the EOB had strong anti-inflammatory, immune modulatory, and wound healing activities. More interestingly, this EOB showed tumor-specific immune boosting activity, a feature of immunotherapy that is a common treatment for many cancers. Findings from this study suggest that essential oils may be a good therapeutic candidate for inflammatory, immune, and cancerous diseases. Advanced exploration of the health benefits of essential oils may lead to viable options for fighting many of these diseases. Thus, this study provides an important stepping stone for further research on essential oils and their health benefits for human beings.
Competing interest
Xuesheng Han and Tory Parker are employees of dōTERRA, where the study agent, EOB, was manufactured.
1. Introduction
Many experimental studies on essential oils and their biological activities have examined only individual essential oils or their constituents in single cell lines or mouse models (Hong et al., Citation2014; Kathirvel & Ravi, Citation2012). However, cell lines alone do not model primary disease biology, and mouse models do not accurately reflect regulatory networks present in human disease (Chong, Alegre, Miller, & Fairchild, Citation2013; Mak, Evaniew, & Ghert, Citation2014). Human cell coculture systems can compensate for these limitations by combining healthy host cells, disease cells (e.g. tumor cells), and disease-relevant stimuli to mimic host-disease microenvironments (Bergamini et al., Citation2012). The combining of multiple essential oils into what is known as an essential oil blend (EOB) is a common practice among aromatherapists, alternative medicine practitioners, and mainstream essential oils companies. It has been assumed that such combining of essential oils can lead to greater therapeutic benefits as a result of the additive or potentially synergistic actions provided by the blended oils. However, this assumption still remains to be tested in systems that mimic human host-disease biology.
For these reasons, we chose to study the effects of an EOB in human cell coculture systems. The present study was designed to assess the biological activities of EOB in several well-validated human cell cocultures that have been successfully used to measure the effects of a variety of chemical compounds on inflammation and other immunomodulatory processes (Berg et al., Citation2010; Bergamini et al., Citation2012). We analyzed the effects of EOB on dozens of protein biomarkers in these cell coculture systems. This experimental approach allowed us to determine whether EOB can modulate a variety of intra- and extracellular regulatory pathways in ways that are not predictable by looking at the individual EOB components and that can potentially benefit human health.
2. Materials and methods
All experiments were conducted in the BioMAP platform, a set of primary human cell systems designed to model disease biology in a robust and reproducible way. The systems consist of three components, a cell type or cell types (many systems involve cocultures), molecular stimuli to create the disease environment, and a set of biomarker (protein) readouts to examine how treatments impact that disease environment (Berg et al., Citation2010).
2.1. Cell cultures
Primary human (H) cells (i.e. neonatal dermal fibroblasts [HNDFs], umbilical venule endothelial cells [HUVECs], peripheral blood mononuclear cells [PBMCs], and B cells) were obtained as previously described (Bergamini et al., Citation2012). HNDFs were plated in low serum conditions 24 h before stimulation with cytokines. HUVECs were obtained from Cascade Biologics (Portland, OR, USA), cultured in endothelial cell growth medium-2 containing manufacturer-provided supplements and 2% heat-inactivated fetal bovine serum (Hyclone, Logan, UT, USA), and then subcultured with 0.05% trypsin/0.53 mM ethylenediaminetetraacetic acid (Mediatech, Herndon, VA, USA) according to the manufacturer’s instructions. PBMCs were prepared from buffy coats (Stanford Blood Bank, Stanford, CA, USA) by centrifugation over Hisopaque-1077 (Sigma-Aldrich, St. Louis, MO, USA) (Bergamini et al., Citation2012). HT-29, a colorectal cancer (CRC) cell line, was obtained from the American Type Culture Collection and maintained according to their recommended protocol.
Stimulatory molecules for these cell coculture systems were as follows: T-cell receptor (TCR) ligands (1×) for SAg (PBMCs + HUVECs), immunoglobulin M antigens and TCR ligands (0.001 ×) for BT (B cells + PBMCs), TCR ligands (0.001×) for HDFSAg (HNDFs + PBMCs), IL-2 and TCR ligands (0.1×) for /TH2 (T helper cell 2 blasts + HUVECs), and TCR ligands (0.001×) for StroHT29 (CRC cell line + PBMCs + HNDFs) and VascHT29 (CRC cell line + PBMCs + HUVECs). Cell culture and stimulation conditions for the BT, SAg, HDFSAg, and /TH2 coculture assays have been described in detail elsewhere and were performed in a 96-well format (Bergamini et al., Citation2012; R Development Core Team, Citation2011). For the StroHT29 system, PBMCs primed with a low level of superantigen were added to a coculture of HNDFs and HT-29 cells and then cultured for 48 h. For the VascHT29 system, PBMCs primed with a low level of superantigen were added to a coculture of HUVECs and HT-29 cells and cultured for 48 h.
2.2. Protein-based readouts
An enzyme-linked immunosorbent assay (ELISA) was used to measure levels of various cell markers. Soluble factors in supernatants were quantified using either homogeneous time-resolved fluorescence detection, bead-based multiplex immunoassay, or capture ELISA. Overt adverse effects of test agents on cell proliferation and viability (cytotoxicity) were measured by sulforhodamine B (SRB) assay for adherent cells and alamarBlue staining for cells in suspension. For proliferation assays, individual cell types were cultured at subconfluence and measured at time points optimized for each system (48–96 h). Detailed information has been described elsewhere (Bergamini et al., Citation2012). Measurements were performed in triplicate wells.
2.3. Reagents
The EOB (DDR Prime™, dōTERRA International LLC, Pleasant Grove, UT, USA) was diluted in dimethyl sulfoxide (DMSO) to 8× the specified concentrations (Final DMSO concentration was no more than 0.1%). Specifically, 25 μL of each 8× solution was added to the cell culture to yield a final volume of 200 μL. DMSO alone (0.1%) served as the vehicle control. The composition of EOB is as follows: frankincense (a mix of Boswellia carterii, B. frereana, and B. sacra) resin oil, sweet orange (Citrus sinensis) peel oil, litsea (Litsea cubeba) fruit oil, thyme (Thymus vulgaris) plant oil, clove (Eugenia caryophyllata) bud oil, summer savory (Satureja hortensis) plant oil, and niaouli (Melaleuca quinquenervia) leaf oil. The gas chromatography-mass spectrometry analysis of EOB showed that it contained about 23–27% limonene, 11–13% alpha-pinene, 6–8% eugenol, 6–8% thymol, 5–7% carvacrol, 5–7% eucalyptol, 4–6% gamma-terpinene, and smaller amounts of other aromatic compounds.
2.4. Statistical analysis
Quantitative biomarker data are presented as the mean log relative expression level (compared to the respective mean vehicle control value) ± standard deviation (SD) of triplicate measurements. Differences in biomarker levels between EOB- and vehicle-treated cocultures were tested for significance with the unpaired Student’s t test. A p-value < 0.05, with an effect size of at least 10% (more than 0.05 log ratio units), was regarded as statistically significant.
3. Results and discussion
See Tables S1 and S2 in Supplementary material for a glossary of cell cocultures and of biomarkers analyzed in the study.
3.1. Bioactivity profile of EOB in immune-oncology coculture systems
We first analyzed four different EOB concentrations (0.1, 0.033, 0.011, and 0.004%, v/v) in two different oncology (CRC) systems (StroHT29 and VascHT29) for biological activity. These concentrations were chosen in an attempt to find a concentration that would be viable for further in vitro studies. The three highest concentrations yielded >50% reduction in cellular protein levels (by SRB assay), and/or >50% reduction in PBMC viability. These values indicate that EOB was overtly cytotoxic to these cells at these concentrations, and therefore they were excluded from further analysis. Only the 0.004% concentration was used for further analysis of key activities, the results of which are discussed below.
In the StroHT29 system (Figure (A)), EOB significantly increased levels of collagen III and matrix metalloproteinase-9 (MMP-9), two biomarkers related to matrix remodeling activities. Another tissue remodeling biomarker, tissue inhibitor of matrix metalloprotease-2 (TIMP-2), was only marginally increased. EOB increased levels of the following immune-related biomarkers: soluble interleukin (sIL)-17A, sIL-2, sIL-6, and soluble tumor necrosis factor-alpha (sTNF-α). Two tumor-related biomarkers, carcinoembryonic antigen-related cell adhesion molecule-5 (CEACAM5) and keratin 20, were elevated by EOB. In addition, EOB increased levels of tissue plasminogen activator (tPA) and urokinase plasminogen activator (uPA) but decreased the mean level of soluble vascular endothelial growth factor (sVEGF). No significant change in the levels of vascular cell adhesion molecule-1 (VCAM-1), the receptor for uPA (uPAR), collagen I, interferon gamma-induced protein-10 (IP-10), plasminogen activator inhibitor-1 (PAI-1), soluble granzyme B (sGranB), soluble interferon gamma (sIFN-γ), sIL-10, or SRB was observed.
Figure 1. Bioactivity profile of EOB (0.004%, v/v) in two immune-oncology coculture systems, StroHT29 (A) and VascHT29 (B).
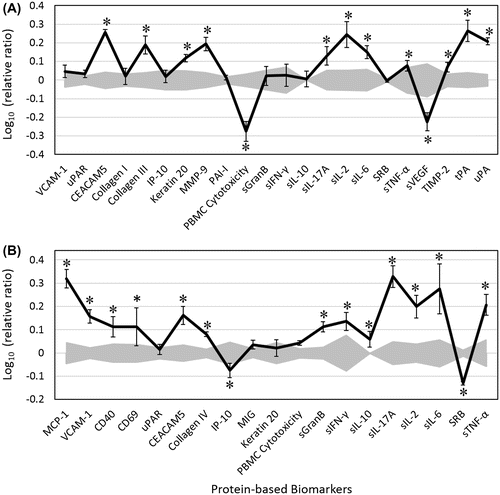
In the VascHT29 system (Figure (B)), EOB significantly increased levels of monocyte chemo-attractant protein-1 (MCP-1), VCAM-1, and cluster of differentiation (CD) proteins (CD40 and CD69), all of which are biomarkers related to immunomodulatory activities. EOB also increased levels of many immune-related biomarkers, including sIFN-γ, sIL-17A, sIL-2, sIL-6, and sTNF-α. Another immune-related biomarker, IP-10, was slightly decreased. Collagen IV (a biomarker for tissue remodeling activity) and CEACAM5 were increased after exposure to EOB. Unlike the StroHT29 system, mean sGranB level was selectively increased in VascHT29 after EOB treatment.
3.2. Bioactivity profile of EOB in autoimmune T cell coculture systems
In the BT system, EOB decreased levels of the immunomodulatory biomarkers, secreted IgG (sIgG), sIL-17A, and sIL-17F, but it slightly increased the level of another immunomodulatory biomarker, sIL-6 (Figure (A)). In the SAg system, EOB was both antiproliferative to T cells and overtly cytotoxic to PBMCs (Figure (B)). EOB also significantly decreased CD40 and slightly but significantly inhibited levels of CD38 (another immune modulatory biomarker) and E-selectin (an inflammation biomarker).
Figure 2. Bioactivity profile of EOB (0.004%, v/v) in the autoimmune T cell coculture systems, BT (A), SAg (B), HDFSAg (C), and /TH2 (D).
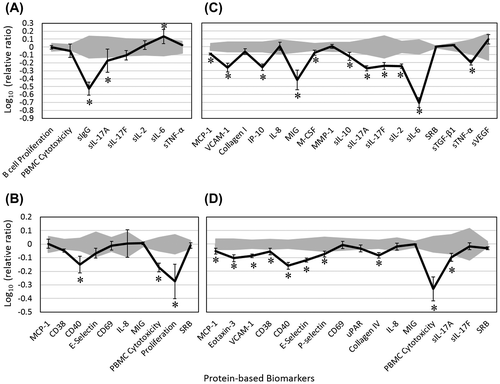
In the HDFSAg system (Figure (C)), EOB inhibited several inflammation-related biomarkers (MCP-1, VCAM-1, IP-10, monokine induced by interferon gamma [MIG] and sTNF-α) as well as immunomodulatory biomarkers (macrophage colony stimulating factor [M-CSF], sIL-17A, sIL-17F, sIL-2, sIL-10, and sIL-6). No significant change in the levels of sIL-8, MMP-1, SRB, or soluble transforming growth factor-beta1 (sTGF-β1) was observed. In addition, EOB decreased the mean level of tissue remodeling biomarker, collagen I, but it slightly increased the mean sVEGF level (both non-significantly). This effect of EOB on sVEGF is the opposite of that observed in the StroHT29 immune-oncology system (Figure A). In the /TH2 system, EOB significantly decreased levels of MCP-1, Eotaxin-3, VCAM-1, E-selectin, and P-selectin, all of which are important inflammation-related biomarkers (Figure (D)). Several immunomodulatory biomarkers, including CD38, CD40, and sIL-17A, were significantly decreased in response to EOB. EOB also decreased levels of collagen IV and was overtly cytotoxic to PBMCs.
3.3. Anti-inflammatory and immune-enhancing properties of EOB
The observed effects of EOB on biomarkers such as VCAM-1, Eotaxin-3, CD40, sIL-17A, and sIL-17F in these preinflamed cell cocultures suggest that EOB might reduce elevated inflammatory responses, such as those that occur in a disease environment. In either murine models or cell cultures, similar anti-inflammatory and immune-enhancing effects of the individual oils or major constituents included in the blend have been reported by other research groups (Chaudhary, Siddiqui, Athar, & Alam, Citation2012; Him, Ozbek, Turel, & Oner, Citation2008; Martin et al., Citation1993; Riella et al., Citation2012; Yoon, Lee, & Hyun, Citation2010). Taken together, the growing literature suggests that essential oils are pharmacologically active and generally inhibitory in multiple models of stimulated inflammatory and immunomodulatory responses, with which the current study is consistent. The finding that EOB significantly impacted these important biomarkers in both cancerous and noncancerous cell cocultures suggests that it may play important roles in both types of disease biology and therefore may provide potential therapeutic benefits to human health.
It is equally important to note that EOB exerted different effects in cancerous and noncancerous cell cultures. Generally, EOB elevated the inflammation- and immunity-related biomarkers (e.g. sIL-17A, sIL-2, sIL-6, VCAM-1, CD40, CD69, sGranB, sTNF-α, and sIFN-γ) in cancerous cell cocultures; however, in the noncancerous cocultures, several of these same biomarkers were inhibited in response to EOB. Specifically, EOB decreased the levels of sIL-17A, sIL-2 and sTNF-α in StroHT29 (CRC cell line + HNDFs + PBMCs), while it increased these levels in HDFSAg (HNDFs + PBMCs) that lacks the cancer cells. Whereas EOB inhibited CD40 production in VascHT29 (CRC cell line + HUVECs + PBMCs), it enhanced CD40 production in SAg (PBMCs + HUVECs). These observations suggest that EOB possesses tumor-specific immune-enhancing potential. The opposite regulatory effects of EOB on these biomarkers in cancerous vs. noncancerous cell cocultures indicate that EOB exerts its effects via different pathways or mechanisms in different disease microenvironments. Further studies are warranted to determine its biological mechanism(s) of action.
4. Conclusions
In primary human cell models of disease, EOB significantly impacted critical biomarkers related to inflammation and immune function. EOB appears to possess tumor-specific immune-enhancing properties, and it may also impact human cells via anti-inflammatory activities and modulation of wound healing. To the best of our knowledge, this is the first study exploring the biological activities of an EOB in complex human cell cocultures. This study provides original and important knowledge of how an EOB affects inflammation- and immune-related biomarkers in validated human cocultures.
Supplementary material
Supplementary material for this article can be accessed here https://doi.10.1080/2331205X.2017.1302909.
Funding
This study was funded by dōTERRA (Pleasant Grove, UT, USA) and conducted at DiscoverX (Fremont, CA, USA).
COGENTMED_-_2016_-_0220_Supplementary_Material.docx
Download MS Word (33.1 KB)Acknowledgments
The authors acknowledge Carsten Smidt, PhD, Jeff Dorsett, MS, Nicole Stevens, MS, and Cody Beaumont, PhD for their critical and constructive reviews of the manuscript.
Additional information
Notes on contributors
Xuesheng Han
At dōTERRA, our group primarily studies the health benefits of essential oils. We are specifically interested in the efficacy and safety of essential oils and their active components. Our studies of essential oils in both in vitro and clinical settings utilize a variety of experimental approaches, including analytical, biological, biochemical, and biomedical methodologies. We work closely with hospitals, clinics, and research institutes towards developing quality essential oils with therapeutic benefits. The research work discussed in this paper represents one part of a large research project, which was designed to extensively examine the impact of essential oils on human cells. This study, along with others, will further the understanding of the health benefits of essential oils for a wide research audience. Dr Han holds a PhD in Biological Sciences and is an elected Fellow of the American College of Nutrition. Dr Price holds a PhD in Pharmacology.
References
- Bergamini, G., Bell, K., Shimamura, S., Werner, T., Cansfield, A., Müller, K., … Neubauer, G. (2012). A selective inhibitor reveals PI3Kγ dependence of TH17 cell differentiation. Nature Chemical Biology, 8, 576–582. doi:10.1038/nchembio.957
- Berg, E. L., Yang, J., Melrose, J., Nguyen, D., Privat, S., Rosler, E., … Ekins, S. (2010). Chemical target and pathway toxicity mechanisms defined in primary human cell systems. Journal of Pharmacological and Toxicological Methods, 61, 3–15. doi:10.1016/j.vascn.2009.10.001
- Chaudhary, S. C., Siddiqui, M. S., Athar, M., & Alam, M. S. (2012). D-Limonene modulates inflammation, oxidative stress and Ras-ERK pathway to inhibit murine skin tumorigenesis. Human & Experimental Toxicology, 31, 798–811. doi:10.1177/0960327111434948
- Chong, A. S., Alegre, M.-L., Miller, M. L., & Fairchild, R. L. (2013). Lessons and limits of mouse models. Cold Spring Harbor Perspectives in Medicine, 3(12), a015495. doi:10.1101/cshperspect.a015495
- Him, A., Ozbek, H., Turel, I., & Oner, A. C. (2008). Antinociceptive activity of alpha-pinene and fenchone. Pharmacologyonline, 3, 363–369.
- Hong, S.-L., Lee, G.-S., Syed Abdul Rahman, S. N., Ahmed Hamdi, O. A., Awang, K., Aznam Nugroho, N., & Abd Malek, S. N. (2014). Essential oil content of the rhizome of Curcuma purpurascens Bl. (Temu Tis) and its antiproliferative effect on selected human carcinoma cell lines. The Scientific World Journal, 2014, 397430. doi:10.1155/2014/397430
- Kathirvel, P., & Ravi, S. (2012). Chemical composition of the essential oil from basil (Ocimum basilicum Linn.) and its in vitro cytotoxicity against HeLa and HEp-2 human cancer cell lines and NIH 3T3 mouse embryonic fibroblasts. Natural Product Research, 26, 1112–1118. doi:10.1080/14786419.2010.545357
- Mak, I. W., Evaniew, N., & Ghert, M. (2014). Lost in translation: Animal models and clinical trials in cancer treatment. American Journal of Translational Research, 6, 114–118.
- Martin, S., Padilla, E., Ocete, M. A., Galvez, J., Jiménez, J., & Zarzuelo, A. (1993). Anti-inflammatory activity of the essential oil of Bupleurum fruticescens. Planta Medica, 59, 533–536. doi:10.1055/s-2006-959755
- R Development Core Team. (2011). R: A language and environment for statistical computing. Vienna: The R Foundation for Statistical Computing. Retrieved from http://www.R-project.org/
- Riella, K. R., Marinho, R. R., Santos, J. S., Pereira-Filho, R. N., Cardoso, J. C., Albuquerque-Junior, R. L. C., & Thomazzi, S. M. (2012). Anti-inflammatory and cicatrizing activities of thymol, a monoterpene of the essential oil from Lippia gracilis, in rodents. Journal of Ethnopharmacology, 143, 656–663. doi:10.1016/j.jep.2012.07.028
- Yoon, W.-J., Lee, N. H., & Hyun, C.-G. (2010). Limonene suppresses lipopolysaccharide-induced production of nitric oxide, prostaglandin E2, and pro-inflammatory cytokines in RAW 264.7 macrophages. Journal of Oleo Science, 59, 415–421.
