Abstract
Introduction: mRECIST were introduced in early 2000 for radiofrequency ablation (RFA) of lung tumours. This retrospective study was performed to evaluate mRECIST in the assessment of lung tumours treated with microwave ablation (MWA).
Material and Methods: All percutaneous CT–guided MWA of lung tumours performed between June 2010 and December 2016 were identified and cases with at least 10 months follow-up were included. The therapeutic response was evaluated based on mRECIST.
Results: Of the 35 patients included 23 had non-small cell lung cancer (NSCLC) and 12 patients had colorectal cancer (CRC) metastases. Compared to the 24-h post-ablation scan, at 24 months the median CT mass size had reduced to 51.1% from baseline. Based on mRECIST, at 12 months 84.8% showed partial response and 15.2% had stable disease. At 24 months 90% (n = 20) showed partial response whereas 5% had stable disease and another 5% showed complete response. A large proportion of patients were alive 12 months after MWA with a partial response based on mRECIST; this partial response rate had not changed at the 24 months mark.
Conclusion: This study challenges the use of mRECIST for the assessment of therapeutic response of lung tumours treated with MWA suggesting that timelines, especially regarding the baseline scan and ablation characteristics, may need to be revised.
Public Interest Statement
Microwave ablation (MWA) has become an established treatment option for solid tumours and proves to be more potent in the treatment of lung cancers than radiofrequency ablation (RFA), a heat-based thermal ablation modality that has been around for approximately a decade longer. The ablation duration with MWA is much shorter, usually shorter than 10 min, and the thermal damage is much larger and more homogeneous. Ensuing inflammatory reaction locally and along the lymphatic drainage path also tends to be stronger and to last longer than with RFA.
The modified response evaluation in solid tumours (mRECIST) for lung lesion ablation, established and published 2007, was relating to thermal damage produced by RFA. Applying the same criteria to lung tumours treated by MWA may lead to misinterpretation of the radiology findings in the wake of the ablation, potentially labelling the ablation result “progressive disease” when in fact the ablation has been successful.
This article challenges mRECIST for the assessment of lung MWA.
1. Introduction
Lung cancer remains the leading cause of cancer death worldwide. (Wong, Lao, Ho, Goggins, & Tse, Citation2017) Metastatic deposits from various primary sites are commonly seen in the lung. Surgical resection is the best curative treatment option for lung cancer. However, many patients with lung tumours have significant medical comorbidities, precluding them from operative management (Shirvani et al., Citation2012). In the last few decades, minimally invasive percutaneous ablative techniques such as radiofrequency ablation (RFA) have been increasingly used as an alternative local therapy to treat both primary and metastatic lung malignancies in selected patients. (Hiraki et al., Citation2007a, Citation2007b; Lencioni et al., Citation2008; Simon et al., Citation2007; Yamakado, Hase, & Matsuoka et al., Citation2007)
RFA has been used for many years in different clinical scenarios including the treatment of cardiac arrhythmias caused by aberrant conduction pathways (Calkins et al., Citation2000). By early 2000, its clinical application in treating lung tumours had been studied and accepted (Dupuy et al., Citation2000; Hiraki et al., Citation2007a, Citation2007b; Lencioni et al., Citation2008; Simon et al., Citation2007; Yamakado et al., Citation2007). After its introduction for the treatment of lung cancer, modified Response Evaluation Criteria in Solid Tumours (mRECIST) had been developed to assess the tumour response to RFA (Table ). (Herrera et al., Citation2003)
Table 1. Modified RECIST criteria for thermal ablation of lung tumours
More recently, microwave ablation (MWA) has gained momentum in treating lung tumours because of its ability to deliver faster and deeper ablation with a uniform distribution of energy (Simon, Dupuy, & Mayo-Smith, Citation2005) creating larger and uniform zones of ablation. (Brace, Hinshaw, Laeseke, Sampson, & Lee, Citation2009) However, this technical advantage over RFA poses a special challenge in using mRECIST in evaluating lung cancers treated with MWA. The more potent MWA creates larger volumes of thermal damage and stronger inflammatory response may result in mis-judgement of the therapeutic response using mRECIST criteria. Hence, this retrospective study was conducted to assess the usefulness of mRECIST in the evaluation of MWA-treated lung malignancies, in particular, to challenge the appropriateness of using pre-ablation mRECIST as a baseline measure.
2. Methods
2.1. Patients and study period
Institutional ethics approval was obtained (HREC/14/QRBW/553). In this retrospective cohort study, searching our departmental thermal ablation database, we identified a total of 70 patients who underwent CT-guided MWA of pulmonary tumours at our institution between June 2010 and December 2016. Inclusion criteria consisted of a follow-up period of more than 10 months. Patients were excluded if they had a repeat ablation of the same lesion within a 12-month period, or other concurrent or sequential therapies including surgery, radiotherapy or chemotherapy. Thirty-five patients were included in this study.
2.2. Instrumentation
All ablations were performed under CT guidance. The Acculis microwave tissue ablation system (AngioDynamics, Latham, NY, USA) was used in 30 cases. The system operates at 2.45 GHz with a maximum power output of 140 W. Three cases were treated with the Amica MWA system (HS hospital Service, Rome, Italy), which also operates at 2.45 GHz with a maximum output of 140 W. The Evident TM system (Covidien, Boulder, CO, USA) was used in two cases, operating at 915 MHz with a maximum power output of 45 W.
2.3. Procedure
One interventional radiologist with more than 15 years of experience in image-guided thermo-ablation performed all ablations. Written informed consent was obtained. Anticoagulation or antiplatelet therapies were stopped for 1 to 7 days prior to the procedure. No antibiotic prophylaxis was administered. Conscious sedation or general anaesthesia was used during all ablation sessions, as per the preference of the anaesthetic team.
Immediately prior to each MWA, a non-contrast CT scan of the target area was obtained for approach planning. Under sterile conditions and CT guidance, the antenna was inserted into the target lesion. Multiplanar reformats were used for confirmation of the final antenna position. Some patients underwent over-lapping ablations, depending on the size and shape of lesions. In addition, power output and ablation duration were carefully considered and chosen based on the lesion size, shape and proximity to vessels.
2.4. Post-procedural follow-up
In addition to the limited non-contrast CT scan immediately after removal of the antenna, a routine chest X-ray was performed 3 h after MWA to assess post-procedure complications. All patients were admitted overnight post ablation and underwent a non-contrast CT scan of the ablation site on the following day to evaluate the thermal damage and assess potential complications not identified on X-ray imaging. This 24-h CT scan was also used as a baseline scan for comparison with all future scans.
Further follow-up imaging with contrast-enhanced CT, unless contra-indicated, was performed at 3, 6, 12, 18, 24 months post-MWA and yearly thereafter for a total of five years. A two-month window either side of each target follow-up was accepted and included as that target interval’s scan.
DICOM data of follow-up scans were uploaded and reviewed on an AFGA IMPAX 6 workstation, and measurements of ablated lesions were performed in the lung window on axial images. The longest diameters of an ablated lesion were compared on subsequent scans. The mass quality was also noted as defined by mRECIST criteria.
Fludeoxyglucose-positron emission tomography (FDG-PET) performed 3 to 24 months after MWA was included. Not all patients had an FDG-PET scan pre- and/or post-ablation, as it often had not been readily available. Post-ablation FDG-PET results were only evaluated in patients with a pre-MWA FDG-PET for comparison. The maximum standard uptake value (SUVmax) was established with syngo.via (Siemens imaging software). The value was calculated from the FDG dose, time of administration and imaging, and patient’s weight and height. The therapeutic response to MWA was then determined using mRECIST criteria.
2.5. Statistical analysis
Patient characteristics and tumour responses were summarised using mean (standard deviation (SD)) or median (interquartile range (IQR)) for continuous variables and frequencies and percentage for categorical variables. Stata15 was used for analyses and graphs.
3. Results
Thirty-five patients met the inclusion criteria with a mean age of 68.2 years (SD 12.5) and 66% male. Twenty-three patients had NSCLC, and 12 patients had CRC metastases. The pre-ablation median size of the treated lesions was 20 mm (IQR 16.0–26.0 mm).
CT mass size was evaluated on serial follow-up CT scans (Table ). Median follow-up was 26 months (range 12 to 68 month). When the pre-ablation size was considered as baseline, the CT mass size increased to 219.7% of the original lesion on the 24-h post-ablation scan (Table ). At 24 months, the median lesion CT mass size was still larger than its baseline.
Table 2. Absolute and percentage CT mass size change over time
Table 3. Tumour response based on mRECIST criteria
On the other hand, when the 24-h post-ablation size was considered as baseline, the lesion was 70.5% of the baseline at 3 months and reached 51.1% at 24 months. Irrespective of the baseline used, the lesion size seemed to reach a plateau by the 24-month period, although there were 15 missing data points at that stage.
CT mass quality was also evaluated over time with comparison to the 24-h post-ablation scan. At 3 months post-ablation, 66.7% were classified as stable disease and 26.7% demonstrated partial response. At 24 months, 85% were stable disease and 10% showed partial response.
Out of 35 patients, there were 17 patients with complete pre- and post-ablation SUVmax measurements. Fourteen (82%) of these patients had a reduction in a SUVmax whereas 3 (18%) had an increase in SUVmax.
Using pre-ablation size as baseline, the final mRECIST outcome (Table ) at 12 months resulted in 84.8% (n = 28) with partial response and 15.2% (n = 5) with stable disease. At 24 months post-ablation (n = 20), 90% had partial response. None of the patients with partial response showed clinical features of disease recurrence or progression.
4. Discussion
Over the past decade, MWA has gained in popularity, as it has theoretical advantages over RFA. Recent studies (Acksteiner & Steinke, Citation2015; Han et al., Citation2015; Sun, Song, Guo, & Sheng, Citation2015) show promising results in local tumour control and overall survival with comparable complication rates. (Egashira, Singh, Bandula, & Illing, Citation2016; Ni, Han, Ye, & Wei, Citation2015; Splatt & Steinke, Citation2015; Vogl et al., Citation2017)
As ablative techniques gain popularity in treating both primary and secondary lung cancers, therapeutic evaluation becomes challenging, as pathological assessment is not an option the ablated lesions show different courses of evolution. When RFA initially gained momentum as a minimally invasive alternative treatment option for pulmonary malignancies, modified RECIST criteria were developed in 2003 (Herrera et al., Citation2003). The new criteria incorporated the size, characteristics, and PET avidity of an ablated lesion to account for the difference in the therapeutic response—opposed to tumours treated with other means, usually chemotherapy, where only unidimensional size is taken into account (RECIST criteria).
mRECIST criteria have failed to unequivocally instruct clinicians when to perform the first post-ablation scan as a baseline for future serial imaging. Multiple studies (Goldberg et al., Citation2009; Zaheer, Whitley, Thomas, & Steinke, Citation2015) have established their baseline scan at different time points, including at 24 h, 1 month and 3 months post-ablation. Our study demonstrates how different baseline scans (pre-ablation and 24hrs post-ablation) can lead to drastically different therapeutic evaluations. The CT mass size dramatically increased from pre-ablation to 24-h post-ablation (Figure a-c) and gradually decreased thereafter (Figure a-c) with many patients’ CT mass size not reaching their pre-ablation levels over the follow-up period (Figure a-c). Furthermore, most lesions showed the greatest size reduction within 3 to 6 months post-ablation (Table ). Thus, if a baseline scan were performed at a time later than 24 h post ablation, the size reduction would be less over time leading to underestimation of the therapeutic response. Therefore, this inconsistency can influence the therapeutic evaluation and conclusions derived using mRECIST.
Figure 1. 68y-year-old male with biopsy proven RUL adenocarcinoma. a. volumetric assessment of target lesion and planned trajectory for MWA antenna. b. antenna in situ, 4min 30sec ablation cycle c. 24-hour CT scan showing extent of thermal damage with mixed consolidation and ground-glass opacity around target lesion.
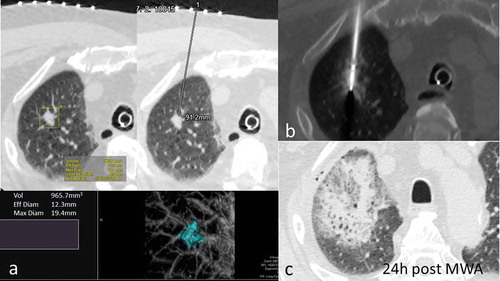
Figure 2. 68y-year-old male with biopsy proven RUL adenocarcinoma, 6-months assessment. a. volumetric assessment of ablated lesion – all three dimensions still significantly larger than pre- ablation b. FDG-PET scan shows very high SUVmax of 13.4 c. density of the ablated focus 48HU; marked pleural tagging present.
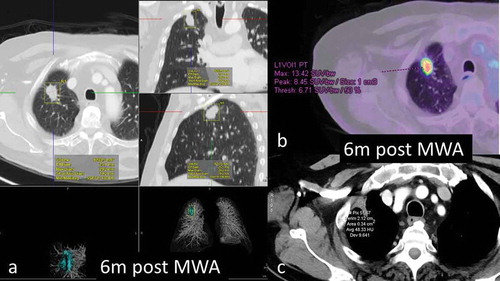
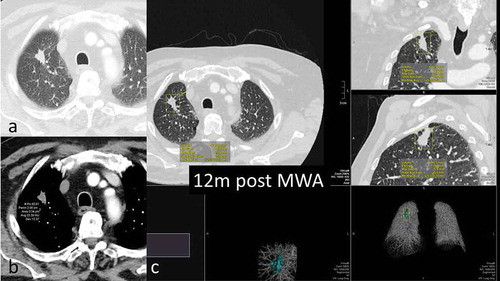
Figure 3. 68y-year-old male with biopsy proven RUL adenocarcinoma, 12-months assessment. a. axial CT scan lung window shows slow shrinkage of the index lesion, still larger than pre-ablation b. density decreased by approximately one third from the 6-month scan c. volumetric assessment – volume 4040mm3 compared to 905mm3 pre ablation; max length 25.8mm compared to 19.4mm pre ablation.
In our study, the use of MWA in lung has demonstrated at times a large circumscribed density at the ablation site that can persist over time. This persistence influences the therapeutic response establishment both in size and mass quality in mRECIST criteria. Our study demonstrates that CT size reaches a plateau after a period of regression. None of the ablated lesions was considered as a complete response by size (Table ). In addition, an increasing proportion of the treated lesions showed stable disease in CT mass quality over time. Our study also demonstrated that a significant proportion of patients were alive and well with no clinical signs of residual disease despite that therapeutic response was evaluated as an overall partial response for more than 2 years post ablation based on mRECIST. These results indicate that mRECIST use in evaluation of MWA treated lung tumours could be misleading.
In addition, FDG-PET, often used to clarify uncertainty in therapeutic response on serial CTs, is not always readily available everywhere. Thus, post-ablative tumour response is often limited to its diameter and mass characteristic using mRECIST. As seen in our study, this has likely resulted in an underestimation of therapeutic response if evaluated by mRECIST, resulting in a partial response or stable disease even years following MWA.
Although SUVmax decreased over time in most cases in our study, suggesting disease response, however only in a minority of the cases Zaheer et al. warned that not all persistent, increased or even new FDG avid uptake at the ablation site is necessarily indicative of local recurrence. (Durick et al., Citation2008)
mRECIST criteria have been developed based on experience with RFA and have been adopted as a guide for monitoring therapeutic response post ablation of lung tumours. However, MWA with a much more powerful output causes larger ablation volumes and associated architectural distortion persisting for a long time. Furthermore, increased inflammatory reactions appear to ensue (personal observation).
Whether treatment response is under- or overestimated will depend on the choice of baseline scan: with pre-ablation size underestimation will happen, whereas with post-24-h ablation size, over-estimation is likely.
The challenge comes from difficulty in differentiating between inflammatory response and treatment response based on CT mass size and quality.
This retrospective study was limited by the fact that not all patients had their follow-up CT scan at the same recommended time interval or had FDG-PET scans pre- and post-MWA to fully assess the appropriateness of mRECIST. A considerable proportion of records were missing from 18 months post-ablation onwards with a decreasing number of patients with serial scans. The exploration of patients’ discharge letters from hospital admissions after the lung tumour ablations often failed to mention the lung cancer treatment all along.
The results should be interpreted with caution as there may be a selection bias owing to missing records.
5. Conclusion
This study challenges the use of mRECIST criteria for the assessment of therapeutic response of lung tumours treated with MWA. It is suggestive that timelines, especially regarding the baseline scan and ablation characteristic criteria may need to be revised. Given the inherent limitations of this retrospective study, prospective studies are required to corroborate these results.
Additional information
Funding
Notes on contributors
Karin Steinke
Karin Steinke is Acting Director of the Department of Medical Imaging at the Royal Brisbane and Women’s Hospital, and Associate Professor at the University of Queensland School of Medicine, Brisbane Australia.
A research fellowship 2002–2003 in Sydney, Australia, was part of her PhD she completed at the University of Basel, Switzerland in 2004, followed by a Master of Surgery at the University of New South Wales, Sydney, Australia in 2005 - both with the topic of Radiofrequency ablation of solid tumors.
Karin’s subspecialties are lung and breast imaging and interventions.
Her field of research is percutaneous thermal ablation of lung tumors, which, together with her interest in thoracic imaging has resulted in over 70 publications and book chapters and over 100 presentations and lectures worldwide.
References
- Acksteiner, A., & Steinke, K. (2015). Percutaneous microwave ablation for early-stage non-small cell lung cancer (NSCLC) in the elderly: A promising outlook. Journal of Medical Imaging and Radiation Oncology, 59, 82–9. doi:10.1111/1754-9485.12251
- Brace, C. L., Hinshaw, J. L., Laeseke, P. F., Sampson, L. A., & Lee, F. T. (2009). Pulmonary thermal ablation: Comparison of radiofrequency and microwave devices by using gross pathologic and CT findings in a swine model. Radiology, 251, 705–711. doi:10.1148/radiol.2513081564
- Calkins, H., Epstein, A., Packer, D., Arria, A. M., Hummel, J., Gilligan, D. M., … Stevenson, W. (2000). Catheter ablation of ventricular tachycardia in patients with structural heart disease using cooled radio-frequency energy: Results of a prospective multicenter study. Cooled RF multi-center investigators group. Journal of the American College of Cardiology, 35, 1905–1914. doi:10.1016/S0735-1097(00)00615-X
- Dupuy, D. E., Zagoria, R. J., Akerley, W., Mayo-Smith, W. W., Kavanagh, P. V., & Safran, H. (2000). Percutaneous radiofrequency ablation of malignancies in the lung. American Journal of Roentgenology, 174, 57–59. doi:10.2214/ajr.174.1.1740057
- Durick, N. A., Laeseke, P. F., Broderick, L. S., Lee, F. T., Sampson, L. A., Frey, T. M., … Brace, C. L. (2008). Microwave ablation with triaxial antennas tuned for lung: Results in an in vivo porcine model. Radiology, 247, 80–87. doi:10.1148/radiol.247106212
- Egashira, Y., Singh, S., Bandula, S., & Illing, R. (2016). Percutaneous high-energy microwave ablation for the treatment of pulmonary tumors: A retrospective single-center experience. Journal of Vascular and Interventional Radiology, 27, 474–479. doi:10.1016/j.jvir.2016.01.001
- Free PMC Article, Shirvani, S. M., Jiang, J., Chang, J. Y., Welsh, J. W., Gomez, D. R., … Smith, B. D.. (2012). Comparative effectiveness of 5 treatment strategies for early-stage non-small cell lung cancer in the elderly. International Journal of Radiation Oncology Biology Physics, 84, 1060–1070. doi:10.1016/j.ijrobp.2012.07.2354
- Goldberg, S. N., Grassi, C. J., Cardella, J. F., Charboneau, J. W., Dodd, G. D., Dupuy, D. E., … Sacks, D. (2009). Image-guided tumor ablation: Standardization of terminology and reporting criteria. Journal of Vascular and Interventional Radiology : JVIR, 20, S377–90. doi:10.1016/j.jvir.2009.04.011
- Han, X., Yang, X., Ye, X., Liu, Q., Huang, G., Wang, J., … Men, M. (2015). Computed tomography-guided percutaneous microwave ablation of patients 75 years of age and older with early stage non-small cell lung cancer. Indian Journal of Cancer, 52, e56–e60. doi:10.4103/0019-509X.172514
- Herrera, L. J., Fernando, H. C., Perry, Y., Gooding, W. E., Buenaventura, P. O., Christie, N. A., & Luketich, J. D. (2003). Radiofrequency ablation of pulmonary malignant tumors in nonsurgical candidates. The Journal of Thoracic and Cardiovascular Surgery, 125, 929–937. doi:10.1067/mtc.2003.18
- Hiraki, T., Gobara, H., Iishi, T., Sano, Y., Iguchi, T., Fujiwara, H., … Kanazawa, S. (2007a). Percutaneous radiofrequency ablation for pulmonary metastases from colorectal cancer: Midterm results in 27 patients. Journal of Vascular and Interventional Radiology : JVIR, 18, 1264–1269. doi:10.1016/j.jtcvs.2007.07.013
- Hiraki, T., Gobara, H., Iishi, T., Sano, Y., Iguchi, T., Fujiwara, H., … Kanazawa, S. (2007b). Percutaneous radiofrequency ablation for clinical stage I non-small cell lung cancer: Results in 20 nonsurgical candidates. The Journal of Thoracic and Cardiovascular Surgery, 134, 1306–1312. doi:10.1016/j.jtcvs.2007.07.013
- Lencioni, R., Crocetti, L., Cioni, R., Suh, R., Glenn, D., Regge, D., … Mussi, A. (2008). Response to radiofrequency ablation of pulmonary tumours: A prospective, intention-to-treat, multicentre clinical trial (the RAPTURE study). The Lancet Oncology, 9, 621–628. doi:10.1016/S1470-2045(08)70155-4
- Ni, X., Han, J., Ye, X., & Wei, Z. (2015). Percutaneous CT-guided microwave ablation as maintenance after first-line treatment for patients with advanced NSCLC. Oncotargets and Therapy, 8, 3227–3235. doi:10.2147/OTT.S90528
- Simon, C. J., Dupuy, D. E., DiPetrillo, T. A., Safran, H. P., Grieco, C. A., Ng, T., & Mayo-Smith, W. W. (2007). Pulmonary radiofrequency ablation: Long-term safety and efficacy in 153 patients. Radiology, 243, 268–275. doi:10.1148/radiol.2431060088
- Simon, C. J., Dupuy, D. E., & Mayo-Smith, W. W. (2005). Microwave ablation: Principles and applications. Radiographics, 25, S69–S83. doi:10.1148/rg.25si055501
- Splatt, A. M., & Steinke, K. (2015). Major complications of high-energy microwave ablation for percutaneous CT-guided treatment of lung malignancies: Single-centre experience after 4 years. Journal of Medical Imaging and Radiation Oncology, 59, 609–616. doi:10.1111/1754-9485.12345
- Sun, Y. H., Song, P. Y., Guo, Y., & Sheng, L. J. (2015). Computed tomography-guided percutaneous microwave ablation therapy for lung cancer. Genetics and Molecular Research : GMR, 14, 4858–4864. doi:10.4238/2015.May.11.18
- Vogl, T. J., Nour-Eldin, N. A., Albrecht, M. H., Kaltenbach, B., Hohenforst-Schmidt, W., Lin, H., … Roman, A. (2017). Thermal ablation of lung tumours: Focus on microwave ablation. RöFo : Fortschritte auf dem Gebiete der Röntgenstrahlen und der Nuklearmedizin, 189, 828–843. doi:10.1055/s-0043-109010
- Wong, M. C. S., Lao, X. Q., Ho, K. F., Goggins, W. B., & Tse, S. L. A. (2017). Incidence and mortality of lung cancer: Global trends and association with socioeconomic status. Scientific Reports, 7(1), 14300. doi:10.1038/s41598-017-14513-7
- Yamakado, K., Hase, S., Matsuoka, T., Tanigawa, N., Nakatsuka, A., Takaki, H., … Sawada, S. (2007). Radiofrequency ablation for the treatment of unresectable lung metastases in patients with colorectal cancer: A multicenter study in Japan. Journal of Vascular and Interventional Radiology : JVIR, 18, 393–398. doi:10.1016/j.jvir.2006.11.003
- Zaheer, S. N., Whitley, J. M., Thomas, P. A., & Steinke, K. (2015). Would you bet on PET? Evaluation of the significance of positive PET scan results post-microwave ablation for non-small cell lung cancer. Journal of Medical Imaging and Radiation Oncology, 59, 702–712. doi:10.1111/1754-9485.12330
