Abstract
Background: Hypermetabolism, defined as an increase in measured resting energy expenditure (mREE) relative to predicted REE (pREE), is recognised as an important feature of amyotrophic lateral sclerosis (ALS). Previous predictions of REE in ALS have not accounted for differences in fat free mass (FFM). This study aimed to investigate the effect of accounting for FFM on pREE in ALS patients and a matched control population. Methodology and findings: Body composition and pREE data were obtained from 50 ALS and 50 age- and sex-matched healthy control participants. We contrast conventional models for predicting REE that rely on anthropometric measures, age, and sex, with models that predict REE relative to FFM. Given that a significantly lower FFM was observed in ALS, models that consider FFM predicted significantly lower REE in ALS participants when compared to controls. Using Bland-Altman analysis, we demonstrate a prediction bias between models that do not account for FFM between the ALS and control populations. We also demonstrate greater agreement in predictions between control and ALS populations when correcting for FFM. Conclusions/significance: Future studies should correct for reductions in FFM when predicting REE in ALS.
Public Interest Statement
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease that is characterised by the irreversible loss of motor neurons in the brain and spinal cord. This results in the loss of muscle function, contributing to progressive paralysis, considerable disability and death usually within 3–5 years following diagnosis. There is currently no effective treatment and no cure for ALS. Research shows that a change in energy use in ALS can impact the course of disease, with negative energy balance and weight loss being associated with a more rapid disease progression. Weight loss in ALS is thought to occur as a consequence of decreased energy intake and increased energy use (also called hypermetabolism). By understanding the cause and consequences of hypermetabolism in ALS, it is hoped that more effective intervention strategies can be developed to slow down and treat ALS.
Competing Interest
The authors declare no competing interest.
1. Introduction
Amyotrophic lateral sclerosis (ALS) is a progressive neuromuscular disease characterised by the death of motor neurones. This results in paralysis and death within an average of 2–5 years following diagnosis (Mitchell & Borasio, Citation2007). In recent years, there has been interest in the role of metabolism in ALS. Weight loss and calorie deficit is associated with shorter survival (Ioannides, Ngo, Henderson, McCombe, & Steyn, Citation2016), whereas intervention strategies that increase energy supply in ALS appear to slow disease progression and improve disease outcome (Wills et al., Citation2014). Underlying these observations are reports of widespread undernutrition (Genton, Viatte, Janssens, Heritier, & Pichard, Citation2011) and the presence of hypermetabolism (an increase in measured resting energy expenditure (mREE) relative to predicted REE (pREE)) in some ALS patients (Bouteloup et al., Citation2009; Desport, Torny, Lacoste, Preux, & Couratier, Citation2005; Desport et al., Citation2001; Funalot, Desport, Sturtz, Camu, & Couratier, Citation2009; Kasarskis, Berryman, Vanderleest, Schneider, & McClain, Citation1996; Kasarskis et al., Citation2014). Given an increased focus on identifying and understanding the factors that compromise energy homeostasis in ALS, improvements in our capacity to predict the energy needs of ALS patients are needed.
Predictions of REE inform research into weight loss and hypermetabolism. However, the use of standard population-based predictions models to determine pREE in ALS may not be valid, with studies reporting large prediction errors when relying on conventional prediction models (Sherman, Pillai, Jackson, & Heiman-Patterson, Citation2004). This is of particular importance when identifying hypermetabolism in ALS, where predictions of REE rely predominantly on the Harris-Benedict equation (Bouteloup et al., Citation2009; Desport et al., Citation2001, Citation2005, Funalot et al., Citation2009; Kasarskis et al., Citation1996, Citation2014). While accounting for weight, height, age, and sex, the Harris-Benedict model does not account for fat free mass (FFM), the greatest determinant of REE (Cunningham, Citation1991; Donahoo, Levine, & Melanson, Citation2004; Heymsfield et al., Citation2012; Johnstone, Murison, Duncan, Rance, & Speakman, Citation2005; Wang et al., Citation2000). Given the loss of muscle mass in ALS (Desport, Preux, Truong, & Courat, Citation2000; Desport et al., Citation1999; Jawaid et al., Citation2010; Kasarskis et al., Citation1996; Nau, Bromberg, Forshew, & Katch, Citation1995), the aim of this study was to investigate the effect of accounting for body composition on predictions of REE in ALS. Using body composition and pREE data from 50 ALS and 50 age- and sex-matched healthy control participants, we contrast prediction models that consider anthropometric data, age and sex, with models that predict REE based on FFM (Table ). We demonstrate that predictions of REE in control and ALS participants are impacted by body composition, and that change in FFM in ALS confounds predictions of REE when using models that do not correct for FFM. Thus, corrections for body composition should be considered in studies that predict REE in ALS.
Table 1. Comparison of key factors considered when predicting resting energy expenditure (pREE) using the Harris Benedict, Mifflin-St Jeor and Nelson prediction models, and models that correct for fat free mass (FFM)
2. Methodology
2.1. Participants
Fifty ALS patients who fulfilled the revised El Escorial criteria for probable or definite ALS (Brooks, Miller, Swash, & Munsat, Citation2000) and electrodiagnostic criteria (de Carvalho et al., Citation2008) were recruited from the Royal Brisbane and Women’s Hospital (RBWH) Motor Neurone Disease research clinic. Exclusion criteria were use of gastrostomy and severe respiratory impairment. Disease duration was defined as the interval between the onset of the first symptom (as reported by the patient) and the day of assessment. The revised ALS functional rating scale (ALSFRS-R) (Cedarbaum et al., Citation1999) was recorded on the day of assessment. Fifty healthy age- and sex- matched adult volunteers were also assessed. Assessments were performed at 8 a.m. after an overnight (>12 h) fast. The fasting status of participants was confirmed by declaration from the individual. The University of Queensland and RBWH human research ethics committees approved this study (201,500,0022, 16th Jan 2016; HREC/14/QRBW/495, 5th Jan 2015). Written informed consent was obtained from all participants prior to assessment. Demographic data for all participants is shown in Table .
Table 2. Demographics, anthropometric, body composition and predicted resting energy expenditure for control and ALS participants
2.2. Body composition assessment
Body composition was determined by whole body air displacement plethysmography (Fields, Goran, & McCrory, Citation2002; Lowry & Tomiyama, Citation2015) using the BodPod system (Cosmed USA Inc.) (von Hurst et al., Citation2016). Following measurement of body mass, two body volume measurements were taken with participants seated inside the BodPod chamber. The predicted thoracic gas volume was subtracted to give the non-gas body volume and, following calculation of body density, fat mass (FM) and FFM were determined using the Siri algorithm (Siri, Citation1961). Full-quality assurance and calibration procedures were carried out according to the manufacturers instructions immediately prior to testing.
2.3. Prediction models of REE
pREE for each participant was derived using three prediction models that are commonly used. Harris and Benedict (Citation1918) and Mifflin et al. (Citation1990) prediction models take account of weight, height, age and sex. We used a proprietary adaptation (Cosmed) of the Nelson prediction model (Nelson, Weinsier, Long, & Schutz, Citation1992) that accounts for measures of FFM and FM, expressing final values in kcal/day. pREE was also determined using 15 prediction models that correct for FFM, as described previously (Wang et al., Citation2000). The mean pREE of these prediction models were used to generate an averaged pREE for predictions using FFM (Table ).
2.4. Statistical methods
Statistical analyses were performed using Prism software version 7 (Graphpad Inc., CA, USA). Comparisons between ALS and control groups were performed using unpaired students t-test. The agreement between pREE generated from each model was assessed by the Bland-Altman approach. Plotting the difference vs. the mean of each prediction model, we evaluated the bias between the mean differences, and presented an estimate of the 95% agreement interval within which all differences fall (Giavarina, Citation2015). Further comparisons between prediction models were performed using one-way ANOVA, with multiple comparisons corrected using Tukey post hoc analysis. For all outcomes, a p value <0.05 was considered statistically significant.
3. Results
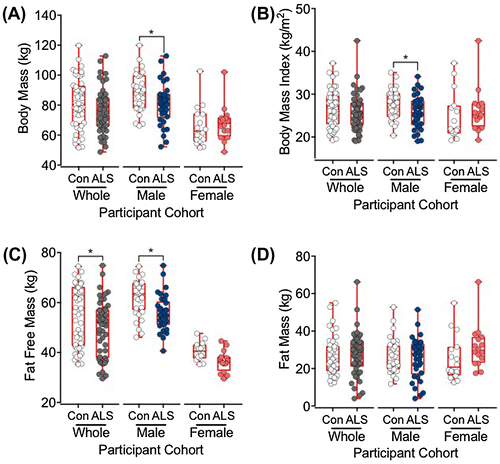
Demographic, anthropometric, body composition and pREE data are shown in Table . ALS and control cohorts were matched for sex (34 male, 16 female; control and ALS cohorts) and age (p = 0.82). In the ALS group, 70% of participants had limb-onset disease and 30% had bulbar-onset disease. As expected (McCombe & Henderson, Citation2010), there was a higher incidence of bulbar-onset disease in the female ALS participants (37.5% of females and 26.5% of males). The mean ALSFRS-R score was 38.2 ± 0.6 (38.7 ± 0.6 male, 37.3 ± 1.2 female). Average disease duration from time of symptom onset was ~25 months. Body mass (p = 0.02) and body mass index (BMI) (p = 0.03) were significantly lower in the male ALS cohort than the male control cohort. FFM was significantly lower in the ALS cohort than the control cohort (p = 0.03) (Table ), and in male ALS patients than the male control participants (p < 0.01). Figure illustrates distributions of measures for body mass (Figure (A)), BMI (Figure (B)) and body composition (fat free mass, Figure (C); fat mass, Figure (D)).
Figure 1. Box and whisker plots showing the distributions of body mass (A), body mass index (BMI; B), fat free mass (C), and fat mass (D) in control (Con) and amyotrophic lateral sclerosis (ALS) participants. The box represents the lower, median and upper quartiles, and the whiskers illustrate the lowest to the highest observation.
3.1. REE prediction model results
Predictions of REE were comparable across the ALS and control cohorts when using the Harris-Benedict and Mifflin-St Jeor models (Table ). The Harris-Benedict and Mifflin-St Jeor models predicted a lower REE in the male ALS cohort when compared to the male control cohort (Table , p = 0.04). This difference is most likely attributed to the difference in body mass and BMI between male ALS and control cohorts. Predictions of REE were significantly lower in all ALS cohorts relative to the control cohorts when using the Nelson prediction model (Table , whole cohort p = 0.02, male cohort p < 0.01, female cohort p = 0.04). These data likely reflect the overall lower FFM observed in ALS participants. Indeed, pREE was lower across all cohorts when correcting for FFM (FFM specific; Table ).
Using Bland-Altman analysis, we demonstrated the impact of correcting for FFM. When contrasted with predictions that correct for FFM, the Harris-Benedict (Figure (A)) and Mifflin-St Jeor (Figure (B)) predictions of REE presented a greater mean bias and a wider range for 95% limits of agreement. The mean bias and range for 95% limits were exaggerated in the ALS population. By contrast, comparable low mean bias and narrow ranges for 95% limits of agreement within control and ALS participants was observed when comparing models that correct for FFM against the Nelson prediction model (Figure (C)). This indicates that correcting for FFM improves predictions of REE. Interestingly, when compared to the Nelson prediction model, models that correct for FFM underestimated REE in all individuals with lower FFM (Figure (C)). This is likely a consequence of corrections for the metabolic load of FM specific to the Nelson prediction model.
Figure 2. Bland-Altman plots illustrating the bias between predicted resting energy expenditure (pREE) outcomes using the Harris-Benedict (A), Mifflin-St Jeor (B), and Nelson (C) prediction models, and predictions of REE following correction for fat free mass (FFM). Plots demonstrate the mean bias (MB, broken red line) of prediction models and estimated 95% agreement intervals (broken grey lines) between prediction models.
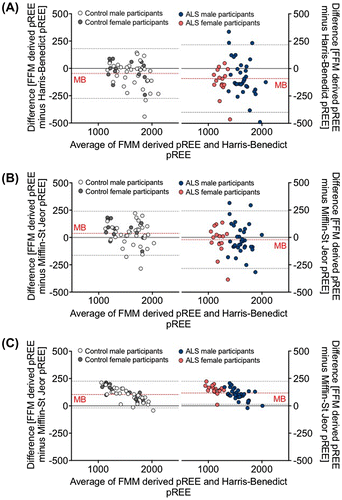
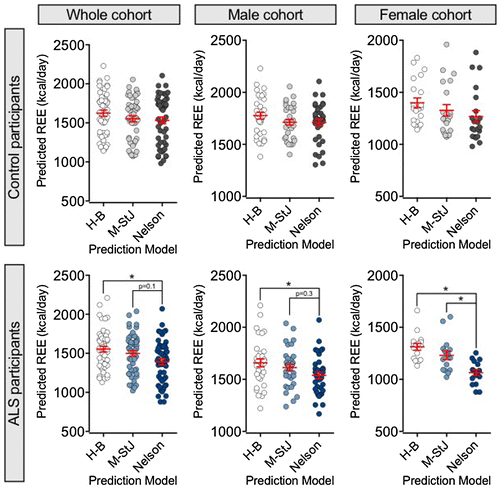
Figure demonstrates differences in prediction outcomes in each cohort and in male and female participants. No significant differences in pREE were observed in the control cohort when using conventional prediction models. By contrast, the Nelson prediction model consistently predicted a lower REE in the ALS cohort when compared to the Harris-Benedict prediction model (p < 0.05 for all participant groups). In female ALS participants, the Nelson prediction model predicted a significantly lower REE than the Mifflin-St Jeor prediction model (p < 0.01), suggesting that the Mifflin-St Jeor model may not accurately correct for FFM in some female ALS participants.
Figure 3. Scatter plots illustrating differences in predicted resting energy expenditure (pREE) outcomes when using the Harris-Benedict, Mifflin-St Jeor and Nelson prediction models in control (top panels) and ALS (bottom panels) participants.
3.2. Bland Altman plots of each pREE model
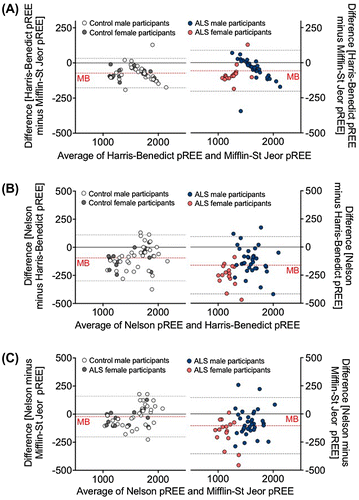
Bland-Altman plots shown in Figure contrast prediction outcomes between the Harris-Benedict and Mifflin-St Jeor models vs. the Nelson prediction model. When comparing pREE derived from the Harris-Benedict and Mifflin-St Jeor predictions (Figure (A)) there was a low mean bias and a narrow range for 95% limits of agreement between the two prediction models. This suggests that the agreement between the Harris-Benedict and Mifflin-St Jeor prediction models was reasonable in both the control and ALS cohort. By contrast, comparison of pREE using the Nelson prediction model with pREE from either the Harris-Benedict (Figure (B)) or Mifflin-St Jeor (Figure (C)) prediction models demonstrate a greater mean bias and wider interval range for 95% limits of agreement for both control and ALS cohorts. A greater mean bias and wider interval range for 95% limits of agreement was also observed in the ALS population relative to the control population when contrasting pREE generated using the Nelson prediction model to the Harris-Benedict (Figure (B)) and Mifflin-St Jeor (Figure (C)) models. Thus, a greater level of disagreement is observed in ALS when contrasting conventional models that consider anthropometric measures, age and sex, with a model that accounts for body composition.
Figure 4. Bland-Altman plots illustrating the bias between predicted resting energy expenditure (pREE) outcomes using the Harris-Benedict and Mifflin-St Jeor (A), Nelson and Harris-Benedict (B), and Nelson and Mifflin-St Jeor prediction models (C). Plots demonstrate the mean bias (MB, broken red line) of prediction models and estimated 95% agreement intervals (broken grey lines) between prediction models.
4. Discussion
Weight loss and hypermetabolism are increasingly recognised as important features of ALS (Ioannides et al., Citation2016). Identification of hypermetabolism in any population is critically dependent on predictions of REE. While various models for predicting REE exist, previous studies have highlighted the complexities of model selection in predicting REE, demonstrating that the choice of prediction model can influence study outcomes (Frankenfield et al., Citation2007; Miller, Milliron, & Woolf, Citation2013; Weijs, Citation2008; Weijs & Vansant, Citation2010). Thus, selection of the most suitable model for predicting REE should consider specific characteristics that modify REE in the population of interest.
We contrasted the Harris-Benedict, Mifflin-St Jeor, and Nelson models in predicting REE within, and between a control and ALS population. Contrasting each model to an average derived from 15 predictions that account for FFM (the main determinant of REE) (Wang et al., Citation2000), we demonstrate that changes in FFM in ALS greatly impact predictions of REE. Mean pREE was highest when using the Harris-Benedict prediction model, followed by the Mifflin-St Jeor prediction model and then the Nelson prediction model. Given that FFM was significantly lower in our ALS cohort, these observations likely reflect an overestimation in pREE by the Harris-Benedict and Mifflin-St Jeor prediction models, which do not correct for changes in FFM. Indeed, when using a prediction model that corrects for FFM (the Nelson prediction model), we found a closer agreement in predictions of REE between control and ALS cohorts. Given that body mass, BMI and FFM were lower in the male ALS cohort when compared to male control cohort, it is not surprising that all models predicted a lower REE in male ALS participants when compared to age-matched male controls. While no differences in body mass, BMI or FFM were observed between the female ALS and female control cohorts, the Nelson model still predicted an overall lower REE for ALS participants compared to control participants.
To further interrogate the differences in prediction outcome between these models we conducted a series of Bland-Altman analyses. We observed close agreement between the Harris-Benedict and Mifflin-St Jeor models in both ALS and control cohorts. In this instance, prediction outcomes likely reflect assumptions centered on weight and height, which are key determinants used to calculate BMI. When comparing the Nelson predictions of REE with the Harris-Benedict and Mifflin-St Jeor predictions, we found a greater disagreement in model outcomes in ALS participants when compared to control participants. Again, differences in pREE generated from these models likely reflect the lower FFM observed in the ALS cohort, and in particular the lower FFM observed in male ALS participants when contrasted to control participants. It should be noted that the Nelson prediction models also corrects for FM, and thus may further adjust for possible differences in FM between ALS and control populations. This was not directly assessed.
This study was not designed to determine the accuracy of each model in predicting REE relative to measured REE. Rather, by contrasting models for predicting REE in a population that is subject to differences in body composition (Desport et al., Citation1999, Citation2000; Jawaid et al., Citation2010; Kasarskis et al., Citation1996; Nau et al., Citation1995), we demonstrate that reductions in FFM critically impact predictions of REE. While the Harris-Benedict prediction model has previously been used to predict REE in studies that identify hypermetabolism in ALS (Bouteloup et al., Citation2009; Desport et al., Citation2001, Citation2005; Funalot et al., Citation2009; Kasarskis et al., Citation1996, Citation2014), significant neurogenic muscle atrophy highlights the need to consider body composition when predicting REE in ALS. Our observations raise the possibility that studies that do not account for body composition in the prediction of REE in ALS might overestimate pREE and lead to an underestimation of the incidence of hypermetabolism. Whether the use of models that consider body composition for predicting REE in ALS will influence reports of the prevalence of hypermetabolism in ALS remains to be determined. It is likely that improvements in predicting hypermetabolism in ALS could better inform research outcomes to advance insights into pathophysiological processes associated with disease.
Funding
This work was funded by Grants-in-Aid from the Motor Neurone Disease Research Institute of Australia (MNDRIA; the Cunningham Collaboration MND research Grant and the Cunningham Family Research Grant) to STN, FJS, PAM and RDH, Royal Brisbane & Women’s Hospital Foundation grants to RDH and the University of Queensland. STN acknowledges the support of a Scott Sullivan MND Research Fellowship funded by The MND and Me Foundation, The Royal Brisbane & Women’s Hospital Foundation, and the Queensland Brain Institute. ZAI is supported by The Australian and New Zealand Association of Neurologists (ANZAN) Education and Research Foundation/National Health and Medical Research Council (NHMRC) Postgraduate Scholarship. We thank Kathryn Thorpe, Susan Heggie and Nicole Hutchinson (RBWH), and Vicki Allen and Lisa Dingwall (CCR) for their support and assistance. We extend our sincerest gratitude to all ALS patients and control volunteers who participated in this study.
Additional information
Notes on contributors
F.J. Steyn
We conduct preclinical and clinical studies, researching factors that modify disease progression in ALS. As part of this process we are studying the impact of altered metabolism on disease progression. Here we address the important issue of altered energy needs in ALS. Existing studies that predict the resting energy needs of ALS patients do not correct for the loss of muscle mass that is seen in ALS. We show that changes in fat free mass (a key component of whole body energy use) in ALS compromises the accuracy of methods that are commonly used for predicting resting energy needs. Using a prediction model that corrects for fat free mass, we provide critical methodological insights to improve identification of altered energy needs in ALS. We are now conducting studies to identify the cause and effect of hypermetabolism in ALS.
References
- Bouteloup, C., Desport, J. C., Clavelou, P., Guy, N., Derumeaux-Burel, H., Ferrier, A., & Couratier, P. (2009). Hypermetabolism in ALS patients: An early and persistent phenomenon. Journal of Neurology, 256, 1236–1242.10.1007/s00415-009-5100-z
- Brooks, B. R., Miller, R. G., Swash, M., & Munsat, T. L. (2000). El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders, 1, 293–299.10.1080/146608200300079536
- Cedarbaum, J. M., Stambler, N., Malta, E., Fuller, C., Hilt, D., Thurmond, B., … 1A complete listing of the BDNF Study Group. (1999). The ALSFRS-R: A revised ALS functional rating scale that incorporates assessments of respiratory function. Journal of the Neurological Sciences, 169, 13–21.10.1016/S0022-510X(99)00210-5
- Cunningham, J. J. (1991). Body composition as a determinant of energy expenditure: A synthetic review and a proposed general prediction equation. The American Journal of Clinical Nutrition, 54, 963.
- de Carvalho, M., Dengler, R., Eisen, A., England, J. D., Kaji, R., Kimura, J., … Swash, M. (2008). Electrodiagnostic criteria for diagnosis of ALS. Clinical Neurophysiology, 119, 497–503.10.1016/j.clinph.2007.09.143
- Desport, J. C., Preux, P. M., Magy, L., Boirie, Y., Vallat, J. M., Beaufrere, B., Couratier, P. (2001). Factors correlated with hypermetabolism in patients with amyotrophic lateral sclerosis. The American Journal of Clinical Nutrition, 74, 328–334.
- Desport, J. C., Preux, P. M., Truong, C. T., & Courat, L. (2000). Nutritional assessment and survival in ALS patients. Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders, 1, 91–96.
- Desport, J. C., Preux, P. M., Truong, T. C., Vallat, J. M., Sautereau, D., & Couratier, P. (1999). Nutritional status is a prognostic factor for survival in ALS patients. Neurology, 53, 1059–1059.10.1212/WNL.53.5.1059
- Desport, J. C., Torny, F., Lacoste, M., Preux, P. M., & Couratier, P. (2005). Hypermetabolism in ALS: Correlations with clinical and paraclinical parameters. Neurodegenerative Diseases, 2, 202–207.
- Donahoo, W. T., Levine, J. A., & Melanson, E. L. (2004). Variability in energy expenditure and its components. Current Opinion in Clinical Nutrition and Metabolic Care, 7, 599–605.10.1097/00075197-200411000-00003
- Fields, D. A., Goran, M. I., & McCrory, M. A. (2002). Body-composition assessment via air-displacement plethysmography in adults and children: A review. The American Journal of Clinical Nutrition, 75, 453–467.
- Frankenfield, D., Hise, M., Malone, A., Russell, M., Gradwell, E., & Compher, C. (2007). Prediction of resting metabolic rate in critically ill adult patients: Results of a systematic review of the evidence. Journal of the American Dietetic Association, 107, 1552–1561.10.1016/j.jada.2007.06.010
- Funalot, B., Desport, J. C., Sturtz, F., Camu, W., & Couratier, P. (2009). High metabolic level in patients with familial amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis, 10, 113–117.10.1080/17482960802295192
- Genton, L., Viatte, V., Janssens, J. P., Heritier, A. C., & Pichard, C. (2011). Nutritional state, energy intakes and energy expenditure of amyotrophic lateral sclerosis (ALS) patients. Clinical Nutrition, 30, 553–559.10.1016/j.clnu.2011.06.004
- Giavarina, D. (2015). Understanding Bland Altman analysis. Biochemia Medica, 25, 141–151.10.11613/issn.1846-7482
- Harris, J. A., & Benedict, F. G. (1918). A biometric study of human basal metabolism. Proceedings of the National Academy of Sciences, 4, 370–373.10.1073/pnas.4.12.370
- Heymsfield, S. B., Thomas, D., Bosy-Westphal, A., Shen, W., Peterson, C. M., & Muller, M. J. (2012). Evolving concepts on adjusting human resting energy expenditure measurements for body size. Obesity Reviews, 13, 1001–1014.10.1111/obr.2012.13.issue-11
- Ioannides, Z. A., Ngo, S. T., Henderson, R. D., McCombe, P. A., & Steyn, F. J. (2016). Altered metabolic homeostasis in amyotrophic lateral sclerosis: Mechanisms of energy imbalance and contribution to disease progression. Neurodegenerative Diseases, 16, 382–397.10.1159/000446502
- Jawaid, A., Murthy, S., Wilson, A., Qureshi, S., Amro, M. J., Wheaton, M., … Schulz, P. E. (2010). A decrease in body mass index is associated with faster progression of motor symptoms and shorter survival in ALS. Neurology, 74, A207–A208.
- Johnstone, A. M., Murison, S. D., Duncan, J. S., Rance, K. A., & Speakman, J. R. (2005). Factors influencing variation in basal metabolic rate include fat-free mass, fat mass, age, and circulating thyroxine but not sex, circulating leptin, or triiodothyronine. The American Journal of Clinical Nutrition, 82, 941–948.
- Kasarskis, E. J., Berryman, S., Vanderleest, J. G., Schneider, A. R., & McClain, C. J. (1996). Nutritional status of patients with amyotrophic lateral sclerosis: Relation to the proximity of death. The American Journal of Clinical Nutrition, 63, 130–137.
- Kasarskis, E. J., Mendiondo, M. S., Matthews, D. E., Mitsumoto, H., Tandan, R., Simmons, Z., … ALS Nutrition/NIPPV Study Group. (2014). Estimating daily energy expenditure in individuals with amyotrophic lateral sclerosis. American Journal of Clinical Nutrition, 99, 792–803.10.3945/ajcn.113.069997
- Lowry, D. W., & Tomiyama, A. J. (2015). Air displacement plethysmography versus dual-energy x-ray absorptiometry in underweight, normal-weight, and overweight/obese individuals. PLoS One, 10, e0115086.10.1371/journal.pone.0115086
- McCombe, P. A., & Henderson, R. D. (2010). Effects of gender in amyotrophic lateral sclerosis. Gender Medicine, 7, 557–570.10.1016/j.genm.2010.11.010
- Mifflin, M. D., St Jeor, S. T., Hill, L. A., Scott, B. J., Daugherty, S. A., & Koh, Y. O. (1990). A new predictive equation for resting energy expenditure in healthy individuals. The American Journal of Clinical Nutrition, 51, 241–247.
- Miller, S., Milliron, B. J., & Woolf, K. (2013). Common prediction equations overestimate measured resting metabolic rate in young hispanic women. Topics in Clinical Nutrition, 28, 120–135.10.1097/TIN.0b013e31828d7a1b
- Mitchell, J. D., & Borasio, G. D. (2007). Amyotrophic lateral sclerosis. The Lancet, 369, 2031–2041.10.1016/S0140-6736(07)60944-1
- Nau, K. L., Bromberg, M. B., Forshew, D. A., & Katch, V. L. (1995). Individuals with amyotrophic lateral sclerosis are in caloric balance despite losses in mass. Journal of the Neurological Sciences, 129, 47–49.10.1016/0022-510X(95)00061-6
- Nelson, K. M., Weinsier, R. L., Long, C. L., & Schutz, Y. (1992). Prediction of resting energy expenditure from fat-free mass and fat mass. The American Journal of Clinical Nutrition, 56, 848–856.
- Sherman, M. S., Pillai, A., Jackson, A., & Heiman-Patterson, T. (2004). Standard equations are not accurate in assessing resting energy expenditure in patients with amyotrophic lateral sclerosis. Journal of Parenteral and Enteral Nutrition, 28, 442–446.10.1177/0148607104028006442
- Siri, W. E. (1961). Body composition from fluid spaces and density: Analysis of methods. Techniques for measuring body composition (pp. 223–224). Washington, DC: National Academy of Sciences, National Research Council.
- von Hurst, P. R., Walsh, D. C. I., Conlon, C. A., Ingram, M., Kruger, R., & Stonehouse, W. (2016). Validity and reliability of bioelectrical impedance analysis to estimate body fat percentage against air displacement plethysmography and dual-energy X-ray absorptiometry. Nutrition & Dietetics, 73, 197–204.10.1111/1747-0080.12172
- Wang, Z., Heshka, S., Gallagher, D., Boozer, C. N., Kotler, D. P., & Heymsfield, S. B. (2000). Resting energy expenditure-fat-free mass relationship: New insights provided by body composition modeling. American Journal of Physiology - Endocrinology and Metabolism, 279, E539–E545.
- Weijs, P. J. M. (2008). Validity of predictive equations for resting energy expenditure in US and Dutch overweight and obese class I and II adults aged 18-65 y. The American Journal of Clinical Nutrition, 88, 959.
- Weijs, P. J. M., & Vansant, G. A. A. M. (2010). Validity of predictive equations for resting energy expenditure in Belgian normal weight to morbid obese women. Clinical Nutrition, 29, 347–351.10.1016/j.clnu.2009.09.009
- Wills, A. M., Hubbard, J., Macklin, E. A., Glass, J., Tandan, R., Simpson, E. P., … Hanes, G. P. (2014). Hypercaloric enteral nutrition in patients with amyotrophic lateral sclerosis: A randomised, double-blind, placebo-controlled phase 2 trial. The Lancet, 383, 2065–2072.10.1016/S0140-6736(14)60222-1
