Abstract
Sacral neuromodulation (SNM) is a minimally invasive therapy for defecatory disorders. PEAK Plasma Blade™ (Medtronic) has been used as an alternative to the conventional electric scalpel and scissors in 3 procedures of neuromodulation. PEAK PlasmaBlade™ uses less total energy, operating at significantly lower temperatures than electrosurgical technology. No damage to the wires or pulse generators or patient complications were detected during the surgery or follow-up. A reduction in the duration of the procedures was noted.
Public Interest Statement
This article describes the use of a new scalpel, PEAK PlasmaBlade™ (Medtronic), during the surgical procedure for sacral neuromodulation. This scalpel uses less total energy and operates at significantly lower temperatures than electrosurgical technology, making it more secure for the implant or exchanges of electrical implantable devices, avoiding possible damages.
Competing Interests
Arantxa Muñoz-Duyos has received honoraria from Medtronic (Minneapolis, MN) as speaker. Laura Lagares-Tena declares no competing interest.
1. Introduction
Sacral neuromodulation (SNM) has been described as an effective treatment for urinary (Van Kerrebroeck & Marcelissen, Citation2012) and fecal incontinence (Altomare et al., Citation2015). Other indications are still under evaluation, such as chronic constipation or chronic pelvic pain.
This method consists in implanting an electrode adjacent to a sacral root and its connection to a pulse generator (PG). The technique has undergone several improvements since its first application in 1981 for urinary incontinence treatment. Initially it required a 10 cm incision to locate the sacral foramina and adjust the electrode. Currently, it is performed with a simpler percutaneous introduction using an acute stimulation needle, a guide and an introducer. Fluoroscopy is used to determine the electrode location and to avoid sacral plexus damage.
SNM therapy comprises two phases: a testing evaluation period or Percutaneal Nerve Evaluation (PNE) and a second stage with the permanent PG implantation. For the PNE phase a tined lead is percutaneously placed to lie adjacent to a sacral nerve root (S4-S3-S2) and it is then tunneled subcutaneously to be connected to an external PG (Verify® External Neurostimulator #3531. Medtronic, Minneapolis, MN, USA). After three weeks, if the PNE has positive results, this tined lead is connected to the implantable PG (InterStim® Neurostimulator #3023 or Interstim® II Neurostimulator #3058. Medtronic, Minneapolis, MN, USA) sited in a subcutaneous pocket created in the superior-lateral part of the buttock. This implantable PG has a shelf life of five years, so a procedure for battery exchange must be performed periodically. The InterStim® Neurostimulator can also be used for dynamic graciloplasty.
The manipulation of the leads and the PG requires a meticulous dissection in order not to cause any damage to the equipment. The procedure could potentially be difficult after the PNE phase when the tissues are still inflamed. The use of electrosurgical scalpel is not recommended as it may cause damage to the protection cover of the lead (Lim et al., Citation2009). Thus, a blunt dissection with scissors is performed when isolating the leads and PG from surrounding tissues, which does not exclude the risk of cutting the wire.
With this study we wanted to evaluate the application of a new dissecting technology, the PEAK PlasmaBlade™ (Medtronic, Minneapolis, MN, USA), which uses less total energy and operates at a significantly lower temperature than the traditional electrosurgical technology.
The PEAK PlasmaBlade™ is a surgical device that uses very brief (40 μs range), high-frequency pulses of radio frequency (RF) energy to induce electrical plasma along the edge of a thin (12.5 μm), 99.5% insulated blade electrode. Due to the low duty cycle from RF pulsing and proprietary Thermal Protection Shield (TPS) insulating technology, the PEAK PlasmaBlade™ uses less total energy.
2. Method and clinical cases
The PlasmaBlade was first put to test in a chicken breast. An electrode was implanted into the meat to try out its dissection (Experimental test). With this test the integrity of the electrode even contacting with the scalpel was demonstrated. Actually, the electrical transmission was automatically stopped once the tip of the scalpel contacted with the electrode. Moreover, PlasmaBlade operated at significantly lower temperatures than electrosurgical technology (40–170°C vs. 200–350°C) (Video 1 ).
).
Video 1. Chicken breast test. The PlasmaBlade operates without damaging the electrode.
Afterwards, three patients underwent PG implant using the PEAK PlasmaBlade™.
Case 1: A 51-year-old woman with obstructive defecation due to rectal hyposensitivity underwent a successful tined lead evaluation of S3 neuromodulation in September 2015. The patient was selected for a PG implant.
Case 2: A 51-year-old woman was diagnosed with severe neurologic fecal incontinence. The patient was implanted after a successful tined lead test in 2010 in the left S3 nerve root. After five years, the patient referred a relapse in her symptoms. Low battery level was detected and a PG exchange was performed.
Case 3: A 55-year-old woman was born with imperforated anus that was corrected by surgery in the perinatal period. With congenital fecal incontinence, the patient underwent non-dynamic graciloplasty (right leg) in 1975 and in 2006 a dynamic graciloplasty (left leg) was implanted with a PG for gracilis muscle training. In 2011, an exchange of the PG was needed due to the low battery level. After a new relapse of symptoms low battery was again detected so a new PG exchange was scheduled.
All patients were fully informed about the procedure and possible side effects and signed an informed consent form.
Surgical time was recorded and compared with three other cases operated on using the standard procedure of scissor dissection. Patients were followed up one week and one month after the procedure in order to record possible complications.
3. Results
An experimental test was first executed to try out an electrode dissection. No damage to the lead was observed after its dissection with the PlasmaBlade.
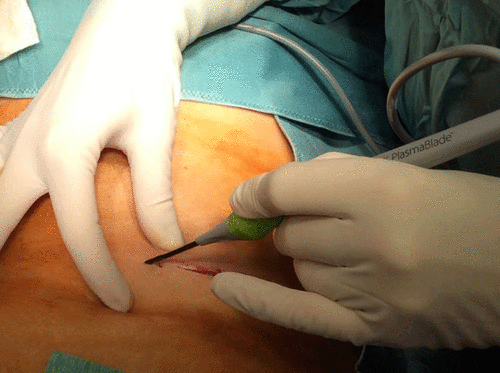
In all three operations, better coagulation was detected using the cut function of the PlasmaBlade when dissecting the skin and subcutaneous tissues compared to our previous experience with electrical scalpel (Figure ).
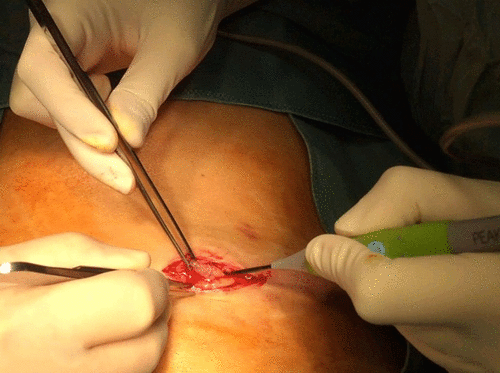
On several occasions there was direct contact between the plasma scalpel and the leads or the neurostimulator (Figure ) with no physical damage to the wire, nor to the electrical circuit, as shown by an impedance test that was run (Figure ).
Figure 3. Impedance test run after implantation of a PG. Impedance values should be between 400 and 4,000 ohms.

Furthermore, the procedure duration was shorter than when using an electrical scalpel and scissors as in previous cases: 25 min with PlasmaBlade compared to 35 min with the classical approach during PG implant; no differences were found during PG replacement.
No surgical wound-related complications were detected during the follow-up.
4. Discussion
The reduced thermal injury depth, inflammatory response, and scar width in healing skin with PlasmaBlade technology compared to electrosurgery has been previously described (Ruidiaz et al., Citation2011).
The lower energy of the plasma scalpel reduces the risk of damage and facilitates the surgeon’s work, reducing the procedure time. This observation is in agreement with the results obtained with the PlasmaBlade in PG replacement for cardiac diseases (Kypta et al., Citation2015). However, no evidence has previously been published about its use in neuromodulation.
5. Conclusion
The PEAK PlasmaBlade™ seems to be a useful tool for PG implantation and replacement in neuromodulation as it reduces the risk of electrode damage.
Funding
The authors received no direct funding for this research.
Additional information
Notes on contributors
Arantxa Muñoz-Duyos
The Colorectal Surgery Unit of Hospital Universitari MútuaTerrassa has been focused on pelvic floor disorders for more than 20 years, being one of the reference centers for these disorders in Spain. The team has a large experience in sacral neuromodulation, which is a minimally invasive therapy for treating fecal incontinence and other defecatory, urinary and pain disorders. This procedure implies the use of very sensible implantable material (wires and batteries) that can benefit from the use of low-energy scalpel as the one described in this paper.
References
- Altomare, D. F., Giuratrabocchetta, S., Knowles, C. H., Muñoz Duyos, A., Robert-Yap, J., Matzel, K. E., on behalf of the European SNS Outcome Study Group. (2015). Long-term outcomes of sacral nerve stimulation for faecal incontinence. British Journal of Surgery, 102, 407–415.10.1002/bjs.2015.102.issue-4
- Kypta, A., Blessberger, H., Saleh, K., Hönig, S., Kammler, J., Neeser, K., & Steinwender, C. (2015). An electrical plasma surgery tool for device replacement-retrospective evaluation of complications and economic evaluation of costs and resource use. Pacing and Clinical Electrophysiology, 38, 28–34.10.1111/pace.2015.38.issue-1
- Lim, K. K., Reddy, S., Desai, S., Smelley, M., Kim, S. S., Beshai, J. F., … Knight, B. P. (2009). Effects of electrocautery on transvenous lead insulation materials. Journal of Cardiovascular Electrophysiology, 20, 429–435.10.1111/jce.2009.20.issue-4
- Ruidiaz, M. E., Messmer, D., Atmodjo, D. Y., Vose, J. G., Huang, E. J., Kummel, A. C., … Gurtner, G. C. (2011). Comparative healing of human cutaneous surgical incisions created by the PEAK PlasmaBlade, conventional electrosurgery, and a standard scalpel. Plastic and Reconstructive Surgery, 128, 104–111.10.1097/PRS.0b013e31821741ed
- Van Kerrebroeck, P. E., & Marcelissen, T. A. (2012). Sacral neuromodulation for lower urinary tract dysfunction. World Journal of Urology, 30, 445–450.10.1007/s00345-011-0780-2