Abstract
Objective: Ascites is one of the most frequent complications of cirrhosis. Our study is to investigate the efficacy and safety of the intermittent drainage of ascites for HBV-related cirrhosis patients with tense ascites. Material and methods: A total of 2,169 HBV-related, decompensated liver cirrhosis inpatients were screened from January 2009–January 2014. Of them, 112 cases were eligible, among whom 55 patients (treated group) were administered paracentesis with central venous catheter and drained of 1–2 L ascites every 1 or 2 days (PCVC therapy). The remaining 57 patients served as controls (control group). Results: Compared with the baseline, the level of mean arterial pressure (MAP) in the control group markedly declined (82.27 ± 8.36 mm Hg vs. 91.25 ± 10.82 mm Hg, p = 0.000), and the levels of MAP (79.14 ± 10.30 mm Hg vs. 90.53 ± 10.97 mm Hg, p = 0.000) and hemoglobin (99.29 ± 16.78 g/L vs. 107.55 ± 21.80 g/L, p = 0.028) in the treated group dropped significantly. There were no significant differences in the levels of creatinine, sodium, eGFR, incidence of complications , weight loss, improvement rate, urine output and dosage of albumin between the two groups. The hospitalization time of the treated group was longer (21.49 ± 13.34 days vs. 16.56 ± 9.07 days, p = 0.025), and the amount of furosemide used in the treated group was greater than that used in the control group (47.72 ± 18.53 mg vs. 27.99 ± 13.32 mg, p = 0.000). Conclusions: Cirrhosis patients with tense ascites who received PCVC therapy showed decreased levels of Hb and MAP, but did not induce renal dysfunction. The efficacy of PCVC therapy is not superior to diuretic treatment.
Public interest statement
Ascites is one of the most comment complications for cirrhosis patients. Repeated large-volume paracentesis plus albumin infusion is an effective therapy. But there are still approximately 18.5% of patients suffer from post-paracentesis circulatory dysfunction, which may result in poor outcomes and economic burden. It was previously reported that administering paracentesis by central venous catheter and intermittently draining small volume ascites (PCVC therapy) is safe and effective for refractory ascites patients. However, the influence of this therapy on circulatory and renal functions has rarely been reported. Our research focused on tense ascites patients who were treated with PCVC therapy, and the results showed that this therapy could decrease the levels of hemoglobin and mean arterial pressure, but did not induce renal dysfunction.
Competing Interest
The authors declare no competing interest.
1. Introduction
In Asia, cirrhosis develops at an estimated annual incidence of 2.1–3.8% in hepatitis B virus (HBV) infected patients (Liaw, Tai, Chu, & Chen, Citation1988; Lin et al., Citation2007). Ascites is one of the most frequent complications of cirrhosis, and the survival rate at 5 years after diagnosis is 20% (Ginès et al., Citation1987; Moreau & Lebrec, Citation1999). For patients with tense ascites, repeated large-volume paracentesis (LVPs) combined with albumin infusion is considered to be effective (Fassio et al., Citation1992; Ginès et al., Citation1988; Salerno et al., Citation1987). Additionally, albumin infusion after ascites removal has proven to be the best plasma expander for the prevention of post-paracentesis circulatory dysfunction (PPCD) (Gines et al., Citation1996). Despite this, approximately 18.5% of patients who received albumin infusions still suffered from PPCD (Gines et al., Citation1996). Since circulatory dysfunction may contribute to an increased risk of renal dysfunction and hyponatremia, leading to shorter survival in cirrhosis patients (Pozzi et al., Citation1994), a new therapy that can further reduce PPCD is needed.
In recent years, given the hypothesis that removing a small amount of ascites intermittently may decrease the risk of circulatory dysfunction, some hospitals in China, including ours, have tried to administer paracentesis by central venous catheter to drain a small volume of ascites (1–2 L/1–2 days) intermittently (PCVC therapy) with albumin infusion in tense ascites patients. The results showed that this was safe, convenient and effective (Shao, Qin, & Jiang, Citation2007). However, very few studies have assessed the influence of this therapy on the circulatory and renal functions in tense ascites patients.
Our research evaluates the influence of PCVC therapy on circulatory and renal functions and the efficacy of this therapy for HBV-related liver cirrhosis patients with tense ascites.
2. Methods
2.1. Patients
The patients were identified retrospectively from electronic case-note records of The Third Affiliated Hospital of Sun Yat-sen University. All patients that were diagnosed with decompensated cirrhosis between January 2009 and January 2014 were screened.
Patients with the diagnosis of decompensated cirrhosis (Liaw et al., Citation2008, Citation2012), positive for serum hepatitis B surface antigen, 16–65 years of age and grade 3 ascites according to criterion of EASL (European Association for the Study of the Liver, Citation2010) were included. Exclusion criteria included the following: superinfection of other hepatitis viruses and human immunodeficiency virus (HIV), concomitant autoimmune diseases, presence of other important organ dysfunction (e.g. renal, heart and pulmonary dysfunction) or malignancies, pregnant and lactating women, excessive drinking or taking hepatotoxic drugs within 6 months, complications with portal thrombosis and gastrointestinal bleeding, receipt of LVPs therapy, ascites due to cardiomyopathy, nephropathy or malnutrition, hypertension, tuberculosis and patients with incomplete clinical data.
Demographic data and details of patient treatment regimens were collected. All eligible patients received treatment with antivirus, according to the guideline of Asian Pacific Association for the study of the liver (APASL) (Liaw et al., Citation2008, Citation2012), restriction of sodium intake (0.5–0.8 g/d), diuretics (spironolactone 60–120 mg/d and furosemide 20–60 mg/d) and a supplement of human serum albumin. Patients who received PCVC therapy served as the treated group. The central venous catheter remained in the abdominal cavity for several days, and it would not be removed until the ascites became too little to drain or the patients complained of discomfort from the catheter. Because very few tense ascites patients have been treated by LVPs in our hospital since 2009, we selected patients who received diuretic therapy only as the control group. The data were collected for variables such as hemoglobin (Hb), mean arterial pressure (MAP), creatinine (Cr), serum sodium (Na), certain complications, hospitalization time, and the dosage of ALB and diuretics.
2.2. Ethics
This study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki strictly and was approved by the Ethics Committee on clinical trials of the Ethics Committee of Third Affiliated Hospital of Sun Yat-sen University.
2.3. Study endpoints and definitions
The primary end points were to evaluate the following: (i) the influence on circulatory function, including changes of Hb and MAP; (ii) the influence on renal function, including changes of Cr, Na and estimated glomerular filtration rate eGFR (calculated by CKD-EPI formula (Levey et al., Citation2009)); and (iii) the incidence of certain complications (e.g. abdominal infection, gastrointestinal bleeding, hepatic encephalopathy, hepatorenal syndrome or electrolyte disturbance) during the treatment.
The secondary end points were to evaluate treatment efficacy, including hospitalization time, improvement rate after treatment and the dosage of ALB and diuretics used during hospitalization.
Improvement from the treatment was defined as the decrease in ascites from grade 3–grade 1.
2.4. Statistical analysis
Continuous variables (e.g. age, weight, levels of renal and liver functions and so on) were expressed as the mean ± standard deviation and compared using a t-test. Categorical variables (e.g. incidence of complications, improvement rate of the treatment and so on) were expressed as frequencies and compared using the χ2-test. All data were analyzed using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA), and a value of p < 0.05 was considered statistically significant.
3. Results
3.1. Study profile and patient baseline characteristics
Our research screened a total of 2,169 HBV-related decompensated liver cirrhosis inpatients at The Third Affiliated Hospital of Sun Yat-sen University from January 2009–January 2014. Of them, 419 suffered grade 3 ascites. 112 cases were eligible, among whom 55 cases received PCVC therapy and the remaining 57 patients served as controls. The study profile is shown in Figure . The baseline characteristics of the two groups are shown in Table . Both groups were similar with respect to the majority of variables (e.g. sex, age, renal and liver functions, complications, etc.) except for lower Na levels and HBV DNA quantification in the treated group.
Figure 1. Study profile. A total of 2,169 HBV-related, decompensated liver cirrhosis inpatients were screened from January 2009–January 2014. Of them, 419 had grade 3 ascites, and 112 cases were eligible, among whom 55 cases served as the treated group and received treatment with intermittent drainage of ascites by central venous catheter (PCVC therapy). The remaining 57 patients who did not receive PCVC therapy served as controls.
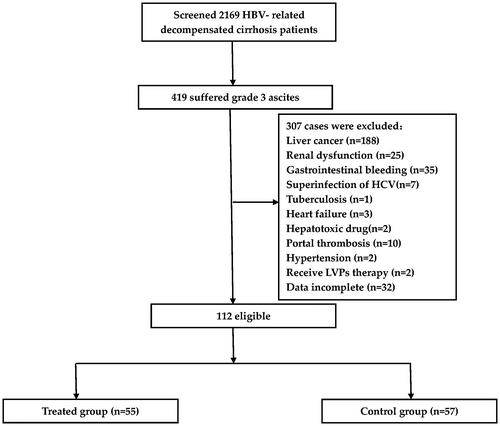
Table 1. Clinical and biochemical indexes of the patients at baseline
3.2. Influence of the treatment on blood volume, renal function and certain complications
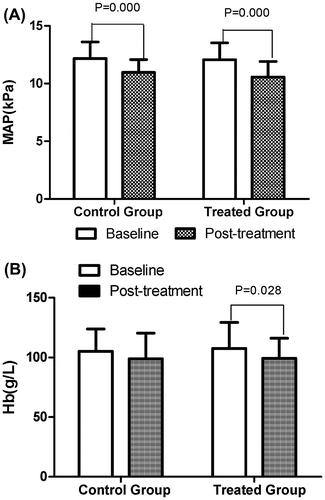
In our study, Hb and MAP were used as the main variables for assessing changes in blood volume. In the control group, the level of MAP dropped significantly (10.97 ± 1.11 mm Hg vs. 12.17 ± 1.44 mm Hg, p = 0.000), while there were no significant differences in the level of Hb compared with the baseline. In the treated group, both Hb (99.29 ± 16.78 g/L vs. 107.55 ± 21.80 g/L, p = 0.028) and MAP (10.55 ± 1.37 mm Hg vs. 12.07 ± 1.46 mm Hg, p = 0.000) levels declined markedly after treatment. No significant differences were found between the two groups in the levels of Hb and MAP after treatment (Figure ).
Figure 2. Comparison of baseline and post-treatment MAP and Hb between the two groups. (A) Compared with the baseline, the levels of MAP dropped significantly after treatment (control group: 82.27 ± 8.36 kPa vs. 91.25 ± 10.82 kPa, p = 0.000; treated group: 79.14 ± 10.30 kPa vs. 90.53 ± 10.97 kPa, p = 0.000) in both groups. (B) Compared with the baseline, the level of Hb in the treated group declined markedly (99.29 ± 16.78 g/L vs. 107.55 ± 21.80 g/L, p = 0.028) after treatment, while no significant difference was found in the control group (98.91 ± 21.44 g/L vs. 105.23 ± 18.57 g/L, p = 0.096).
Compared with the baseline, there were no remarkable differences in the levels of Cr, Na and eGFR in either group (Figure ). Additionally, there were no significant differences in the levels of Cr (77.44 ± 19.80 μmol/L vs. 78.36 ± 21.93 μmol/L, p = 0.491) and eGFR (108.37 ± 36.70 ml/min/1.73 m2 (Lin et al., Citation2007) vs. 107.07 ± 35.14 ml/min/1.73 m2 (Lin et al., Citation2007), p = 0.849) between the two groups after treatment. No significant difference was found in the incidence of complications (e.g. abdominal infection, gastrointestinal bleeding, hepatic encephalopathy, hepatorenal syndrome and electrolyte disturbance) between the two groups during hospitalization (Table ).
Figure 3. Comparison of baseline and post-treatment renal function between the two groups. (A) Compared with the baseline, there was no significant difference in the level of Cr after treatment (control group: 71.81 ± 16.34 μmol/L vs. 77.44 ± 19.80 μmol/L, p = 0.100; treated group: 75.59 ± 20.31 μmol/L vs. 78.36 ± 21.93 μmol/L, p = 0.495). (B) Compared with the baseline, there was no significant difference in the level of eGFR after treatment (control group: 117.06 ± 34.86 ml/min/1.73 m2 vs. 108.37 ± 36.70 ml/min/1.73 m2, p = 0.197; treated group: 110.47 ± 33.12 ml/min/1.73 m2 vs. 107.07 ± 35.14 ml/min/1.73 m2, p = 0.602). (C) Compared with the baseline, there was no significant difference in the level of Na after treatment (control group: 138.26 ± 3.51 mmol/L vs. 137.70 ± 3.88 mmol/L, p = 0.417; treated group: 135.54 ± 4.53 mmol/L vs. 135.96 ± 4.18 mmol/L, p = 0.614).
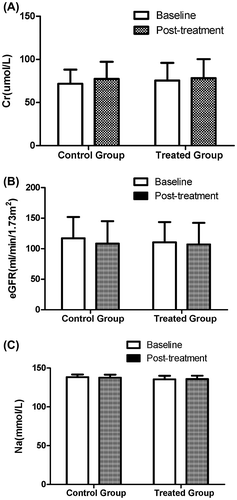
Table 2. Comparison of complications between the 2 groups during the treatment
3.3. Efficacy of the therapy
We compared the improvement rate, hospitalization time, weight loss and urine output between the two groups to evaluate the efficacy of the treatment. The results showed that there were no marked differences in the improvement rate of ascites or average urine output between the two groups. The average weight loss of the two groups was similar. The average hospitalization time of the treated group was longer (21.49 ± 13.34 days vs. 16.56 ± 9.07 days, p = 0.025) than the control group (Table ).
Table 3. Comparison of efficacy between the 2 groups
3.4. Dosage of ALB and diuretics
There were no significant differences in the dosages of ALB and spironolactone between the two groups. However, the dose of furosemide used in the treated group was greater than that of the control group (47.12 ± 18.53 mg/d vs. 27.99 ± 13.32 mg/d, p = 0.000) (Table ).
Table 4. Comparison of dosage of ALB and diuretics between the two groups
4. Discussion
In China, cirrhosis is mainly caused by HBV infection. It was reported that 5–10% cirrhosis patients will develop into refractory ascites (Zeng, Lin, & Xie, Citation2011). In our study, 19.32% cirrhosis patients suffered tense ascites. The percentage is a little higher, but it is reasonable. For that not all the tense ascites patients will progress to refractory ascites. Cirrhosis patients with ascites are associated with poor outcomes. Although antiviral therapy can improve the outcomes of cirrhosis patients (Liaw et al., Citation2011; Shim et al., Citation2010), diuretics and/or paracentesis are still needed for patients with grade 2 or 3 ascites. For grade 3 ascites patients, LVPs is the first treatment of choice. However, the risk of post-paracentesis renal dysfunction and hyponatremia exists, which may be induced by a reduction in effective blood volume and the activation of endogenous vasoconstrictive and anti-natriuretic mechanisms (Schrier et al., Citation1988). Although albumin infusion after LVPs has proven to be effective for decreasing PPCD, the incidence is still as high as 18.5% (Gines et al., Citation1996). We attempted to treat tense ascites patients with PCVC therapy with the purpose of reducing the occurrence of PPCD, and the results showed that the levels of Hb and MAP were significantly reduced in the treated group, but no difference was found in the control group. Additionally, PCVC therapy did not induce renal dysfunction. However, in terms of efficacy, it is not superior to diuretic treatment.
In a study by Pozzi et al. (Citation1994), it was shown that systemic vascular resistance existed and MAP decreased after rapid total paracentesis in tense ascites patients. In a study by Gines et al. Citation1996, PPCD was defined as an increase in plasma renin activity of more than 50% of the pre-treatment value on the sixth day after paracentesis. In our study, Hb and MAP were chosen as the primary variables to evaluate the change in blood volume. Although no differences were found in the levels of Hb and MAP after treatment, the levels of MAP in both groups were reduced compared with the baseline. The level of Hb in the treated group also declined after treatment. Data from five patients (four in the control group and one in the treated group) were complicated by gastrointestinal bleeding during their hospitalization and were not involved in the statistical analysis. The results indirectly indicated that both diuretic and PCVC therapy could lead to a decreased blood volume, which is similar to the results of Pozzi et al. (Citation1994). The main reasons may include the decrease in abdominal pressure after ascites removal and the accentuation of arteriolar vasodilation. The decrease in blood volume may reflexively activate the renin-angiotensin system (Panos et al., Citation1990; Simon et al., Citation1987). Furthermore, patients in the treated group did not receive PCVC therapy alone, as they received diuretic therapy as well. The average urine output of the treated group was similar to that of the control group (Table ). Therefore, we speculated that the volume of ascites removed and urine output may be too large to affect the circulatory blood volume.
The study by Ginès et al. (Citation1988) showed that LVPs plus intravenous albumin did not significantly affect the levels of BUN, Cr and Na after treatment. Our study showed that there were no significant differences in the levels of Cr, Na and eGFR in each group compared with the baseline. Moreover, two patients in each group developed hepatorenal syndrome, and no marked difference was found. Our results were consistent with those of Ginès et al. (Citation1988). For many years, albumin has been used as a plasma expander. Our study showed that albumin infusion after LVPs can effectively prevent the complication of PPCD. Maintaining stable circulatory function is beneficial for renal function. Our results showed that PCVC therapy may decrease the blood volume of patients, but the influence on renal function was mild, which may be mainly related to intravenous albumin injection.
To evaluate the efficacy of this treatment for cirrhosis patients with tense ascites, we recorded and compared the improvement rate, hospitalization time, average weight loss, urine output and the dosage of diuretics between the two groups. The results showed that the hospitalization time of the treated group was longer and the dosage of furosemides used in treated group was greater compared with the control group, which indicated that PCVC therapy for tense ascites cirrhosis patients is not superior to diuretic treatment. The main reasons may be as follows: (i) cirrhosis patients with tense ascites often have complications with portal hypertension and hypoproteinemia, which can generate large amounts of ascites daily, whereas drainage of 1–2 L ascites every 1 or 2 days is not enough to effectively remove ascites and may prolong hospitalization time and increase the dosage of diuretic medicine required; (ii) the amount of albumin infused and the urine output were similar in the two groups, while the total output (including urine output and ascites) in the treated group was greater, indicating that the amount of albumin used in the treated group may be relatively inadequate; and (iii) the catheter in the abdominal cavity may stimulate the peritoneal area, which may increase the generation of ascites. Moreover, there is a risk of infection with the presence of a catheter in the abdominal cavity for long durations (although no significant difference was found in the incidence of abdominal infection).
In summary, using PCVC therapy for liver cirrhosis patients with tense ascites will decrease the levels of Hb and MAP, but will not induce renal dysfunction. Its efficacy is not superior to diuretic treatment. A prospective randomized study, which includes patients receiving LVPs as controls, is needed to make a comprehensive assessment of PCVC therapy.
Funding
This work was supported by Guangzhou Major Project in collaborative innovation of industry [grant number 1561000157].
| List of abbreviations | ||
| HBV | = | hepatitis B virus |
| HIV | = | human immunodeficiency virus |
| WBC | = | white blood cell |
| Hb | = | hemoglobin |
| PLT | = | platelet |
| ALT | = | alanine aminotransferase |
| ALB | = | albumin |
| TBIL | = | total bilirubin |
| INR | = | international normalized ratio |
| CTP | = | Child-Turcotte-Pugh |
| MAP | = | mean arterial pressure |
| MELD | = | model for end-stage liver disease |
| eGFR | = | estimated glomerular filtration rate |
| Cr | = | creatinine |
| APASL | = | Asian Pacific Association for the study of the liver; mean arterial pressure |
| HR | = | heart rate |
| Na | = | serum sodium |
| HBeAg | = | hepatitis B e-antigen |
Acknowledgements
We thank all the patients who participated in this research study.
Author contributions
Jun-feng Chen and Miao Huang contributed equally to this work. Bing-liang Lin, Jun-feng Chen and Miao Huang participated in the design of the study, analysis and interpretation of the data and drafting of the manuscript. Miao Huang participated in the collection, analysis and interpretation of the data. Jun-feng Chen, Shao-quan Zhang, Hui-juan Cao, Wei-zhen Weng, Jing Xiong collected the data. Dong-ying Xie, Zhi-liang Gao participated in the interpretation of data and critical review of the manuscript for important intellectual content.
Additional information
Notes on contributors
Bing-liang Lin
Bing-liang Lin has published peer-reviewed articles in the area of liver failure, liver cirrhosis and liver carcinoma. He and his research team focus on both of the mechanism of liver failure and the new therapy for liver failure, especially in stem cell and immunologic treatment.
References
- European Association for the Study of the Liver. (2010). EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. Journal of Hepatology, 53, 397–417.
- Fassio, E., Terg, R., Landeira, G., Abecasis, R., Salemne, M., Podesta, A., … Kravetz, D. (1992). Paracentesis with dextran 70 vs. paracentesis with albumin in cirrhosis with tense ascites: Results of a randomized study. Journal of Hepatology, 14, 310–316.10.1016/0168-8278(92)90176-P
- Gines, A., Fernandez-Esparrach, G., Monescillo, A., Vila, C., Domenech, E., Abecasis, R., … Gines, P. (1996). Randomized controlled trial comparing albumin, dextran-70 and polygelin in cirrhotic patients with ascites treated by paracentesis. Gastroenterology, 111, 1002–1010.10.1016/S0016-5085(96)70068-9
- Ginès, P., Quintero, E., Arroyo, V., Terés, J., Bruguera, M., Rimola, A., … Rozman, C. (1987). Compensated cirrhosis: Natural history and prognostic factors. Hepatology, 7, 122–128.10.1002/(ISSN)1527-3350
- Ginès, P., Titó, L., Arroyo, V., Planas, R., Panés, J., Viver, J., … Badalamenti, S. (1988). Randomized comparative study of therapeutic paracentesis with and without intravenous albumin in cirrhosis. Gastroenterology, 94, 1493–1502.10.1016/0016-5085(88)90691-9
- Levey, A. S., Stevens, L. A., Schmid, C. H., Zhang, Y. L., Castro, A. F., Feldman, H. I., … Coresh, J. (2009). A new equation to estimate glomerular filtration rate. Annals of Internal Medicine, 150, 604–612.10.7326/0003-4819-150-9-200905050-00006
- Liaw, Y. F., Kao, J. H., Piratvisuth, T., Chan, H. L. Y., Chien, R. N., Liu, C. J., … Amarapurkar, D. (2012). Asian-Pacific consensus statement on the management of chronic hepatitis B: A 2012 update. Hepatology International, 6, 531–561.10.1007/s12072-012-9365-4
- Liaw, Y. F., Leung, N., Kao, J. H., Piratvisuth, T., Gane, E., Han, K. H., … Locarnini, S. (2008). Asian-pacific consensus statement on the management of chronic hepatitis B: A 2008 update. Hepatology International, 2, 263–283.10.1007/s12072-008-9080-3
- Liaw, Y. F., Sheen, I. S., Lee, C. M., Akarca, U. S., Papatheodoridis, G. V., Suet‐Hing Wong, F., … Buti, M. (2011). Tenofovir disoproxil fumarate (TDF), emtricitabine/TDF and entecavir in patients with decompensated chronic hepatitis B liver disease. Hepatology, 53, 62–72.10.1002/hep.23952
- Liaw, Y. F., Tai, D. I., Chu, C. M., & Chen, T. J. (1988). The development of cirrhosis in patients with chronic type B hepatitis: A prospective study. Hepatology, 8, 493–496.10.1002/(ISSN)1527-3350
- Lin, S. M., Yu, M. L., Lee, C. M., Chien, R. N., Sheen, I. S., Chu, C. M., & Liaw, Y. F. (2007). Interferon therapy in HBeAg positive chronic hepatitis reduces cirrhosis and hepatocellular carcinoma. Journal of Hepatology, 46, 45–52.10.1016/j.jhep.2006.08.021
- Moreau, R., & Lebrec, D. (1999). Management of patients with cirrhosis and ascites. Gastroentérologie Clinique Et Biologique, 23, 379–387.
- Panos, M. Z., Moore, K., Vlavianos, P., Chambers, J. B., Anderson, J. V., Gimson, A. E., … Williams, R. (1990). Single, total paracentesis fortense ascites: Sequential hemodynamic changes and right atrial size. Hepatology, 11, 662–667.10.1002/(ISSN)1527-3350
- Pozzi, M., Osculati, G., Boari, G., Serboli, P., Colombo, P., Lambrughi, C, … D’amico, P. (1994). Time course of circulatory and humoral effects of rapid total paracentesis in cirrhotic patients with tense, refractory ascites. Gastroenterology, 106, 709–719.10.1016/0016-5085(94)90706-4
- Salerno, F., Badalamenti, S., Incerti, P., Tempini, S., Restelli, B., Bruno, S., … Roffi, L. (1987). Repeated paracentesis and i.v. albumin infusion to treat ‘‘tense ascites” in cirrhotic patients: A safe alternative therapy. Journal of Hepatology, 5, 102–108.10.1016/S0168-8278(87)80067-3
- Schrier, R. W., Arroyo, V., Bernardi, M., Epstein, M., Henriksen, J. H., & Rodés, J. (1988). Peripheral arterial vasodilatation hypothesis. A proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology, 8, 1151–1157.10.1002/(ISSN)1527-3350
- Shao, L., Qin, X., & Jiang, L. (2007). Application of drainage with central venous catheterization set in hepatic cirrhosis patients with refractory ascites. Journal of Practical Diagnosis and Therapy, 21, 911–913.
- Shim, J. H., Lee, H. C., Kim, K. M., Lim, Y. S., Chung, Y. H., Lee, Y. S., & Suh, D. J. (2010). Efficacy of entecavir in treatment-naive patients with hepatitis B virus-related decompensated cirrhosis. Journal of Hepatology, 52, 176–182.10.1016/j.jhep.2009.11.007
- Simon, D. M., McCain, J. R., Bonkovsky, H. L., Wells, J. O., Hartle, D. K., & Galambos, J. T. (1987). Effects of therapeutic paracentesis on systemic and hepatic hemodynamics and on renal and hormonal function. Hepatology, 7, 423–429.10.1002/(ISSN)1527-3350
- Zeng, X., Lin, Y., & Xie, W.-f. (2011). Management of ascites caused by cirrhosis. Chinese Journal of Digestion, 25, 757–758.
