Abstract
Objectives: We aimed to study if interleukin (IL) 8 and hepatocyte growth factor (HGF) predict development of severe acute pancreatitis (SAP) among patients without organ dysfunction (OD) at presentation, and if they discriminate transient OD from persistent OD among patients presenting with OD. Methods: From prospectively collected cohort of 176 AP patients and 32 healthy controls, plasma levels of IL-8 and HGF were determined within 5 days after symptom onset using an enzyme-linked immunosorbent assay. Results: AP was severe in 23 patients, of whom 10 did not have clinical signs of OD at presentation. IL-8 and HGF levels increased along with the severity of AP (P < 0.001). In patients without OD at study entry, IL-8 and HGF values predicted the development of SAP with the AUCs of 0.73 (95% CI, 0.56–0.91) and 0.79 (95% CI, 0.66–0.93), respectively. Of all patients, 22 presented with OD, and among them IL-8 predicted persistence of OD with the AUC of 0.88 (95% CI 0.69–1.0). Combining IL-8 and HGF did not improve the models. Conclusions: In AP patients without OD at presentation, circulating levels of IL-8, or HGF, may predict the development of SAP. In patients presenting with OD, IL-8 level may discriminate the patients with transient OD from those with persistent OD.
Public Interest Statement
Severe acute pancreatitis (SAP) is potentially a life-threatening disease, but early identification of such patients may improve the prognosis. However, about half of such patients have only mild symptoms on admission to hospital, which may cause a delay in starting the optimal care. At the moment, we are lacking a reliable laboratory marker that could help in these situations. The levels of inflammatory mediators, such as cytokines, rise early in the blood after onset of acute pancreatitis and may function as such early predictors. In the present study, we evaluated interleukin 8 (IL-8) and hepatocyte growth factor (HGF) as early predictors of SAP. Our main finding was, that IL-8, or HGF, predict development of SAP before clinical signs of organ dysfunction have developed. Thus, determining IL-8 or HGF levels in the blood on admission to hospital may help in identifying the patients who need special attention.
Competing Interests
The authors declare no competing interest.
1. Introduction
Most patients with acute pancreatitis (AP) have a mild disease, as defined by the revised Atlanta classification, and recover uneventfully with conservative management (Banks et al., Citation2013). Morbidity and mortality are largely associated with severe forms of AP including moderately severe AP and severe AP (SAP). Moderately severe AP is characterized by local or systemic complication, with or without the presence of transient organ dysfunction (OD) resolving within 48 h follow-up. Mortality is rare (Vege et al., Citation2009). In SAP, OD is persistent (>48 h) with a mortality as high as 36–50% (Buter, Imrie, Carter, Evans, & McKay, Citation2002; Johnson & Abu-Hilal, Citation2004). In the case of multiple organ dysfunction (MOD) the mortality may be even higher (Halonen et al., Citation2002; Mc Kay & Buter, Citation2003).
The pathogenesis of AP remains elusive. The theory of premature trypsinogen activation leading to acinar cell damage and further promotion of inflammation has been challenged by recent experimental data suggesting novel pathways, such as the activation of NF-κB being responsible for the inflammatory response in AP (Rakonczay, Hegyi, Takacs, McCarroll, & Saluja, Citation2008; Sah, Dawra, & Saluja, Citation2013). Consequently, local inflammatory cells become activated and a variety of inflammatory mediators are generated. The local response may lead to a systemic one, presented as systemic inflammatory response syndrome (SIRS), which is usually self-limiting, but in a small group of patients it may deteriorate to an excessive and uncontrolled systemic inflammatory reaction, as occurs in SAP (Kylanpaa, Rakonczay, & O’Reilly, Citation2012).
Close monitoring and early moderately aggressive fluid resuscitation may improve the prognosis of SAP (Haydock et al., Citation2013). Although numerous biomarker candidates, as well as clinical and radiological scoring systems have emerged (Bollen et al., Citation2012; Lee et al., Citation2016; Staubli, Oertli, & Nebiker, Citation2015), in terms of clinical utility, an ideal marker to predict SAP during the first 48 h after admission is yet to be found. Previously, IL-8 and HGF have been shown to predict OD, but these studies include also the patients having SAP already on admission (Aoun et al., Citation2009; Espinosa et al., Citation2011; Sporek et al., Citation2013; Ueda et al., Citation1996, 1997; Zhang, Niu, & Yang, Citation2014). Using novel Multiplex detection technology, we recently found that out of the 48 cytokines tested, IL-8, HGF, and granulocyte colony-stimulating factor (G-CSF) predicted the development of SAP in a subgroup of AP patients who presented without OD (Nieminen et al., Citation2014). Here, we focused on IL-8 and HGF, and studied, in an independent cohort of AP patients, if they (i) predict SAP, and (ii) provide a means to discriminate transient OD from persistent OD in AP patients presenting with OD.
2. Materials and methods
2.1. Patients and definitions
The study cohort consists of 176 non-consecutive AP patients admitted to Helsinki University Hospital between March 2011 and August 2014, within 96 h after onset of symptoms. The patients with a history of chronic pancreatitis were excluded. The physicians at the emergency department enrolled the 165/176 patients into the study on admission to hospital. Out of 176 patients, 11 SAP patients, who had participated in another study (Nisula et al., Citation2013), were enrolled upon admission to ICU. Thirty-two adult healthy subjects (20 men and 12 women, median age 46 years, range 21–71) recruited within the study period served as controls.
The study was approved by the Ethical Committee of the Department of Surgery at Helsinki University Hospital, and each patient, or their next of kin, gave their written informed consent to the study. A verbal informed consent was obtained from the voluntary healthcare professionals serving as controls. From the cohort, the circulating levels of matrix metalloproteinase 8 have been studied in relation to the severity of AP (Nukarinen et al., Citation2016).
AP was diagnosed if two of the following three features were present: (1) acute onset of epigastrial pain, (2) plasma amylase level more than three times over the upper limit of the normal range and/or (3) characteristic features in computed tomography. AP treatment followed the international guidelines including early moderately aggressive fluid resuscitation, no routine use of prophylactic antibiotics, nasojejunal tube for enteral feeding in SAP, and endoscopic retrograde cholangiopancreatography if biliary obstruction and concurrent cholangitis were present (Working Party of the British Society of Gastroenterology, Association of Surgeons of Great Britain & Ireland, Pancreatic Society of Great Britain & Ireland, & Association of Upper GI Surgeons of Great Britain & Ireland, Citation2005).
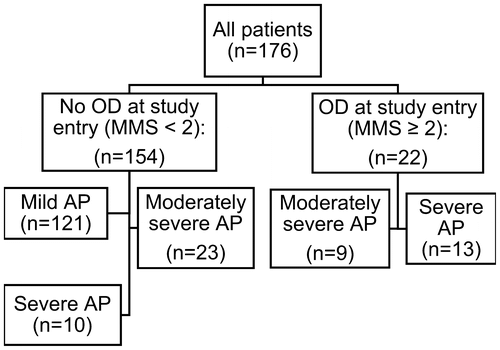
Demographic and clinical characteristics of patients were collected from medical charts. The severity of AP was retrospectively analyzed according to the revised Atlanta classification (Banks et al., Citation2013). The presence of OD was assessed at study entry according to the Modified Marshall Score (MMS), where three organ systems (respiratory, cardiovascular and renal) are evaluated and OD is present if a patient receives ≥2 points from at least one of the organ systems (Banks et al., Citation2013; Marshall et al., Citation1995). The flow chart of the patients is shown in Figure .
Figure 1. Flow chart of the patients. The presence or absence of organ dysfunction (OD) at study entry was assessed according to modified Marshall score (MMS), (Marshall et al., Citation1995) and the severity of acute pancreatitis (AP) according to the revised Atlanta classification (Banks et al., Citation2013).
2.2. Samples
Non-fasting plasma samples were taken after enrollment to commercial EDTA tubes and the samples were stored at 80°C until analyzed. Sampling occurred within 24 h after hospital admission in 91% of patients, and among the rest within the first three days. Among all patients the sampling occurred within five days after onset of symptoms. The delay in sampling, calculated from the onset of symptoms or from hospital admission, did not differ significantly between mild, moderately severe or severe AP (Table ). In addition, the delay in sampling did not correlate with the IL-8 or HGF levels (Table ).
Table 1. Characteristics of patients categorized according to the severity of acute pancreatitis
The IL-8 and HGF levels were measured by enzyme-linked immunosorbent assay (ELISA) using reagents from BD Biosciences, Erembodegem, Belgium (IL-8) and R&D Systems Europe Ltd, Abingdon, UK (HGF). The detection limits and inter-assay coefficients of variation were 0.8 pg/mL and 5.0% for IL-8 and 7.8 pg/mL and 5.6% for HGF, respectively. In 17 of the 176 patients, the HGF levels were below the detection limit and therefore these values have been extrapolated as 0.002 ng/mL (one fifth of the lowest HGF value determined). IL-8 from all patients fell within the standard range of the IL-8 ELISA. Evaluating only IL-8 and HGF levels, and not G-CSF, was based on the results of our previous cytokine study (see Table 6 in Nieminen et al., Citation2014), showing that out of those three cytokines either IL-8 or HGF found all the identified SAP patients presenting without OD (MMS < 2).
C-reactive protein (CRP) and creatinine were chosen for comparison, because they belong to routine follow-up of AP patients and have shown to have prognostic value in AP (Muddana, Whitcomb, Khalid, Slivka, & Papachristou, Citation2009; Puolakkainen, Valtonen, Paananen, & Schroder, Citation1987). Their plasma values were determined according to the hospital’s routine laboratory practice, where normal reference range for CRP is less than 10 mg/L and for creatinine 50–90 μmol/L.
2.3. Statistics
We used IBM SPSS® Statistic version 19 (SPSS, Chicago, Illinois, USA) statistical software for statistical analysis. The nonparametric tests were used because of the skewness of the data, and the results are given as medians and interquartile ranges (IQR) or number of patients and percentages. Comparisons between two groups were made using the Mann-Whitney U test, and those between three groups with the Jonckheere-Terpstra test for trend. Correlations between two continuous variables were done using the Spearman rank correlation. In the analyses, p values < 0.05 were considered significant and double-sided tests were used.
Receiver operator characteristic (ROC) curve analysis with respective areas under the ROC curves (AUCs) were obtained to compare the overall performance of each biomarker and the combined logistic regression model of IL-8 and HGF. The clinically optimal cutoff values, used for post hoc analysis, were chosen from the curves using a similar method as in our previous study (Nieminen et al., Citation2014). In short, we determined the specificity of ≥90%, and chose the point on the ROC curve, where the longest increase in the sensitivity of the slope declines. This method was used to mimic an everyday hospital life, where the limited ICU resources should be aimed to only those who will reliably develop SAP. We figured, that an acceptable rate of a false positive test result would be ≤10%. For each cutoff value we determined sensitivity, specificity, positive and negative likelihood ratios (LR), and diagnostic odds ratio (DOR), with 95% confidence intervals (CI) (Newcombe, Citation1998). Finally, using a univariate logistic regression analysis we analyzed if the odds ratio to predict SAP was significant among the biomarkers.
3. Results
3.1. Patients
The characteristics of patients are shown in Table . All the patients with SAP were treated in the ICU and developed either respiratory or renal failure needing invasive mechanical ventilation and/or haemodialysis. The most common etiology for AP was alcohol (63%). Two patients, both with SAP, died.
3.2. IL-8 and HGF predict SAP among patients without OD (MMS < 2) at study entry
The levels of IL-8, HGF, CRP, and creatinine are given in Supplemental online material, Table S1. The circulating levels of IL-8 and HGF of patients were significantly higher than those of controls, and they correlated with the severity of AP (p < 0.001 for both) and also with each other (Spearman’s r = 0.514, p < 0.001). The highest IL-8 levels were found in two non-survivors. The IL-8 and HGF levels predicted SAP among all AP patients (Supplemental online material, Figure S1 and Table S2). Of the SAP patients 10 did not have OD at study entry (MMS < 2) and were analyzed further.
The HGF and IL-8 values were significantly higher among the 10 patients who developed SAP than among patients who did not (Table ). The AUCs of IL-8, HGF and the combined logistic regression model of IL-8 and HGF were 0.73, 0.79 and 0.82, respectively (Figure (A), Table ). At the clinically optimal cutoff level, sensitivity, specificity, LRs, and DOR of IL-8 and HGF were much alike in predicting SAP (Table ). The ROC curves in Figure (A) show that combining IL-8 and HGF did not improve the model. At the clinically optimal cutoff level, the univariate regression analysis revealed IL-8, HGF, and CRP, but not creatinine, as significant predictors of SAP (Table ).
Table 2. Biomarker levels in relation to the severity of acute pancreatitis among patients without and with organ dysfunction at study entry
Figure 2. The ROC curves of IL-8 and HGF and the combined logistic regression model of IL-8 and HGF in prediction of severe acute pancreatitis (A) among patients without organ dysfunction at study entry (MMS < 2) compared to CRP, and (B) among patients with OD at study entry (MMS ≥ 2). The arrows point to optimal cutoff values used to calculate the statistical parameters of each biomarker for Table .
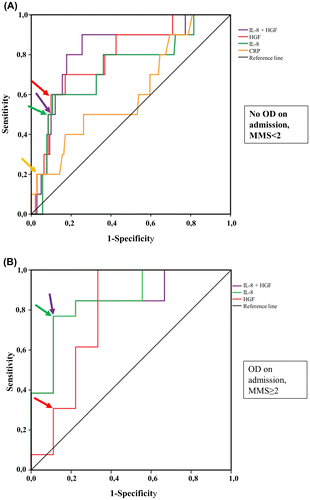
Table 3. Statistical performance of the biomarkers to predict severe acute pancreatitis at the clinically optimal cutoff level among patients without and with organ dysfunction at study entry
Table 4. Univariate analysis of clinical factors and biomarkers in predicting severe acute pancreatitis among patients without and with organ dysfunction at study entry
3.3. IL-8 predicts development of persistent OD among patients with OD (MMS ≥ 2) at study entry
Of all patients 22 had OD at study entry (Figure ). In 9 of them OD resolved within 48 h (moderately severe AP), but in 13 of them OD was persistent and, thus, AP was classified as severe. The levels of both IL-8 and HGF were significantly higher in patients in whom OD did not resolve within 48 h than in those in whom it resolved, but there was no significant difference in the CRP or creatinine values (Table ).
The AUCs of IL-8, HGF and the combined regression model of IL-8 and HGF were 0.86, 0.78 and 0.84, respectively (Figure (B), Table ). In the ROC curve analysis CRP and creatinine did not predict SAP with the AUCs of 0.43 (95% CI, 0.18–0.68) and 0.36 (95% CI, 0.13–0.60), respectively, and thus we excluded them from the further analysis. As Figure (B) shows, combining IL-8 and HGF did not improve the model compared to IL-8 alone. In the univariate regression analysis, IL-8 was a significant predictor of SAP (Table ).
4. Discussion
In this study we show, using cytokine-specific ELISA, that IL-8 and HGF predict the development of SAP among an independent cohort of AP patients without OD (MMS < 2) at study entry. The results confirm the results of our screening study using Multiplex detection technology (Nieminen et al., Citation2014). A great number of previous studies show IL-8 as a predictor of SAP (Aoun et al., Citation2009; Zhang et al., Citation2014), whereas HGF studies are only few consisting a limited number of patients (Espinosa et al., Citation2011; Sporek et al., Citation2013; Ueda et al., Citation1996, 1997). A pitfall in the previous studies is, that they usually contain all patients, i.e. also the patients with OD at study entry, which may distort the results, when a true predictive marker is wanted. Reliable early predictive markers are needed, since assessment of clinical severity in AP patients on admission to hospital is difficult. Indeed, about half of the SAP patients present without clinical signs of OD (Buter et al., Citation2002; Maksimow et al., Citation2014; Nieminen et al., Citation2014; Penttila et al., Citation2016). Among such patients close monitoring and early treatment with moderately aggressive intravenous hydration is often delayed, which may worsen the outcome. Additionally, in the future, this patient group may in a therapeutic window for immunomodulatory therapies (Buter et al., Citation2002; Norman, Citation1998). So far, along with IL-8 and HGF, granulocyte colony-stimulating factor (Nieminen et al., Citation2014), adenosine-generating ecto-5′-nucleotidase/CD73 (Maksimow et al., Citation2014), and circulating nucleosomes (Penttila et al., Citation2016) have shown predictive value in the subgroup of patients without OD (MMS < 2) at presentation.
Another finding in the current study was that IL-8 may predict development of SAP (persistent OD) among patients with OD (MMS ≥ 2) at study entry. In our previous study (Nieminen et al., Citation2014), the IL-8 levels were higher in persistent OD group than in transient OD group, but the difference was not statistically significant. In the current study, both IL-8 and HGF levels were significantly higher among patients in whom the OD was persistent compared to those with transient OD, but a logistic regression analysis showed only IL-8 ≥ 130.9 pg/mL as a significant predictor of SAP. Additionally, the highest IL-8 levels were found in two SAP patients with OD (MMS ≥ 2) at study entry, who died later during hospitalization. In clinical practice, however, distinguishing between transient and persistent OD may not be crucial at present, because all the patients presenting with OD need immediate intensive monitoring and optimal treatment preferably in the ICU (Tenner, Baillie, DeWitt, Vege, & American College of Gastroenterology, Citation2013; Working Group IAP/APA Acute Pancreatitis Guidelines, Citation2013), and it is not possible to assess whether the quickly (within 48 h) resolving OD is due to ICU treatment or the natural course of AP.
Although our aim was to perform a reliable statistical analysis according to the recommendations for predictive marker studies (Windsor, Citation2008, 2010), including not only sensitivity and specificity, but also LRs and DOR with 95% CI, the statistics of the current study has limitations. The number of patients, especially among patients with OD at study entry (MMS ≥ 2) is limited, resulting in wide 95% CI. Another limitation is, that a post hoc analysis, where the cutoff values are obtained from the same population where their predictive value was analyzed, is known to exaggerate the results. The third limitation involves sepsis, which may induce significant rise in IL-8 levels (Hack et al., Citation1992; Rau et al., Citation1997).We however evaluated carefully if any of the patients had sepsis at study entry and found none. This, and the finding that sepsis is uncommon in an early stage of AP (Beger, Bittner, Block, & Buchler, Citation1986) strongly suggest that sepsis may not explain our results.
Despite different analytical methods used in the current study and in our previous study (Nieminen et al., Citation2014), IL-8 and HGF showed similar predictive value in both of the studies according to the similar AUCs and + LRs. However, the predictive values of IL-8 and HGF are not perfect if clinically optimal cutoff values are used. Using a conventional method by choosing a cutoff value on the ROC curve closest to the upper left corner results in the maximal sum of sensitivity and specificity. We wanted to use another method, and chose a cutoff point with the high specificity (≥90%), to assess if a biomarker is useful in everyday hospital life in differentiating reliably the patients who will develop SAP and should be admitted to ICU without delay. The results show that +LRs of both IL-8 and HGF are less than 10 suggesting that the likelihood of SAP is too low, and therefore the clinical value of the markers in predicting SAP is limited. The reason for the limited performance of the cytokines may derive e.g. from the individual differences in the immune system and cytokine response. Also, the time of presentation after onset of symptoms may affect the cytokine levels due to their rapid kinetics. Combining cytokines, or other predictive markers, may reduce the impact of these effects (Mentula et al., Citation2005). Although in our previous study (Nieminen et al., Citation2014) the combined model of IL-8 and HGF showed better predictive value compared to a single cytokine, here it showed no additional benefit, possibly due to the moderately strong correlation between IL-8 and HGF.
In conclusion, the results of the current study support our preliminary findings (Nieminen et al., Citation2014) that the circulating levels of IL-8, or HGF, may act as predictive markers of SAP in patients without OD at presentation, i.e. the patient group that may be in a therapeutic window for immunomodulatory treatment modalities. Combining the markers did not improve the model. Second, in patients with OD at presentation, IL-8 level may help to distinguish the patients with transient OD from those who will develop persistent OD.
Supplementary material
Supplemental material for this article can be accessed here https://doi.org/10.1080/2331205X.2017.1396634.
Funding
The study was supported by the Sigrid Juselius Foundation, Helsinki, Finland, and the Helsinki University Hospital Research Funds, Helsinki, Finland, and the competitive research funding of Tampere University Hospital, Tampere, Finland.
| List of abbreviations | ||
| AP | = | acute pancreatitis |
| APACHE | = | acute physiology and chronic health evaluation |
| AUC | = | area under the curve |
| CI | = | confidence interval |
| CRP | = | C-reactive protein |
| DOR | = | diagnostic odds ratio |
| ELISA | = | enzyme-linked immunosorbent assay |
| HGF | = | hepatocyte growth factor |
| ICU | = | intensive care unit |
| IQR | = | interquartile range |
| IL | = | interleukin |
| LOS | = | length of hospital stay |
| LR | = | likelihood ratio |
| MMS | = | modified Marshall score |
| MOD | = | multiple organ dysfunction |
| OD | = | organ dysfunction |
| ROC | = | receiver operator characteristic |
| SAP | = | severe acute pancreatitis |
Supplemental_online_material.doc
Download MS Word (613 KB)Additional information
Notes on contributors
Anne K. Penttilä
The research activities of our group are focused on immunopathogenic mechanisms behind acute and chronic inflammatory diseases, such as acute pancreatitis, sepsis and rheumatoid arthritis. We have a pleasure of working in a close collaboration between scientists and medical doctors, which facilitates the conduct of clinical studies. Our group has a wide experience in carrying out studies on predictive markers of severe acute pancreatitis. The current study is a part of the first author’s PhD dissertation on predicting development of severe acute pancreatitis under the supervision of the last two co-authors.
References
- Aoun, E., Chen, J., Reighard, D., Gleeson, F. C., Whitcomb, D. C., & Papachristou, G. I. (2009). Diagnostic accuracy of interleukin-6 and interleukin-8 in predicting severe acute pancreatitis: A meta-analysis. Pancreatology, 9, 777–785.10.1159/000214191
- Banks, P. A., Bollen, T. L., Dervenis, C., Gooszen, H. G., Johnson, C. D., Sarr, M. G., … Vege, S. S. (2013). Classification of acute pancreatitis–2012: Revision of the Atlanta classification and definitions by international consensus. Gut, 62, 102–111.10.1136/gutjnl-2012-302779
- Beger, H. G., Bittner, R., Block, S., & Buchler, M. (1986). Bacterial contamination of pancreatic necrosis. A prospective clinical study. Gastroenterology, 91, 433–438.
- Bollen, T. L., Singh, V. K., Maurer, R., Repas, K., Van Es, H. W., Banks, P. A., & Mortele, K. J. (2012). A comparative evaluation of radiologic and clinical scoring systems in the early prediction of severity in acute pancreatitis. The American Journal of Gastroenterology, 107, 612–619.10.1038/ajg.2011.438
- Buter, A., Imrie, C. W., Carter, C. R., Evans, S., & McKay, C. J. (2002). Dynamic nature of early organ dysfunction determines outcome in acute pancreatitis. British Journal of Surgery, 89, 298–302.10.1046/j.0007-1323.2001.02025.x
- Espinosa, L., Linares, P. M., Bejerano, A., Lopez, C., Sanchez, A., Moreno-Otero, R., & Gisbert, J. P. (2011). Soluble angiogenic factors in patients with acute pancreatitis. Journal of Clinical Gastroenterology, 45, 630–637.10.1097/MCG.0b013e31820d3533
- Hack, C. E., Hart, M., van Schijndel, R. J., Eerenberg, A. J., Nuijens, J. H., Thijs, L. G., & Aarden, L. A. (1992). Interleukin-8 in sepsis: Relation to shock and inflammatory mediators. Infection and Immunity, 60, 2835–2842.
- Halonen, K. I., Pettilä, V., Leppäniemi, A. K., Kemppainen, E. A., Puolakkainen, P. A., & Haapiainen, R. K. (2002). Multiple organ dysfunction associated with severe acute pancreatitis. Critical Care Medicine, 30, 1274–1279.10.1097/00003246-200206000-00019
- Haydock, M. D., Mittal, A., Wilms, H. R., Phillips, A., Petrov, M. S., & Windsor, J. A. (2013). Fluid therapy in acute pancreatitis: Anybody’s guess. Annals of Surgery, 257, 182–188.10.1097/SLA.0b013e31827773ff
- Johnson, C. D., & Abu-Hilal, M. (2004). Persistent organ failure during the first week as a marker of fatal outcome in acute pancreatitis. Gut, 53, 1340–1344.10.1136/gut.2004.039883
- Kylanpaa, L., Rakonczay, Jr., Z., & O’Reilly, D. A. (2012). The clinical course of acute pancreatitis and the inflammatory mediators that drive it. International Journal of Inflammation, 360685.
- Lee, K. J., Kim, H. M., Choi, J. S., Kim, Y. J., Kim, Y. S., & Cho, J. H. (2016). Comparison of predictive systems in severe acute pancreatitis according to the revised Atlanta classification. Pancreas, 45, 46–50.10.1097/MPA.0000000000000433
- Maksimow, M., Kyhälä, L., Nieminen, A., Kylänpää, L., Aalto, K., Elima, K., ... Jalkanen, S. (2014). Early prediction of persistent organ failure by soluble CD73 in patients with acute pancreatitis. Critical Care Medicine, 42, 2556–2564.10.1097/CCM.0000000000000550
- Marshall, J. C., Cook, D. J., Christou, N. V., Bernard, G. R., Sprung, C. L., & Sibbald, W. J. (1995). Multiple organ dysfunction score: A reliable descriptor of a complex clinical outcome, 23. Critical Care Medicine, 1638–1652.10.1097/00003246-199510000-00007
- Mc Kay, C. J., & Buter, A. (2003). Natural history of organ failure in acute pancreatitis. Pancreatology, 3, 111–114.10.1159/000070078
- Mentula, P., Kylänpää, M. L., Kemppainen, E., Jansson, S. E., Sarna, S., Puolakkainen, P., ... Repo, H. (2005). Early prediction of organ failure by combined markers in patients with acute pancreatitis. British Journal of Surgery, 92, 68–75.10.1002/bjs.v92:1
- Muddana, V., Whitcomb, D. C., Khalid, A., Slivka, A., & Papachristou, G. I. (2009). Elevated serum creatinine as a marker of pancreatic necrosis in acute pancreatitis. The American Journal of Gastroenterology, 104, 164–170.10.1038/ajg.2008.66
- Newcombe, R. G. (1998). Two-sided confidence intervals for the single proportion: Comparison of seven methods. Statistics in Medicine, 17, 857–872.10.1002/(ISSN)1097-0258
- Nieminen, A., Maksimow, M., Mentula, P., Kyhälä, L., Kylänpää, L., Puolakkainen, P., … Salmi, M. (2014). Circulating cytokines in predicting development of severe acute pancreatitis. Critical Care, 18, R104.10.1186/cc13885
- Nisula, S., Kaukonen, K. M., Vaara, S. T., Korhonen, A. M., Poukkanen, M., Karlsson, S., … Laurila, J. J. (2013). Incidence, risk factors and 90-day mortality of patients with acute kidney injury in Finnish intensive care units: The FINNAKI study. Intensive Care Medicine, 30, 420–428.10.1007/s00134-012-2796-5
- Norman, J. (1998). The role of cytokines in the pathogenesis of acute pancreatitis. The American Journal of Surgery, 175, 76–83.10.1016/S0002-9610(97)00240-7
- Nukarinen, E., Lindström, O., Kuuliala, K., Kylänpää, L., Pettilä, V., Puolakkainen, P., … Hästbacka, J. (2016). Association of matrix metalloproteinases -7, -8, -9 and TIMP -1 with disease severity in acute pancreatitis. PLoS One, 11(8), e0161480.10.1371/journal.pone.0161480
- Penttila A. K., Rouhiainen A., Kylanpaa L., Mustonen, H., Puolakkainen, P., Rauvala, H., & Repo, H. (2016). Circulating nucleosomes as predictive markers of severe acute pancreatitis. Journal of Intensive Care, 4, 14. 14-016-0135-6.
- Puolakkainen, P., Valtonen, V., Paananen, A., & Schroder, T. (1987). C-reactive protein (CRP) and serum phospholipase A2 in the assessment of the severity of acute pancreatitis. Gut, 28, 764–771.10.1136/gut.28.6.764
- Rakonczay, Jr., Z., Hegyi, P., Takacs, T., McCarroll, J., & Saluja, A. K. (2008). The role of NF-kappaB activation in the pathogenesis of acute pancreatitis. Gut, 57, 259–267.10.1136/gut.2007.124115
- Rau, B., Steinbach, G., Gansauge, F., Mayer, J. M., Grunert, A., & Beger, H. G. (1997). The potential role of procalcitonin and interleukin 8 in the prediction of infected necrosis in acute pancreatitis. Gut, 41, 832–840.10.1136/gut.41.6.832
- Sah, R. P., Dawra, R. K., & Saluja, A. K. (2013). New insights into the pathogenesis of pancreatitis. Current Opinion in Gastroenterology, 29, 523–530.10.1097/MOG.0b013e328363e399
- Sporek, M., Kolber, W., Kusnierz-Cabala, B., Dumnicka, P., Gurda-Duda, A., Kuzniewski, M., … Kulig, J. (2013). Determination of hepatocyte growth factor at early phase of acute pancreatitis. Folia medica Cracoviensia, 53, 87–95.
- Staubli, S. M., Oertli, D., & Nebiker, C. A. (2015). Laboratory markers predicting severity of acute pancreatitis. Critical Reviews in Clinical Laboratory Sciences, 52, 273–283.10.3109/10408363.2015.1051659
- Tenner, S., Baillie, J., DeWitt, J., Vege, S. S., & American College of Gastroenterology (2013). American College of Gastroenterology guideline: Management of acute pancreatitis. The American Journal of Gastroenterology, 108, 1400–1415.10.1038/ajg.2013.218
- Ueda, T., Takeyama, Y., Hori, Y., Nishikawa, J., Yamamoto, M., & Saitoh, Y. (1997). Hepatocyte growth factor in assessment of acute pancreatitis: Comparison with C-reactive protein and interleukin-6. Journal of Gastroenterology, 32, 63–70.10.1007/BF01213298
- Ueda, T., Takeyama, Y., Toyokawa, A., Kishida, S., Yamamoto, M., & Saitoh, Y. (1996). Significant elevation of serum human hepatocyte growth factor levels in patients with acute pancreatitis. Pancreas, 12, 76–83.10.1097/00006676-199601000-00010
- Vege, S. S., Gardner, T. B., Chari, S. T., Munukuti, P., Pearson, R. K., Clain, J. E., … Sarr, M. G. (2009). Low mortality and high morbidity in severe acute pancreatitis without organ failure: A case for revising the atlanta classification to include “moderately severe acute pancreatitis”. The American Journal of Gastroenterology, 104, 710–715.10.1038/ajg.2008.77
- Windsor, J. A. (2008). Assessment of the severity of acute pancreatitis: No room for complacency. Pancreatology, 8, 105–109.10.1159/000123604
- Windsor, J. A. (2010). A better way to predict the outcome in acute pancreatitis? The American Journal of Gastroenterology, 105, 1671–1673.10.1038/ajg.2010.145
- Working Group IAP/APA Acute Pancreatitis Guidelines (2013). IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology, 13, e1–e15.
- Working Party of the British Society of Gastroenterology, Association of Surgeons of Great Britain and Ireland, Pancreatic Society of Great Britain and Ireland, Association of Upper GI Surgeons of Great Britain and Ireland. (2005). UK guidelines for the management of acute pancreatitis. Gut, 54, iii1–iii9.
- Zhang, J., Niu, J., & Yang, J. (2014). Interleukin-6, interleukin-8 and interleukin-10 in estimating the severity of acute pancreatitis: An updated meta-analysis. Hepato-Gastroenterology, 61, 215–220.
